Abstract
Context: Alchornea triplinervia (Spreng.) Müll. Arg. (Euphorbiaceae) is a tree widespread in many Brazilian states. This plant naturally occurs in different ecosystems including tropical Atlantic forest, Amazon rain forest, moist tropical mixed forest, savanna forest, among others. Local populations traditionally use it in tea form to treat gastric disturbances.
Objective: The objective of this research was to evaluate the plant A. triplinervia as a potential inhibitor of some macrophage functions involved in the inflammatory process.
Materials and methods: The effects of Alchornea triplinervia ethyl acetate fraction (AtF) on hydrogen peroxide (H2O2), nitric oxide (NO) and tumor necrosis factor-α (TNF-α) production in peritoneal macrophages were investigated using phenol red, Griess reagent and a sandwich immunoassay, respectively.
Results: AtF chromatographic analyses indicate the presence of flavonoids as majority compounds. The fraction also showed an intense inhibition of H2O2 and NO production. The inhibitory effects of the fraction in H2O2 and NO production ranged from 72.25 ± 4.68 to 69.64 ± 4.21 and from 47.8 ± 8.96 to 76.77 ± 8.11%, respectively in the two tested concentrations, 15.62 and 62.5 μg/mL. TNF-α production was partially inhibited in the tested concentrations and the inhibitory rate was around 18%.
Discussion and conclusion: It is supposed that the elevated biological potential of A. triplinervia is related to the presence of phenolic compounds in the plant leaves. According to the results observed in this study, it is suggested that AtF presents anti-inflammatory activity, supporting the traditional use of A. triplinervia in Brazilian folk medicine.
Introduction
Plants from tropical rainforests represent a rich source of potential immunomodulating substances and leads from ethnobotanical practices have been the primary source of plant selection in the last few years (CitationWilliams, 2001).
The Euphorbiaceae family contains 72 genera and 1,100 species in Brazil (CitationBarroso, 1984). Species from the Alchornea genus have traditionally been used in the treatment of bacterial, fungal, parasitic, and inflammatory disorders. These plants showed in vitro cytotoxic activity against Hep-G2 and A-431 human cancer cell lines, inhibited topoisomerase II and HIV-1 strain HTLVIIIB cytopathicity (CitationSetzer et al., 2000; CitationAyisi & Nyadedzor, 2003). Flavonoids, alkaloids, and other phenolic compounds have been isolated from species of Alchornea (CitationBraca et al., 2002).
Alchornea triplinervia (Spreng.) Müll. Arg (Euphorbiaceae) is a tree that can be found in many Brazilian states, such as Amazonas, Acre, Rondônia, Roraima, Bahia, Mato Grosso, Mato Grosso do Sul, Rio de Janeiro, São Paulo and Rio Grande do Sul (CitationStannard, 1995; CitationDurigan et al., 1999; CitationVaccaro et al., 1999; CitationSecco, 2004). This plant has high ecological plasticity, naturally occurring in different ecosystems including tropical Atlantic forest, Amazon rain forest, moist tropical mixed forest and savanna forest, among others (CitationDurigan et al., 1999; CitationSilva & Marconi, 1990; CitationSiqueira, 1994). An ethnopharmacological survey was carried out with the plant species A. triplinervia, locally known as tapiá and the folk lore sustains the traditional use of its leaves and aerial parts in tea form to treat gastric disturbances (CitationSilva et al., 2000).
However, there are just two scientific-based studies regarding A. triplinervia, one describing the isolation of amentoflavone, isocorilagin, gallic acid, and methyl gallate from leaves of this plant and the other one, from our research group, focused on the pharmacological aspects of the plant. In this study, Alchornea triplinervia ethyl acetate fraction (AtF) displayed antibacterial activity against H. pylori with gastroprotective action (CitationLima et al., 2008).
In contrast, pharmacology studies have been performed with other species of the Alchornea genus before. Alchornea glandulosa Poepp. & Endl., Alchornea cordifolia (Schum. & Thonn.) Müll. Arg. and Alchornea castaneifolia (Willd.) Juss. already exhibited strong anti-inflammatory activities (CitationDunstan et al., 1997; CitationOsadebe & Okoye, 2003; CitationManga et al., 2004; CitationLopes et al., 2005). Thus, as the anti-inflammatory activity could contribute to the alleged therapeutic effects of A. triplinervia, the present study aimed to evaluate this plant species as a potential inhibitor of some macrophage functions involved in the inflammatory process. The activities of AtF in the murine immune system were studied through the determination of H2O2, NO and TNF-α production by peritoneal macrophages activated with phorbol myristate acetate (PMA) and lipopolysaccharide (LPS).
Methods
Plant material
Leaves of A. triplinervia were collected from the Jardim Botânico, Instituto de Biociências, UNESP, Botucatu, São Paulo state, Brazil (June 2003). They were authenticated by Jorge Tamashiro. A voucher specimen (14873) is deposited at the Herbarium of Botucatu, UNESP.
Ethyl acetate fraction
The A. triplinervia leaves (500 g) were air-dried (7 days at 40°C) and powdered. The powdered dried leaves were exhaustively macerated with methanol at room temperature (3 times, 72 h). At the end of the process, after solvent evaporation in a vacuum rotary evaporator, 75 g (15%) of methanol extract was obtained. Then, the methanol extract (28 g) was partitioned between ethyl acetate and water 1:1 (v/v), resulting in 7.5 g (27%) of the ethyl acetate fraction and 15 g (54%) of the aqueous fraction.
Phytochemical analysis
AtF was analyzed by thin-layer chromatography (TLC) [silica gel plates, chloroform/methanol/n-propanol/water (5:6:1:4 by volume), organic phase]. The plates were sprayed with specific reagents and after that were observed under UV light: Dragendorff’s reagent and iodoplatinate (alkaloids), NP/PEG (diphenylamine borate/polyethylene glycol) reagent (flavonoids), ammonia vapors (phenolic compounds), anisaldehyde-sulfuric acid reagent (saponins and triterpenes), 5% ferric chloride solution in methanol and with 1% gelatin solution and iodine vapors (tannins) (CitationWagner et al., 1986).
Preparation of the samples
The ethyl acetate fraction obtained from A. triplinervia was initially dissolved in dimethyl sulfoxide (DMSO). After that, the sample was reconstituted in the culture medium RPMI-1640. The choice of the tested concentrations of AtF (15.62 and 62.5 μg/mL) in the in vitro assays was based on previous research of our group (unpublished data). None of the samples had more than 0.5% of DMSO.
Animals
Forty Male Swiss mice (6–8 weeks old, weighing 18 to 25 g), supplied by the animal house of the Faculty of Pharmaceutical Science of Araraquara were maintained in a polycarbonate box (at 23 ± 1°C, 55 ± 5% humidity, 10-18 circulations/h and a 12 h light/dark cycle), with water and food available ad libitum. The UNESP Institutional Animal Care and Use Committee approved all of the employed protocols (06/2005).
Peritoneal macrophages
Thioglycollate-elicited peritoneal exudate cells (PEC) were harvested from Swiss mice using 5 mL of sterile PBS, pH 7.4. The cells were washed twice by centrifugation at 400 g for 5 min at 4°C. Then, the cells were counted and adjusted to the concentration required for each assay. Subsequently, the cells were re-suspended in RPMI-1640 (Sigma, St. Louis, MO) containing 5% heat inactivated fetal bovine serum, 100 IU/mL penicillin, 100 µg/mL streptomycin and 50 mM 2-mercaptoethanol. After that, PEC were seeded in plates and incubated for 60 min at 37°C in a 7.5% CO2 atmosphere for macrophage adherence. Non-adherent cells were removed by washing the culture with culture medium.
Cell viability
In the first part, PEC (5 × 106) was utilized and the adherent cells were incubated with the fraction and LPS (1 μg/mL) for 24 h. Then, in the second assay, PEC (2 × 106) was also utilized and the adherent cells were incubated for 1 h with the fraction and PMA (0.2 μM). After incubation, the medium was poured off and the macrophages were incubated with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide 1 mg/mL) for 3 h. The formazan formed was dissolved in acidic 2-propanol and the optical density was measured using a microplate reader (Multiskan, Labsystem, Vartaa, Finland) equipped with a 540 nm filter and 620 nm reference filter. The optical density of dissolved formazan in the control (untreated cells) was taken as 100% of viability (CitationMosmann, 1983).
H2O2 measurement
For this assay, PEC at 2 × 106 cells/mL was used. Then, 100 µL of complete buffer containing 140 mmol NaCl, 10 mmol potassium phosphate buffer, pH 7.0, 5.5 mmol dextrose, 0.56 mmol phenol red, and 0.01 mg/mL type II horseradish peroxidase was added to each of the wells of a 96-well plate containing the adherent cells. Next, the adherent cells were exposed to the fraction (50 µL) and PMA 0.2 μM (50 µL). Cells incubated only with PMA were used as a positive control. The cells were incubated for 1 h at 37°C in a 7.5% CO2 atmosphere. The reaction was stopped with 10 µL of 4 N NaOH and the samples were read at 620 nm with a Multiskan Ascent ELISA reader (Labsystems) against a blank containing phenol red solution and 4 N NaOH. The results were expressed as nanomoles of H2O2/2 × 105 cells, from a standard curve established in each test consisting of known molar concentrations of H2O2 in buffered phenol red (CitationPick & Keisari, 1980; CitationPick & Mizel, 1981).
Measurement of NO production
NO production was determined by assaying culture supernatants for nitrite using Griess reagent. PEC at 5 × 106 cells/mL was used, and then the adherent cells were incubated with the fraction and LPS (1 μg/mL) for 24 h at 37°C in a 7.5% CO2 atmosphere. Cell-free supernatant (100 μL) was mixed with 100 μL of Griess reagent (sulfanilamide 0.1%, phosphoric acid 3%, naphthylethylenediamine 0.1%) and incubated at room temperature for 10 min. Cells incubated only with LPS were used as a positive control. After incubation, the absorbance of the wells was determined by using a microplate reader (Multiskan, Labsystem) equipped with a 540 nm filter. Nitrite concentration was determined using dilutions of sodium nitrite in culture medium as standard (CitationGreen et al., 1982).
Measurement of TNF-α production
PEC at 5 × 106/mL was used for the cytokine immunoassay. Then the adherent cells were incubated for 24 h with the fraction and LPS (1 μg/mL) at 37°C in a 7.5% CO2 atmosphere. Supernatants were removed and TNF-α production was quantified by a sandwich immunoassay kit (BD Biosciences, Pharmingen) following the manufacturer’s instructions.
Inhibition percentages rates
The inhibition percentage rates were calculated in the assays where the fraction showed inhibitory action in H2O2, NO and TNF-α production. The obtained results in the assays were compared to the controls. As previously described by our group, the next equation was utilized to calculate the rate (CitationLopes et al., 2005).
In this equation, A = PMA or LPS (+), fraction (−); B = PMA or LPS (+), fraction (+); C = PMA or LPS (−), fraction (−).
Statistical analysis
The results are expressed as means ± SD. Each experiment was performed at least five times. One-way ANOVA with Dunnett’s post test was performed using GraphPad InStat version 3.0 for Windows 95, (GraphPad Software, San Diego, CA). Values of p <0.05 were considered statistically significant.
Results
Initially, phytochemical studies were carried out in this research. AtF analysis by TLC indicated the presence of several constituents such as gallic acid derivatives, glycoside flavonoids, and phenolic acids ().
Table 1. Major constituents of A. triplinervia ethyl acetate fraction (AtF). The symbol (×) indicates the presence and (–) the absence of the classes of compounds.
After that, the cytotoxic effect of AtF was evaluated using MTT assay in the presence of PMA and LPS (). Viability levels higher than 85% were observed in the two concentrations of AtF (15.62 and 62.5 μg/ml) incubated with PMA. The viability percentage of the same concentrations of the fraction incubated with LPS ranged from 91.95 to 78.19%.
Figure 1. Effects of A. triplinervia ethyl acetate fraction (AtF) on the viability of peritoneal macrophages in the presence of PMA and LPS. For the test using PMA, PEC (2 × 106) was utilized and the adherent cells were incubated for 1h with the fraction and PMA (0.2 μM). For the test using LPS, PEC (5 × 106) was utilized and the adherent cells were incubated with the fraction and LPS (1 μg/mL) for 24 h. Cells in culture medium (control) correspond to 100% of viability. The cell viability was determined by MTT assay as described previously. Results are the means ± SD of five separate experiments. One-way ANOVA with Dunnett’s post test was performed.*p <0.01 versus control; **p <0.05 versus control.
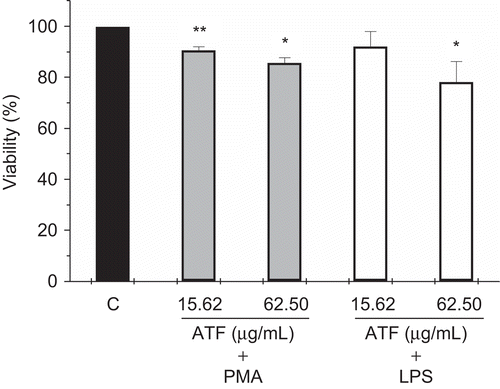
The question of viability is essential when a cell is explanted from its normal environment and the small cytotoxic effect of AtF under the experimental conditions described above allowed the development of all experiments.
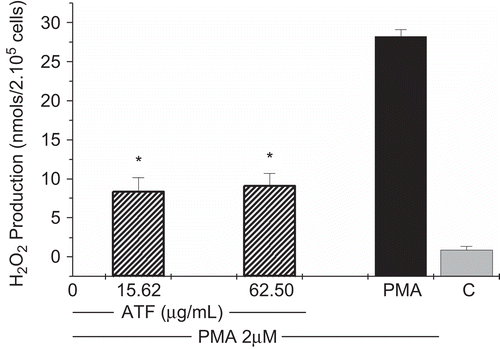
In this study, AtF showed an intense inhibition of H2O2 production in PMA-stimulated macrophages (). The inhibitory effects of the fraction in H2O2 production ranged from 72.25 ± 4.68 to 69.64 ± 4.21%, respectively, in the two tested concentrations, 15.62 and 62.5 μg/mL ().
Table 2. Inhibitory effects of A. triplinervia ethyl acetate fraction (AtF) on H2O2, NO and TNF-α production.
Figure 2. Effects of A. triplinervia ethyl acetate fraction (AtF) on H2O2 production in peritoneal macrophages. PEC at 2 × 106cells/mL was used and complete buffer was added to the adherent cells. The cells were exposed to the fraction and PMA 0.2 μM. Cells incubated only with PMA were used as a positive control and cells in complete buffer as a negative control (C). Results are the means ± SD of five separate experiments. One-way ANOVA with Dunnett’s post test was performed. *p <0.01 versus PMA control.
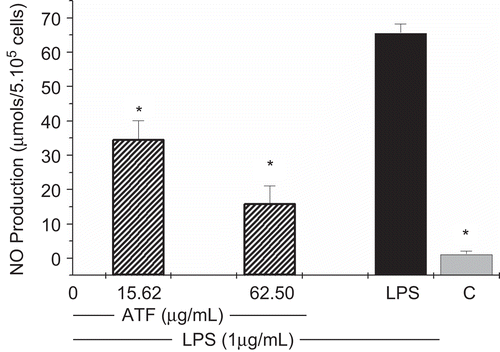
As shown in and , NO production was strongly inhibited in a concentration-dependent manner by AtF. The NO inhibition percentage ranged from 47.8 ± 8.96 to 76.77 ± 8.11%, respectively in the concentrations of 15.62 and 62.5 μg/mL.
Figure 3. Effects of A. triplinervia ethyl acetate fraction (AtF) on NO production in peritoneal macrophages. PEC at 5 × 106 cells/mL was used and the adherent cells were incubated for 24 h with the fraction and LPS (1 μg/mL). Cell-free supernatant was mixed with Griess reagent. Cells incubated only with LPS were used as a positive control and cells in culture medium (RPMI-1640) as a negative control (C). Results are the means ± SD of five separate experiments. One-way ANOVA with Dunnett’s post test was performed. *p <0.01 versus LPS control.
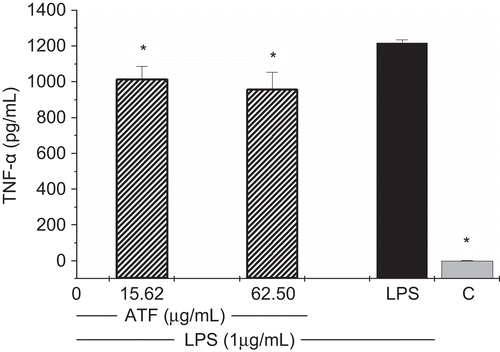
Furthermore, TNF-α production was quantified by a sandwich immunoassay kit and the values showed a mild inhibition of TNF-α production by AtF ( and ). The inhibitory rate was around 18% in the two tested concentrations, 15.62 and 62.5 μg/mL.
Figure 4. Effects of A. triplinervia ethyl acetate fraction (AtF) on TNF-α production in peritoneal macrophages. For the cytokine immunoassay, PEC at 5 × 106/mL was used. Adherent cells were incubated for 24 h with the fraction and LPS (1 μg/mL). Cells incubated only with LPS were used as a positive control and cells in culture medium (RPMI-1640) as a negative control (C). Results are the means ± SD of five separate experiments. One-way ANOVA with Dunnett’s post test was performed. *p<0.01 versus LPS control.
Discussion
Traditional medicine has a long history of serving people all over the world. Medicinal plant is an important element of indigenous medical systems in many regions of our planet (CitationKong et al., 2003). The use of medicinal plants, especially in South America, contributes significantly to primary healthcare (CitationHoletz et al., 2002).
Reliable reports showed that about 80% of the world population use plants for medical purposes (CitationSchulz et al., 2002). Even though less than 1% of the plant species from tropical forest have been researched for medicinal activity (CitationDi Stasi et al., 2002). The plant A. triplinervia was selected for this work based on ethnopharmacological analysis. Studies showed that research guided by the traditional use of plants tends to be more successful (CitationTrotter et al., 1982; CitationElisabetsky & Wannmacher, 1993).
It is known that during an inflammatory response cells of the innate and acquired immune systems release a variety of mediators. These mediators serve to trigger or enhance specific aspects of the inflammatory response and they can be released by different kinds of cells as mast cells, neutrophils and macrophages (CitationGoldsby et al., 2000). Reactive oxygen species as hydrogen peroxide (H2O2) and nitric oxide (NO) exert multiple modulating effects on inflammation and play a key role in the regulation of immune responses (CitationGuzik et al., 2003). H2O2 has a variety of functions in the human body, acting as a signaling molecule, as a cytotoxic agent in the defense system and it can also cause diseases (CitationHalliwell, 1987; CitationClement et al., 1998; CitationForman & Torres, 2001). It is also important to note that enhanced formation of NO following the induction of inducible nitric oxide synthase (iNOS) has been implicated in the pathogenesis of a number of conditions, including various forms of circulatory shock and inflammation (CitationSouthan & Szabo, 1996).
Moreover, cytokines are low-molecular-weight regulatory proteins or glycoproteins secreted in response to a number of stimuli by cells in the body. Tumor necrosis factor-α (TNF-α) is a cytokine that exerts a key role in the cytokine network with regard to the pathogenesis of many infectious and inflammatory diseases (CitationEigler et al., 1997).
Macrophage is a hematopoietic cell that plays an essential role in inflammation and has the capability to produce both reactive oxygen species (ROS) and reactive nitrogen species (RNS). These phagocytic cells can produce and release reactive species in response to stimulation with various agents. In addition to participating in bacterial killing, reactive species have been implicated in inflammation and tissue injury (CitationForman & Torres, 2001).
Reactive oxygen species affect virtually every step of the development of inflammation. Superoxide (O2_) produced by NADPH oxidases may lead to toxic effects, when produced at high levels during oxidative burst. Similarly, large amounts of NO, generated primarily by iNOS can be toxic and pro-inflammatory (CitationGuzik et al., 2003).
In this study, AtF could strongly inhibit H2O2 production in PMA-stimulated macrophages. The inhibitory rate was approximately 70%. It’s an important effect since long exposures to high concentrations of H2O2 can destroy biological structures and lead to irreversible cell damage (CitationRamasarma, 1990).
Our results are consistent with those demonstrated by CitationBenencia et al. (1999), who observed a diminution in the respiratory burst response of murine peritoneal macrophages when these cells were treated in vitro with Trichilia glabra L. (Meliaceae) leaf aqueous extract. This study indicated the presence of anti-inflammatory principles in the T. glabra extract similar to those described for other members of the same family.
The controlled production of NO has an important role in the immune system. However, unregulated excessive production of NO may cause abnormal physical conditions or diseases (CitationLee et al., 2002). In this regard, AtF was investigated to evaluate if it is an inhibitor of NO production. Therefore, in the present investigation, NO release from LPS-stimulated murine macrophages was quantitatively analyzed using Griess reaction. Our study showed that AtF could intensely inhibit NO production in a concentration-dependent manner. Possibly, AtF could also inhibit the induction of iNOS in LPS-stimulated murine macrophages at the transcriptional level, because LPS-induced NO production was inhibited by it in a concentration-dependent manner.
The activation of iNOS provides a prolonged exposure to a large amount of NO and inhibits the activity of several enzymes such as aconitase, cytochrome c oxidase and ribonucleotide reductase. Thus, NO may becomes cytotoxic or cytostatic (CitationAchike & Kwan, 2003). In this case, agents that modulate the activity of NO may be of considerable therapeutic value. In particular, those that reduce the formation of NO may be beneficial in pathophysiological conditions where excessive production of NO is a contributory factor. These include diseases such as septic shock, neurodegenerative disorders and inflammation (CitationHobbs et al., 1999).
A reaction between O2_ and NO results in peroxynitrite formation. This strong oxidant occurs in cells that produce these two reactive intermediates simultaneously, like macrophages (CitationForman & Torres, 2001). In the present study, the inhibition of H2O2 and NO production contributes to reduced peroxynitrite development. In fact, the known toxicity of NO in, for example, the neurotoxicity of stroke and ischemia, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, motor neuron disease and inflammatory diseases, is probably mediated by peroxynitrite (CitationBeckman & Koppenol, 1996).
Many extracts, fractions or isolated compounds obtained from plants have already been evaluated for the inhibition of NO production (CitationKobuchi et al., 1997; CitationDirsch et al., 1998; CitationSeo et al., 2001). Compounds that interfere with NO synthesis, especially inhibiting iNOS, are powerful candidates to lead the development of anti-inflammatory drugs (CitationYunes & Calixto, 2001).
The inflammatory response involves the production of pro-inflammatory oxidants and cytokines (CitationD’Alessandro et al., 2003). TNF-α is a multifunctional cytokine that mediates key roles in acute and chronic inflammation. It has the capability to induce the expression of other pro-inflammatory cytokines, such as interleukin-1 (IL-1) and several chemokines (CitationPalladino et al., 2003). In vivo and in vitro studies support the concept that plant-derived compounds can exert their effects through the modulation of the cytokines system (CitationCalixto et al., 2004).
Additionally, the fraction caused a mild reduction of TNF-α levels, around 18%. Despite the percentage of inhibition not being as high, it is a significant result because TNF-α induces a number of pro-inflammatory changes in endothelial cells, including cytokine production, expression of adhesion molecules, releasing procoagulatory substances and induction of iNOS. These alterations may lead to septic shock, rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease (CitationEigler et al., 1997; CitationPalladino et al., 2003).
It is important to verify whether NF-кB activation can be inhibited by AtF, whereas NF-кB is one of the most important transcription factors in the control of pro-inflammatory genes as TNF-α and iNOS. Also, TNF-α secretion by macrophages in response to LPS stimulation can be regulated by mRNA inducible, mRNA stability and proteolytic processing (CitationClark, 2000; CitationMoss et al., 2001). Therefore, it is possible that AtF might affect some of those processes.
In order to adapt to environmental insults, plants produce a vast number of natural products that have antimicrobial and immunomodulating potential. These include isoflavonoids, indoles, phytosterols, polysaccharides, sesquiterpenes, alkaloids, glucans, tannins, vitamins and trace minerals that function as antioxidants and co-enzymes, and many other phytochemical substances (CitationWilliams, 2001).
CitationLima et al. (2008) determined by high-performance liquid chromatography ultraviolet (UV)-photodiode array that the total concentration of flavonoids in AtF is 19.73% (197.3 ± 0.19 mg/g), being the majority compound of this fraction. They demonstrated also that ethyl acetate fraction from AtF contained primarily five phenolic compounds: ellagic acid, quercetin-3-O-galactopyranoside, quercetin-7-O-glucopyranoside, quercetin-3-O-glucopyranoside, and quercetin-3-O-arabinopyranoside. In our research, AtF qualitative phytochemical analysis by TLC is in agreement with the compounds described by CitationLima et al. (2008). Phenolic compounds found in AtF may be responsible for the effects observed in our study.
Flavonoids are 3-ring phenolic compounds that have been reported to exhibit a wide range of biological effects including antibacterial, antiviral, anti-inflammatory and antiallergic (CitationCook & Samman, 1996). They inhibit enormous numbers of enzymes such as cyclooxygenase and lipoxygenase and scavenge superoxide anions. Flavonoids are anti-inflammatory agents as the result of diminished formation of pro-inflammatory mediators such as prostaglandins, leukotrienes, reactive oxygen species and NO (CitationRobak & Gryglewski, 1996).
CitationAlvarez et al. (2002) investigated a flavonoid, (-)-epigallocatechin-3-gallate (EGCG), on the respiratory-burst responses of rat peritoneal macrophages. EGCG was capable of modulating ROS production during the respiratory burst of rat peritoneal macrophages by acting as a superoxide anion scavenger. In the same way, CitationShen et al. (2002) showed that wogonin and quercetin, structurally related flavonoids that exist extensively in the diet, dose-dependently suppressed lipopolysaccharide-induced NO production in RAW 264.7 macrophages and primary peritoneal macrophages. No notable cytotoxic effect was observed on either cell type associated with a decrease in iNOS protein expression.
An important study describes the anti-inflammatory activities exerted by another member of the Alchornea genus, A. cordifolia, but the exact mechanism behind this effect is still unknown. A. cordifolia methanol extract reduced croton oil-induced edema in mouse ear, after topical application. A bioassay guided liquid–liquid fractionation of the methanol extract gave four active fractions. The authors believe that the activity of two fractions may be at least in part explained by the presence of the anti-inflammatory flavonoids hyperoside and quercitrin (CitationManga et al., 2004).
At this moment, we need to understand the precise mechanisms of AtF and how it works to inhibit inflammatory diseases. Nevertheless, further experiments using isolated compounds and in vivo pharmacological tests are needed to prove the anti-inflammatory activity of A. triplinervia. Even so, our findings may suggest a real capacity of this plant to affect macrophage functions involved in the inflammatory process, indicating a possible scientific basis for the traditional use of A. triplinervia.
Conclusions
AtF attenuated the inflammatory mediators produced by activated macrophages in vitro. The discovery of new pharmacological agents of plant origin that can prevent an overproduction of H2O2, NO and TNF-α is relevant to medicine. Our results contribute to a better understanding of the beneficial effects of A. triplinervia.
Declaration of interest
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for funding from Biota-Fapesp Program and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for grants to Flávia Cristine Mascia Lopes (F.C.M.L).
References
- Abad MJ, Bermejo P, Alvarez M, Guerra JA, Silvan AM, Villar AM (2004): Flavonoids and a sesquiterpene lactone from Tanacetum microphyllum inhibit anti-inflammatory mediators in LPS-stimulated mouse peritoneal macrophages. Planta Med 70: 34–38.
- Achike FI, Kwan CY (2003): Nitric oxide, human diseases and the herbal products that affect the nitric oxide signaling pathway. Clin Exp Pharmacol Physiol 30: 605–615.
- Alvarez E, Leiro J, Orallo F (2002): Effect of (-)-epigallocatechin-3-gallate on respiratory burst of rat macrophages. Int Immunopharmacol 2: 849–855.
- Ayisi NK, Nyadedzor C (2003): Comparative in vitro effects of AZT and extracts of Ocimum gratissimum, Ficus polita, Clausena anisata, Alchornea cordifolia, and Elaeophorbia drupifera against HIV-1 and HIV-2 infections. Antiviral Res 58: 25–33.
- Barroso GM (1984): Sistemática de Angiospermas do Brasil. Viçosa, Brazil, UFV Imprensa Universitária.
- Beckman JS, Koppenol WH (1996): Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol 271: C1424–1437.
- Benencia F, Courrèges MC, Coulombié FC (1999): Trichilia glabra: Effect on the phagocytic activity and respiratory burst response of peritoneal macrophages. Immunopharmacol 41: 45–53.
- Braca A, Mendez J, Menichini F, Morelli I (2002): Constituents of Alchornea triplinervia (Euphorbiaceae). Biochem Syst Ecol 30: 1109–1111.
- Calixto JB, Campos MM, Otuki MF, Santos ARS (2004): Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines, adhesion molecules. Planta Med 70: 93–103.
- Clark A (2000): Post-transcriptional regulation of pro-inflammatory gene expression. Arthritis Res 2: 172–174.
- Clement M, Ponton A, Pervaiz S (1998): Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. Febs Letters 440: 13–18.
- Cook CN, Samman S (1996): Flavonoids – Chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem 7: 66–76.
- D’Alessandro T, Prasain J, Benton MR, Botting N, Moore R, Darley-Usmar V, Patel R, Barnes S (2003): Polyphenols, inflammatory response, and cancer prevention: Chlorination of isoflavones by human neutrophils. J Nutr 133: S3773–3777.
- Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz Junior M, Tien OS, Kakinami SH, Reis MS (2002): Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 73: 69–91.
- Dirsch VM, Kiemer AK, Wagner H, Vollmar AM (1998): Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis 139: 333–339.
- Dunstan CA, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L (1997): Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosysthesis and rat ear oedema assays. J Ethnopharmacol 57: 35–56.
- Durigan G, Bacic MC, Franco GADC, Siqueira MF (1999): Inventário Florístico do Cerrado na Estação Ecológica de Assis, SP. Hoehnea 26: 149–172.
- Eigler A, Sinha B, Hartmann G, Endres S (1997): Taming TNF: Strategies to restrain this proinflamatory cytokine. Immunol Today 18: 487–492.
- Elisabetsky E, Wannmacher L (1993): The status of ethnopharmacology in Brazil. J Ethnopharmacol 38: 137–143.
- Forman HJ, Torres M (2001): Redox signaling in macrophages. Mol Aspects Med 22: 189–216.
- Goldsby RA, Kindt TJ, Osborne BA (2000): Kuby Immunology. New York, W.H. Freeman.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982): Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126: 131–138.
- Guzik TJ, Korbut R, Adamek-Guzik T (2003): Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54: 469–487.
- Halliwell B (1987): Oxidants and human disease: Some new concepts. FASEB J 1: 358–364.
- Hobbs AJ, Higgs A, Moncada S (1999): Inhibition of nitric oxide synthase as a potential therapeutic target. Ann Rev Pharmacol Toxicol 39: 191–220.
- Holetz FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP (2002): Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 97: 1027–1031.
- Kobuchi H, Droy-Lefaix MT, Christen Y, Packer L (1997): Ginkgo biloba extract (EGb 761): Inhibitory effect on nitric oxide production in the macrophage cell line RAW 264.7. Biochem Pharmacol 53: 897–903.
- Kong JM, Goh NK, Chia LS, Chia TF (2003): Recent advances in traditional plant drugs and orchids. Acta Pharmacol Sin 24: 7–21.
- Lee HS, Kim BS, Kim MK (2002): Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem 50: 7700–7703.
- Lima ZP, Calvo TR, Silva EF, Pellizzon CH, Vilegas W, Brito AR, Bauab TM, Hiruma-Lima CA (2008): Brazilian medicinal plant acts on prostaglandin level and Helicobacter pylori. J Med Food 11: 701–708.
- Lopes FC, Calvo TR, Vilegas W, Carlos IZ (2005): Inhibition of hydrogen peroxide, nitric oxide and TNF-alpha production in peritoneal macrophages by ethyl acetate fraction from Alchornea glandulosa. Biol Pharm Bull 28: 1726–1730.
- Manga HM, Brkic D, Marie DEP, Quetin-Leclercq J (2004): In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. (Euphorbiaceae). J Ethnopharmacol 92: 209–214.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- Moss ML, White JM, Lambert MH, Andrews RC (2001): TACE and other ADAM proteases as targets for drug discovery. Drug Discov Today 6: 417–426.
- Osadebe PO, Okoye FB (2003): Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J Ethnopharmacol 89: 19–24.
- Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL (2003): Anti-TNF-α therapies: The next generation. Nat Rev Drug Discov 2: 736–746.
- Pick E, Keisari Y (1980): A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38: 161–170.
- Pick E, Mizel DJ (1981): Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods 46: 211–226.
- Ramasarma T (1990): H2O2 has a role in cellular regulation. Indian J Biochem Biophys 27: 269–274.
- Robak J, Gryglewski RJ (1996): Bioactivity of flavonoids. Pol J Pharmacol 48: 555–564.
- Schulz V, Hänsel R, Tyler VE (2002): Fitoterapia Racional: Um Guia de Fitoterapia para as Ciências da Saúde. Barueri, Brazil, Manole.
- Secco RS (2004): Alchorneae (Euphorbiaceae: Alchornea, Aparisthmium e Conceveiba). Flora Neotropica 93: 1–195.
- Seo WG, Pae HO, Oh GS, Chai KY, Yun YG, Kwon TO, Chung HT (2001): Inhibitory effect of ethyl acetate fraction from Cudrania tricuspidata on the expression of nitric oxide synthase gene in RAW 264.7 macrophages stimulated with interferon-γ and lipopolysaccharide. Gen Pharmacol 35: 21–28.
- Setzer WN, Shen X, Bates RB, Burns JR, Mcclure KJ, Zhang P, Moriarity DM, Lawton RO (2000): A phytochemical investigation of Alchornea latifolia. Fitoterapia 71: 195–198.
- Shen SC, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC (2002): In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E2 production. Eur J Pharmacol 446: 187–194.
- Silva EM, Hiruma-Lima CA, Lólis SF (2000): Etnobotânica no município de Porto Nacional [abstract]. In: Symposium of Brazilian Medicinal Plants, Cuiabá, Federal University of Mato Grosso (UFMT), p.106.
- Silva FC, Marconi LP (1990): Fitossociologia de uma floresta com araucária em Colombo-PR. Boletim de Pesquisa Florestal 20: 23–38.
- Siqueira MF (1994): Análise florística e ordenação de espécies arbóreas da Mata Atlântica através de dados binaries. MSc thesis, Unicamp, Campinas, Brazil.
- Southan GJ, Szabo C (1996): Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol 51: 383–394.
- Stannard BL (1995): Flora of the Pico das Almas, Chapada Diamantina −Bahia, Brazil. London, Royal Botanic Gardens, Kew.
- Trotter RT, Logan MH, Rocha JM, Boneta JL (1982): Ethnography and bioassay: Combined method for a preliminary screen of home remedies for potential pharmacological activity. J Ethnopharmacol 8: 113–119.
- Vaccaro S, Longhi SJ, Brena DA (1999): Aspectos da composição florística e categorias sucessionais do estrato arbóreo de três subseres de uma floresta estacional decidual, no Município de Santa Tereza - RS. Ciência Florestal 9: 1–18.
- Wagner HM, Bladt S, Zgainski EM (1986): Plant Drug Analysis. Berlin, Germany: Springer.
- Williams JE (2001): Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Una de Gato and Sangre de Grado. Altern Med Rev 6: 567–579.
- Yunes RA, Calixto JB (2001): Plantas Medicinais sob a Ótica da Química Medicinal Moderna. Chapecó, Brazil: Argos.
