Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a substantial contributor to morbidity and mortality. In search of a natural products capable of inhibiting this multidrug resistant bacteria, we have investigated the antimicrobial activity of emodin (EM) isolated from Rheum palmatum L. (Polygonaceae) against 17 different strains of the bacterium. New antimicrobial activity was found using the paper disc diffusion method, agar dilution as well as checkerboard method. Against the 17 strains, the disc diffusion test was in the range of 18–30 mm, and the minimum inhibitory concentrations (MICs) of EM were in the range of 1.5–25 μg/mL. From those results we performed the checkerboard test to determine the synergism of EM in combination with ampicillin (AM) or oxacillin (OX) against all strains. The combined activity of EM and two antimicrobial agents (AM, OX) against all strains resulted in a fractional inhibitory concentrations index (FICI) ranging from 0.37–0.5 and from 0.37–0.75, respectively. The effect of EM with AM and OX was found to be synergistic or partially synergistic. We found that EM reduced the MICs of AM and OX. EM and in combination with AM or OX could lead to the development of new combination antibiotics against MRSA infection.
Keywords::
Introduction
Staphylococcus aureus (S. aureus) is a public, fatal pathogen associated with a variety of infections (CitationBaltch et al., 2007). It is the principal cause of several infections, such as skin, soft tissue, surgical site and catheter, pneumonia, bacteremia, and osteoarticular infections (CitationDenis et al., 2006). Moreover, because S. aureus generally is an intracellular pathogenic organ, infections caused by this microbe can be difficult for medical treatment and can survive and relapse. Also, the overuse of drugs causes resistance (CitationBaltch et al., 2007). Infections caused by methicillin or other β-lactam antibiotic-resistant S. aureus are generally nosocomial and are reported from most countries (CitationPatel, 2009).
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged worldwide and has become one of the most important hospital and community pathogens (CitationPatel, 2009; CitationSchito, 2006; CitationBaltch et al., 2007). The pharmacological tools available to cure MRSA being limited today, new agents to treat MRSA-associated infections are greatly needed (CitationLiu et al., 2009). First, positive detection of the mecA gene for MRSA, or methicillin-resistant gene product, namely penicillin binding protein PBP2a, is an advance preparation for demonstration of MRSA (CitationWitte et al., 2007). Second, the treatment guidelines for these infections recommend combinations of antibiotics against these pathogenic organisms (CitationKoga et al., 2008). To meet this need, various natural products were screened for new antibiotic substances. During the screening for antimicrobial agents against MRSA, extraction from Rheum palmatum L. (Polygonaceae) was identified as a candidate for further studies.
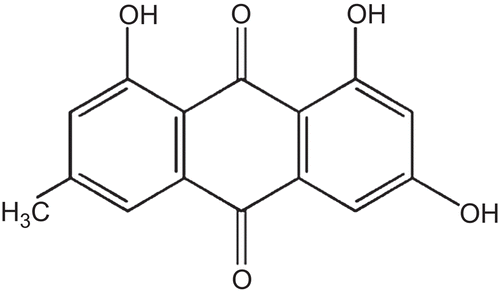
Rheum palmatum, popularly known as Dahuang, has traditionally been used as an oriental folk medicine. The emodin (EM) is biologically active and naturally occurs in anthraquinone (1,3,8-trihydroxy-6-methyl anthraquinone) found in Rheum palmatum and related plants such as rhubarb (CitationShuangsuo et al., 2006; CitationYan et al., 2009). The anthraquinone components including EM, aloe-emodin, rhein, chrysophanol and physcion, have been reported (CitationYan et al., 2009). It is an active compound known for the medicinal and therapeutic specific of many plant-derived drugs (CitationBasu et al., 2005). The anthraquinones have shown anti-inflammatory and anti-cancer effects and since ancient times, EM has been an active component of herbal extracts used in medical treatments. Previous work has shown that EM inhibits the growth of certain bacteria (CitationBasu et al., 2005; CitationLi et al., 2006). However, little is known about its antimicrobial effects on MRSA. The aloe-emodin was reported, with different EM structure (CitationHatano et al., 2005). The screening of Rheum palmatum roots for antibacterial activity, showed EM to have antibacterial activity against MRSA.
Thus, here we present a study that shows the antimicrobial activity of EM against MRSA and Methicillin-sensitive Staphylococcus aureus (MSSA) strains, as well as its ability to lower the MICs of β-lactam antibiotics.
Materials and methods
Plant material and isolation
The extraction was performed with minor modification of the method previously described by CitationKang et al. (2008). The dried roots of Rheum palmatum (500 g), purchased from the oriental drug store Daehak Hanyak Kuk (Iksan, Korea), was authenticated by Dong-Yeul Kwon. A voucher specimen was also deposited in the Laboratory of Herbology, College of Pharmacy, Wonkwang University, Iksan, Korea. The EtOH extracts (60 g) were partitioned using organic solvents with different polarities to yield n-hexane (2.5 g), CH2Cl2 (1.2 g), EtOAc (30 g), n-BuOH (10 g) and H2O (13.5 g) fractions in sequence. The CH2Cl2 (800 mg) fraction was separated by Sephadex LH-20 column chromatography (3 × 15 cm, CHCl3:MeOH, 10:1) to obtain two fractions (A, B). Each fraction was tested for bioassay. The inactive fraction A (50-100 ml, 160 mg) was chromatographed on silica gel (200–300 mesh, 10 g) column with n-hexane-EtOAc (lower layers, by volume, 15:1, 3:1) to obtain chrysophanol (40–50 mL, 25 mg). The active fraction B (287 mg, 200–300 mL) was chromatographed on a Sephadex LH-20 column (20 × 10 cm) with CHCl3:MeOH (10:1, 800 mL) to give two fractions (B1, B2). Fraction B2 (215 mg, 300–400 mL) was separated by a silica gel chromatography (n-hexane:EtOAc, 5:1–1:1, each volume, 500 mL) to afford emodin (50–80 mL, 20 mg). The structure of the compound was determined by its physico-chemical and spectral data, which agreed with those previously reported (CitationKang et al., 2008). Emodin was deposited at the Standardized Material Bank for New Botanical Drugs (No. NNMBP004), Wonkwnag University (Republic of Korea).
Bacterial strains and growth conditions
Among the 17 S. aureus strains used in this study, 15 clinical isolates (MRSA) were obtained from 15 different patients at Wonkwang University Hospital (Iksan, South Korea). The other two strains were S. aureus ATCC 33591 (methicillin-resistant strain) and S. aureus ATCC 25923 (methicillin-susceptible strain). ATCC 25923 (American Type Culture Collection, Manassas, VA) and ATCC 33591 were commercially purchased. Before use, all bacteria were stored in 30% glycerol and frozen at −70°C. The bacteria were cultured in Mueller-Hinton broth (MHB) and Mueller-Hinton agar (MHA) (Difco, Baltimore, MD). The 17 S. aureus strains were used to test the antibacterial activity of ampicillin (AM), oxacillin (OX) and EM.
Determination of the mecA gene
Detection of the mecA gene in the MRSA strains was performed by PCR (Polymerase chain reaction) amplification (). Prior to the DNA extraction, bacteria stock cultures were subcultured twice on to MHA plates. For rapid extraction, one to five bacterial colonies were suspended in 300 μL of cell lysis buffer and heated at 100°C for 20 min. After centrifugation at 12 000 rpm for 10 min, 2 μL of the supernatant was used for the DNA extraction. PCR reactions were performed using a MRSA Primer Mix Kit (Genotek, Daejeon, Korea). The PCR amplification consisted of 30 cycles (94°C, 60 s; 55°C, 60 s; 72°C, 60 s). The final PCR products were separated on 2% agarose gel. We have previously reported on these primers (CitationLee et al., 2008).
Table 1. Sequences of the primers used in PCR.
Table 2. The S. aureus strains used in the experiments.
Determination of antibacterial activity by the disc diffusion method
The paper disc diffusion method was used to determine antibacterial activity (CitationVeljic et al., 2008; CitationWitte et al., 2007). Sterile paper discs (6 mm; Toyo Roshi Kaisha, Tokyo) were loaded with 20 μL of EM (varying concentrations: 10 and 50 μg/disc, ) dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO), and were left to dry for 12 h at 37°C in a sterile room. Before the experiment the stock solution was diluted with distilled water to obtain a 50% DMSO stock solution (CitationVeljic et al., 2008). The bacterial suspensions were diluted to match the 0.5 McFarland standard scale (approximately 1.5 × 108 cfu/mL), and were further diluted to obtain the final inoculum. The MHA was poured into Petri dishes and inoculated with 100 μL of the suspension containing 1.5 × 108 cfu/mL of bacteria. AM and OX were used as positive control, and the discs treated with 50% DMSO were used as negative controls (50% DMSO was not active against all strain). The plates were placed in an incubator (Vision, Bucheon-city, Korea) at 37°C for 24 h. The inhibition zone diameter around each of the discs was measured and recorded at the end of the incubation period. The experiment was done in triplicate.
Determination of minimal inhibitory concentrations (MICs)
The EM and the two antimicrobial agents were dissolved in MHA with 10% DMSO. Agar dilution method was used to determine the MICs of ampicillin, oxacillin or emodin. Samples were diluted in MHA to a final volume of 25 mL using microtiter plates. The 17 strains were suspended in MHB from MHA plates incubated for 48 h. All strains suspensions were adjusted to the 0.5 McFarland standards (approximately 1 × 108 cfu/mL). Final inoculums were adjusted to the 106 cfu/spot. The MHA was supplemented with EM concentrations ranging from 1.56–25 μg/mL, with AM at concentrations from 0.9–125 μg/mL and with OX at concentrations from 1.9 to 1 mg/mL in plates. These serially diluted cultures were then incubated at 37°C for 24 h. The MIC was defined as the lowest concentration that completely suppressed colony growth. The MIC of AM and OX were also determined, and similarly defined as the lowest antibiotic concentration at which no visible bacterial growth was observed.
Determination of in vitro combinations
The antimicrobial effects that resulted from combination inhibiting the two antimicrobial agents were assessed by the checkerboard test. The antimicrobial combination assayed included EM plus AM or OX. Serial dilutions of the EM with AM or EM with OM antimicrobial agents were mixed in cation-supplemented MHA. The inocula were prepared from colonies that had been grown on MHA overnight. The final bacterial concentration after inoculation was 5 × 106 cfu/mL. The MIC was determined after 24 h of incubation at 37°C. The MIC was defined as the lowest concentration of drug alone or in combination inhibiting the visible growth. Each experiment was repeated three times. The in vitro interaction was quantified by determining the fractional inhibitory concentration (FIC). The FIC index was determined using the following formula:
where [A] is the concentration of drug A, MICA is its MIC, and FICA is the FIC of drug A for the organism, and [B], MICB, and FICB are defined in the same way for drug B. The FIC index thus obtained was interpreted as follows: <0.5, synergy; 0.5–0.75, partial synergy; 0.76–1, additive effect; >1–4, indifference; and >4, antagonism (CitationChoi et al., 2008). Finally, the varying rates of synergy between the two agents were determined.
Results
The antimicrobial efficacy of EM against the 16 MRSA strains and the single MSSA strain was evaluated by the disc diffusion method via determination of the surrounding inhibition zones, as well as by evaluating the MIC using the agar dilution method. shows the antimicrobial activity of EM. The mean of inhibition zones produced against the tested bacteria ranged between 12 and 30 mm. The growth of all the tested strains of MRSA and MSSA was inhibited at 10 and 50 μg/disc.
Table 3. The antimicrobial activity (as inhibition zone diameters) of EM, AM and OX against S. aureus strains.
The MICs for EM, AM and OX against the the 17 strains; 15 strains of clinically-isolated of MRSA, on standard MRSA, and one standard MSSA are shown in . 15 strains of MRSA, one standard MRSA, and one standard MSSA strain are shown in . The MICs we determined using the agar dilution method confirmed the antimicrobial effects we found by the disc diffusion method. EM showed antimicrobial activity against all the tested strains of MRSA as well as the MSSA strain. The MICs of EM against S. aureus ranged from 1.56–25 μg/mL.
Table 4. The MICs of EM, AM, OX against S. aureus strain.
For the determination of in vitro combinations test we selected a clinical MRSA isolate, the standard MRSA strain, and the standard MSSA strain. This test was performed to determine the action of EM alone as well as its synergistic action with AM or OX against the MRSA clinical isolate, the standard MRSA strain, or the standard MSSA strain. The EM markedly lowered the MICs of AM and OX against the MRSA strains. For the standard MSSA strain the EM lowered the MICs of both AM and OX. The combined activity of EM and two antimicrobial agents (AM, OX) against all strains resulted in fractional inhibitory concentrations index (FICI) ranging from 0.37–0.5 and from 0.37–0.75, respectively (). So the combination effect of EM with AM and OX was found to be synergistic or partially synergistic. We found that EM reduced MICs of AM and OX.
Table 5. Result of the combined effect of EM and AM against S. aureus.
Table 6. Result of the combined effect of EM and OX against S. aureus.
Discussion
Since MRSA bacteria possess multi-drug resistance, MRSA infections are dangerous and present serious problems for hospitals. Many researchers are studying natural products that could potentially be used against MRSA. In the present study, we have investigated the antimicrobial activity of EM against clinical isolates of MRSA and a standard MSSA strain.
To our knowledge this is the first report showing that EM can lower the MICs of β-lactam antibiotics. Others have reported that β-lactam antibiotics inhibit bacterial cell wall biosynthesis. In this study EM exhibited antimicrobial activity and lowered the MICs of β-lactam antibiotics against MRSA and a standard MSSA strain.
The isolated compound was characteristic of EM by its structure. The presence of two peri-hydroxy and three hydroxyl groups are an indication. In addition, the presence of the methoxy group confirmed to be EM. More importantly, the three phenol groups are on 1, 3 and 8 positions (CitationShia et al., 2009). This EM has been used as a traditional oriental medicine for the treatment of skin burn, infection, gallstone, hepatitis, inflammation, and osteomyelitis. Moreover, this is a safe agent even in long exposure in mice (CitationMuto et al., 2007). CitationHatano et al. (1999) reported that EM, aloe-emodin, and the others, showed strong antibacterial activity against MRSA. Here we report that EM agent also showed synergistic activity with AM and OX against MRSA. While these results obtained here cannot be applied currently in clinical treatment, we consider that the combination treatment of EM isolated with AM or OX will prove to be helpful to treat MRSA. Further medicinal, clinical and mechanism studies are needed to verify how EM enhances the antibacterial activity. Today, the ongoing emergence of multi-drug resistant bacteria and the infectious diseases caused by them are serious global problems.
Therefore we will be able to reduce the use of existing antibacterial drugs and increase the use of natural product drugs such as EM. At this stage, the product is still under investigation.
The emodin markedly lowered the MICs of AM and OX against the two MRSA strains and one MSSA. While the product is still under investigation, the present results are promising and may enhance the use of natural products instead of antibiotics.
Declaration of interest
This paper was supported by Wonkwang University in 2010.
References
- Baltch AL, Ritz WJ, Bopp LH, Michelsen PB, Smith RP (2007): Antimicrobial activities of daptomycin, vancomycin, and oxacillin in human monocytes and of daptomycin in combination with gentamicin and/or rifampin in human monocytes and in broth against Staphylococcus aureus. Antimicrob Agents Chemother 51: 1559–1562.
- Basu S, Ghosh A, Hazra B (2005): Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: Isolation of emodin and physcion as active antibacterial agents. Phytother Res 19: 888–894.
- Shia CS, Hou YC, Tsai SY, Huieh PH, Leu YL, Chao PD (2009): Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J Pharm Sci 17: 2185–2195.
- Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, Kim JH, Sohn DH, Shin DW, Park H, Kwon DY (2008): In vitro activity of methyl gallate isolated from Galla Rhois alone and in combination with ciprofloxacin against clinical isolates of Salmonella. J Microbiol Biotechnol 18: 1848–1852.
- Denis O, Deplano A, Nonhoff C, Hallin M, De Ryck R, Vanhoof R, De Mendonça R, Struelens MJ (2006): In vitro activities of ceftobiprole, tigecycline, daptomycin, and 19 other antimicrobials against methicillin-resistant Staphylococcus aureus strains from a national survey of Belgian hospitals. Antimicrob Agents Chemother 50: 2680–2685.
- Kang SC, Lee CM, Choung ES, Bak JP, Bae JJ, Yoo HS, Kwak JH, Zee OP (2008): Anti-proliferative effects of estrogen receptor-modulating compounds isolated from Rheum palmatum. Arch Pharm Res 31: 722–726.
- Koga T, Masuda N, Kakuta M, Namba E, Sugihara C, Fukuoka T (2008): Potent in vitro activity of tomopenem (CS-023) against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52: 2849–2854.
- Lee YS, Kang OH, Choi JG, Oh YC, Chae HS, Kim JH, Park H, Sohn DH, Wang ZT, Kwon DY (2008): Synergistic effects of the combination of galangin with gentamicin against methicillin-resistant Staphylococcus aureus. J Microbiol 46: 283–288.
- Li JM, Jin ZX, Chen T, Gu QP (2006): Correlation of anti-bacterial activity with secondary metabolites content in Sargentodoxa Cuneata tables. Zhejiang Da Xue Xue Bao Yi Xue Ban 35: 273–280.
- Liu MH, Otsuka N, Noyori K, Shiota S, Ogawa W, Kuroda T, Hatano T, Tsuchiya T (2009): Synergistic effect of kaempferol glycosides purified from Laurus nobilis and fluoroquinolones on methicillin-resistant Staphylococcus aureus. Biol Pharm Bull 32: 489–492.
- Muto A, Hori M, Sasaki Y, Saitoh A, Yasuda I, Maekawa T, Uchida T, Asakura K, Nakazato T, Kaneda T, Kizaki M, Ikeda Y, Yoshida T (2007): Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol Cancer Ther 6: 987–994.
- Patel M (2009): Community-associated meticillin-resistant Staphylococcus aureus infections: Epidemiology, recognition and management. Drugs 69: 693–716.
- Schito GC (2006): The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin Microbiol Infect 1: 3–8.
- Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, Yan’an C (2006): Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit 12: BR302–306.
- Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T (1999): Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull (Tokyo) 47: 1121–1127.
- Hatano T, Kusuda M, Inada K, Ogawa TO, Shiota S, Tsuchiya T, Yoshida T (2005): Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 66: 2047–2055.
- Veljic M, Tarbuk M, Marin PD, Ciric A, Sokovic M, Marin M (2008): Antimicrobial activity of methanol extracts of mosses from Serbia. Pharm Biol 46: 871–875.
- Witte W, Pasemann B, Cuny C (2007): Detection of low-level oxacillin resistance in mecA-positive Staphylococcus aureus. Clin Microbiol Infect 13: 408–412.
- Yan D, Ma Y, Shi R, Xu D, Zhang N (2009): Pharmacokinetics of anthraquinones in Xiexin decoction and in different combinations of its constituent herbs. Phytother Res 23: 317–323.