Abstract
Aim: Growing evidence indicates that the glutamatergic system, especially the abnormalities of glutamate and N-methyl-D-aspartate (NMDA) receptors contribute to the pathophysiology and possibly the pathogenesis of major depressive disorders. This study is to evaluate the effect of gan mai da zao (GMDZ) decoction on glutamate and NMDA receptor in unpredictable chronic mild stress (UCMS) rats.
Materials and methods: Sucrose preference test and open field test were used to estimate the depressive-like behaviors of UCMS rats. Glutamate levels and NMDA receptor subunits (NR1, NR2A and NR2B) in the frontal cortex and hippocampus were determined by HPLC-FLD and by western-blot respectively.
Results: 32 days UCMS induced depressive-like behaviors, increased glutamate concentration and decreased NMDA receptor subunits NR2A and NR2B in the frontal cortex and hippocampus of rats. However, NR1 expression remained constant in stressed rats compared with normal. The GMDZ decoction alleviated the depressive-like behavior, decreased glutamate level, and increased expression of NMDA receptor subunit NR2A and NR2B in the frontal cortex and hippocampus of stressed rats.
Conclusions: These results suggest that GMDZ treatment reversed chronic unpredictable stress-induced depressive-like behaviors in UCMS rats, possibly via reducing glutamate levels and increasing the NMDA receptor subunits NR2A and NR2B in frontal cortex and hippocampus.
Introduction
Depression is a severe illness with a lifetime prevalence of about 10% according to large epidemiological studies (CitationShen et al., 2009). At present, nearly all medications available for the treatment of depression have been developed based on the monoaminergic deficit hypothesis of depression that developed in the mid 1960s (CitationSchildkraut, 1965). Although this approach has led to great advances in our ability to treat depression, the limitations of our current armamentarium of antidepressant drugs are becoming increasingly evident. Meanwhile, even though newer types of antidepressants are better tolerated and have wider therapeutic windows than the older tricyclic compounds, they still produce troublesome side-effects (CitationCassano & Fava, 2004). Thus, newer antidepressants targeting other neurotransmitters with fewer side effects are needed.
Recently a renewed interest in the glutamatergic system as a treatment option for major depression emerged with the finding that the N-methyl-D-aspartate (NMDA) antagonist ketamine leads to a rapid improvement of depressive symptoms (CitationZarate et al., 2006). Magnetic resonance (MR) spectroscopy studies demonstrated in healthy subjects that ketamine leads to an alternation in glutamine concentration in the anterior cingulated cortex (CitationRowland et al., 2005). Glutamate is the primary excitatory neurotransmitter in the mammalian brain. Glutamatergic neurotransmission may be modulated in the brain by different receptor types, including ionotropic and metabotropic receptors. In this context, studies have pointed to the ionotropic glutamate NMDA receptor as an important player in the etiology of psychopathologies, such as anxiety and major depression (CitationCunha et al., 2008).
Traditional Chinese prescription (TCP) has been commonly recognized as a safe and effective prescription in the treatment of various depressive disorders in China (CitationGuo et al., 2009). GMDZ decoction is a TCP that consists of three crude drugs: Glycyrrhiza uralensis Fisch. (Leguminosae, Jujube), Triticum aestivum L. (Poaceae, Wheat), and Ziziphus jujuba Mill. (Rhamnaceae, Jujube), with a unique effect on depression and has been used to treat depressive patients for centuries in China. Clinical studies have shown that it is an efficacious and well-tolerated antidepressant prescription (CitationWu, 2002; CitationYang et al., 2009). However, the mechanisms underlying the antidepressant effect of GMDZ decoction remain elusive. Recent studies showed that the main component isolated from GMDZ increased plasma level of total amino acids in piglets (CitationHe et al., 2008; CitationShuang et al., 2001). Therefore, it was interesting to investigate whether the GMDZ decoction altered levels of glutamate and NMDA receptor in depression.
The NMDA receptor is a member of the glutamate ionotropic receptors, which are ligand-gated cation channel complexes. This receptor consists of four or five subunits, and its functionality requires the presence of at least one NR1 subunit, which is essential for the NMDA-receptor-channel complex (CitationSuen et al., 1998). In turn, NR2 (NR2A–NR2D) family subunits are related to modulatory properties of the receptor. The NR2 subunits incorporated in the NMDA receptor determine its functional and pharmacological properties (CitationNeyton & Paoletti, 2006) and the expression of NR2A and NR2B transcripts was drastically reduced in major depression (CitationBeneyto et al., 2007; CitationFeyissa et al., 2009). Meanwhile, the hippocampus and prefrontal cortex are very vulnerable to stress and harmful stimuli. There are morphological and functional alterations in these two brain regions in stressed animals and depressed humans, including the alternation of glutamate and NMDA-receptor-channel complex. Thus, we investigated effect of GMDZ decoction and known antidepressant fluoxetine on expression of NMDA receptor subunit NR1, NR2A and NR2B at hippocampus and frontal cortex in depressive-like rats.
Materials and methods
Preparation of extracts of the GMDZ decoction
Three crude drugs: Glycyrrhiza uralensis (10 g), Triticum aestivum (15 g) and Ziziphus jujuba (15 g). were purchased from the Medicinal Materials Company of Hangzhou Yanqingtan, and authenticated by Xi-Lin Chen, Department of Botany, Zhejiang Chinese Medical University, based on their micro and macroscopic characteristics. The quality of these crude drugs is controlled and processed according to the Chinese CitationPharmacopoeia (2005). Drug samples were collected as voucher specimens and kept with the records. About 2000 g of the dry crude herbs were mixed at the ratio stated in the prescription, ground into powder and boiled in distilled water (40 g/320 mL, reflux, 2 h × 2). The extract was filtered and dried under reduced pressure at a temperature below 60°C. The doses were expressed in terms of the dried weight of the GMDZ decoction extract per unit body weight of the experimental animals (g/kg).
Animals and drug treatment
Male Wistar rats (250 ± 16 g) were purchased from the Laboratory Animal Center of Zhejiang Chinese Medical University. Before the experiments the rats were allowed one week acclimation period in the animal quarters under air conditioning (22° ± 1°C, humidity 50–60%) and an automatically controlled photoperiod of 12 h light daily, fed with standard rodent chow and tap water ad libitum. The experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (CitationNIH, 1996), and were approved by the Animal Care and Use Committee of the Zhejiang Chinese Medical University.
To observe the effects of GMDZ decoction on depression model rats, fluoxetine was chosen for the positive control drug and 51 rats were randomly divided into five groups: The first group (control) received a once-daily oral gavage (p.o.) administration of distilled water. The second group (UCMS) received a once-daily p.o. administration of distilled water. The third group (UCMS and fluoxetine, 10 mg/kg) received a once-daily p.o. administration of fluoxetine. The dosage of 10 mg/kg for fluoxetine has been reported to show antidepressant action in a previous work (CitationFornal et al., 2001). The fourth group (UCMS and GMDZ, 4 g/kg) received a once-daily p.o. administration of GMDZ. By using allometric calculation, 4 g/kg of GMDZ for rat corresponds to the daily clinical dose for a 60 kg man (40 g/day). The fifth group (UCMS and GMDZ, 16 g/kg) received a once-daily p.o. administration of GMDZ for 18 days. All groups were administered two weeks after UCMS, and lasted for 18 days.
Unpredictable chronic mild stress procedure
The UCMS procedure was modified from procedures used by a previous study (CitationWillner et al., 1987). The repeated mild stressors including: clip tail 2 min, bandage stress 30 min, heat stress 45°C, 10 min, cold swim 4°C, 5 min, inversion of the light/dark cycle, water deprivation 24 h, food deprivation 24 h and foot shock (rats were given 36 V shocks for 10 s at intervals of 1 min for 30 times). One of these stressors was applied daily following a semi-random schedule. Because of the nature of the UCMS treatment (e.g. light/dark cycle modification), UCMS treated and control animals were placed on shelving racks in two different but otherwise similar rooms. The UCMS procedure was applied as soon as the experimental groups were formed, and continued during the different behavioral tests. However, no stressors were applied on testing days.
Open field test
Locomotor activity was assessed by open field test every week after UCMS as previously reported (CitationWillner, 2005). Briefly, the open field apparatus consisted of a black box surrounded by planks with a height of 40 cm, the floor was divided into 25 squares (25 × 25 cm) by white stripes. Testing was carried out in dimmed white light. Each animal was tested individually and only once. The rat was gently put into the central grid. The locomotor activity was scored within 3 min, including the following behaviors: ambulation – the number of grid lines it crossed with all the four paws, rearing – the number of times the animal stood on its hind limbs.
Sucrose preference test
The sucrose preference test was used to operationally define anhedonia. Specifically, anhedonia is defined as a reduction in sucrose intake and sucrose preference relative to the intake and preference of the control group. A sucrose preference test consists of first removing the food and water from each rat’s cage for a period of 20 h. Water and 1% sucrose were then placed in the cages in pre-weighed glass bottles, and animals were allowed to consume the fluids freely for a period of 24 h. Two baseline preference tests were performed, separated by at least 5 days, and the results were averaged. A preference test was also conducted following the 32 days chronic unpredictable stress period. On the last stress day rats were deprived of water and food for 20 h, and from the next day on rats were given a 24 h window sucrose preference test (24 h after the last drug treatment). Sucrose and water consumption (g) was measured and the sucrose preference was calculated as the sucrose preference (%) = sucrose consumption/(sucrose consumption + water consumption).
Sample preparation
Following the chronic unpredictable stress period and post-chronic unpredictable stress sucrose preference test, rats were sacrificed via decapitation. The animals were anesthetized with sodium pentobarbital (40 mg/kg body weight, i.p.) and decapitated. The brain was rapidly separated from the skull and divided into the two hemispheres. Each hemisphere was rinsed with oxygenated ice-cold artificial cerebral spinal fluid and the frontal cortex and hippocampus region were rapidly dissected on an ice-cold glass dish, aliquotted into tubes, and stored in liquid nitrogen until analysis.
Glutamate detection
The concentrations of amino acid glutamate were measured by OPA-β-mercaptoethanol precolumn derivation, reversed phase gradient elution and fluorescence detection (CitationBegley et al., 1994). The hippocampus and frontal cortex were taken out of liquid nitrogen, put into chilled tubes, homogenized in 0.8 μL perchloric acid (0.1 M) on ice. The homogenates were centrifuged at 11,000 g for 20 min and 500 μL of the supernatant were ultrafiltrated for another 20 min. The amino acid in the homogenate was first derived to its fluorescent isoindoles. Homogenate (20 μL) and 10 μL OPA deriving fluid were allowed to react for 45 s at room temperature. The HPLC employed buffer A: 0.1 M KH2PO4 buffer (adjusted to pH 6.6): methanol = 65: 35, v/v; and buffer B:0.01 M KH2PO4 buffer (adjusted to pH 6.6):methanol = 10:90, v/v. Buffer A was ultrasonically degassed, buffer B was filtered and degassed through a 0.2 mm nitrocellulose membrane. The above two-buffer HPLC system (Shimadzu-10AVP, Japan) was coupled to a fluorescent detector (RF-10AXL, Shimadzu, Japan). Separation was achieved on a C18 column (Hypersil, BDS, 5 mm). The reaction mixture (20 μL) was injected into the column. The gradient conditions were as follows: initial conditions are 100% mobile phase A; from time 0 to 13.5 min the gradient changes to 70% mobile phase A and 30% mobile phase B; from 13.51 to 20 min the gradient changes to 20% mobile phase A and 80% mobile phase B; from 20.01 to 30 min the gradient changes to 100% mobile phase A and remains at this condition until the next injection. The flow rate was set to 1 mL/min. Excitatory wavelength: 357 nm; emission wavelength: 455 nm.
The hippocampus and frontal cortex were taken out of liquid nitrogen, then put into chilled tubes treated with an enzyme inhibitor, then they were lysed with the lysis buffer (50 mM Tris-HCl, pH 8.0; 20 mM EDTA; 1% SDS; and 100 mM NaCl). Protein concentration was measured using a protein assay according to the manufacturer’s procedure. Lysate samples were applied to 10% SDS-polyacrylamide gels, electrophoresed, and transferred to nitrocellulose membranes for 3 h at 60 V constant voltage at 4°C. After blocking with 5% nonfat dry milk in a buffered solution (10 mM Tris-HCl, pH 7.5; 100 mM NaCl; and 0.1% Tween 20) at 4°C overnight, the membrane was exposed to one of the following antibodies: rabbit NMDA NR2A, rabbit NMDA NR2B and rabbit NMDA NR1 at 1:200 dilution (Santa Cruz Biotech, CA) for 3 h at room temperature on a shaker. Membranes were washed with the buffer solution and incubated with secondary horseradish peroxidase-conjugated antibody for 1 h at room temperature. Immunoreactivity was visualized by ECL. NR1, NR2A and NR2B protein expression were quantified by densitometry using the Scion Image Beta 4.02 software and are shown as density relative to β-actin.
Statistical analysis
Statistical analysis was made using SPSS for windows 16.0 software. Open field tests were analyzed using a repeated measurement ANOVA with treatment (control, UCMS, UCMS+ fluoxetine and UCMS + GMDZ decoction) and week (baseline, weeks 1, 2, 3, and 4) as two factors. Data of sucrose tests, glutamate level and expression of NMDA receptor subunit were performed by using one-way ANOVA. One-way or two-way ANOVAs were followed by the Bonferroni post hoc tests for multiple comparisons. Values were presented as means ± SEM, with p <0.05 considered significant.
Results
Open field test
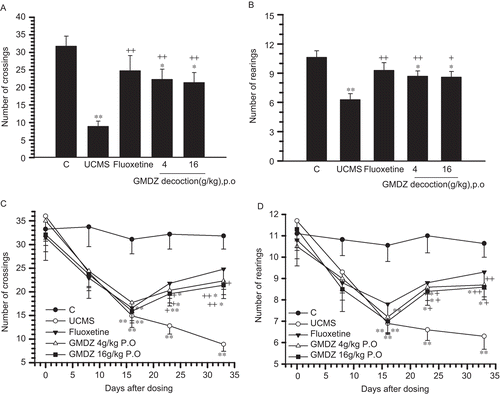
As shown in , a repeated measurement ANOVA revealed that UCMS significantly reduced the ambulation (F(4,184) = 3.899, p <0.001). Compared with the control group, the frequency of ambulation and rearing in the UCMS exposed group, GMDZ low-dose group, GMDZ high-dose group and fluoxetine group reduced significantly 2 weeks after UCMS procedure (F(4,46) = 5.614, p <0.001). The UCMS exposed group kept decreasing in ambulation and rearing during the 32 days of UCMS treatment. GMDZ decoction significantly increased UCMS induced decrease in ambulation and rearing after administration for 18 days (p <0.05), as well as the fluoxetine treated group (p <0.05).
Figure 1. Effect of GMDZ decoction on the scores of ambulation (A) and rearing (B) on day 33 in the open field test. The changes of ambulation before and during the UCMS protocol (C), and the changes of rearing before and during the UCMS protocol (D). The stress was initiated at day 0. No significant difference between animals in the four groups was present at day 0 (base line).Each column represents the mean ± SEM of 9–11 animals. +p <0.05; ++p <0.01 compared with UCMS group.*p <0.05; **p <0.01 compared with the vehicle-treated control.
Sucrose preference tests
Before the UCMS period, rats from different groups showed no significant difference in sucrose solution intake, sucrose preference and total liquid intake (). Exposure to UCMS for 32 days induced a decrease in sucrose preference and sucrose solution intake (p <0.01), which is indicative of operationally defined anhedonia (). GMDZ decoction significantly suppressed the UCMS induced decrease in sucrose preference and sucrose solution intake (p <0.01, p <0.05, respectively). Fluoxetine also significantly increased UCMS induced decrease in sucrose preference and sucrose solution intake (p <0.01, p <0.05, respectively). The total liquid intake did not differ among groups ().
Table 1. The sucrose preference test before unpredicted chronic mild stress (![]() − SE).
− SE).
Table 2. Effects of GMDZ decoction in sucrose preference test in UCMS treated rats (![]() − SE).
− SE).
Glutamate level in the hippocampus and frontal cortex
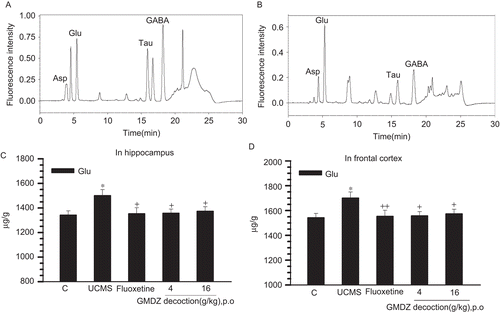
As shown in , one way ANOVA test indicated that the concentrations of glutamate in frontal cortex and hippocampus significantly differed among groups respectively (F(4,45) = 3.09, p <0.05; F(4,45) = 2.854, p <0.05). The concentrations of glutamate in frontal cortex and hippocampus in the UCMS exposed group were significantly higher than those in the control group (p <0.05). A significant decrease in glutamate concentration was observed in the fluoxetine group compared with the control group (p <0.05). GMDZ decoction markedly inhibited the UCMS induced increase in glutamate concentration (p <0.05).
Figure 2. Effect of GMDZ decoction on glutamate levels in the frontal cortex and hippocampus. (A) Typical chromatograms of amino acid standard; concentrations of amino acids in the standard solution are 0.6 μg/mL. (B) Chromatograms of rat frontal cortex and hippocampus sample. (C) Glutamate levels in the hippocampus. (D) Glutamate levels in the frontal cortex. The measured values for glutamate level in brain regions were expressed in μg/g wet tissue weight. Each column represents the mean ± SEM of 9–11 animals. +p <0.05; + +p <0.01 compared with UCMS group;*p <0.05; **p <0.01 compared with the vehicle-treated control; Glu, glutamate.
NR1, NR2A and NR2B protein levels in the frontal cortex and hippocampus
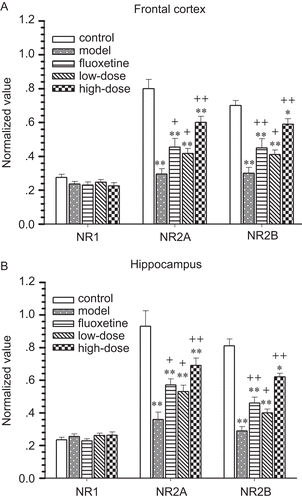
In Western blots, the NR1, NR2A and NR2B antibodies detected three prominent bands at about 120 kDa,180 kDa and 180 kDa, respectively (). One way ANOVA test indicated that protein levels of NR2A and NR2B significantly differed among groups in hippocampus(F(4,35) = 14.124, p <0.001, F(4,35) = 43.153, p <0.001), and in frontal cortex(F(4,35) = 22.115, p <0.001, F(4,35) = 18.141, p <0.001) respectively. In the chronic unpredictable stress group, the NR2A and NR2B protein levels decreased (p <0.01) as compared to the control level (). Mild increases in NR2A and NR2B protein levels were observed in the fluoxetine group as compared to the control group (p <0.05). GMDZ decoction treatment markedly increased the NR2A and NR2B protein levels in these two brain regions (p <0.05).
Figure 3. Western blots were carried out to analyze the expression of NMDA receptor subunits (NR1; NR2A; NR2B) and β-actin in crude membranes isolated from rat frontal cortex (A) and hippocampus (B) after administration of GMDZ decoction. Results shown are immunoblots from single representative experiments. (1) Control group, (2) UCMS group, (3) UCMS + fluoxetine, (4) UCMS + low-dose GMDZ decoction, (5) UCMS + high-dose GMDZ decoction.
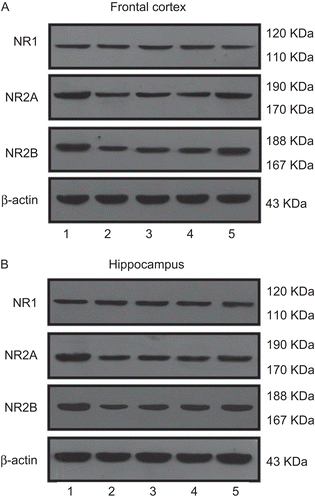
Figure 4. Expression of NMDA receptor subunits in crude membranes isolated from rat frontal cortex (A) and hippocampus (B) after administration of GMDZ decoction. Western blots were used to analyze the expression of NMDA receptor subunits. The relative expression values were normalized with β-actin value. Data are presented as mean ± SEM; +p <0.05; ++p<0.01 compared with UCMS group;*p <0.05; **p <0.01 compared with the vehicle-treated control.
Discussion
Chronic unpredictable stress acts as a predisposing and participating factor in the onset of depression in humans, and chronic unpredictable stress paradigm produces behavioral deficits thought to model aspects of depression (CitationWillner, 2005). In this model, various stressors were applied in an unpredictable order, simulating conditions in the natural environment for the main purpose of inducing anhedonia-like behavioral change, i.e. inability to experience pleasure, which is the core symptom of human major depression. Consistent with previous reports (CitationWillner et al., 1987), the present study showed that the UCMS procedure decreases the sensitivity to rewards as indicated by a reduction in the consumption of 1% sucrose solution and could be attenuated by known antidepressant fluoxetine. GMDZ treatment significantly increased the UCMS induced decrease in sucrose intake. This suggests that GMDZ has an antidepressant activity in behavioral despair animal models. Meanwhile, GMDZ increased scores in the open field test in UCMS rats. Thus, the results from this current study verified and strengthened previous findings that GMDZ has an antidepressant activity.
Glutamate is a major excitatory amino acid neurotransmitter that plays a prominent role in synaptic plasticity, learning, and memory, and is a potent neuronal excitotoxin under a variety of experimental conditions, triggering either rapid or delayed neurotoxicity (CitationGreene & Greenamyre, 1996). An abnormality in glutamate neurotransmission has also been proposed to be the basis of some forms of depression. The brain glutamate levels were significantly increased in patients with major depressive disorder (CitationHashimoto et al., 2007). Likewise, elevated serum levels of glutamate were also observed in major depression patients, and 5-week treatment with antidepressants significantly decreased the levels of glutamate in sera (CitationMaes et al., 1998). The present finding of increased glutamate in the hippocampus and frontal cortex in UCMS exposed rats may reinforce previous reports, which indirectly suggest the involvement of glutamate in the pathophysiology of major depression, although there have also been reports demonstrating decreased glutamate levels in the brains of adults with major depression (CitationRosenberg et al., 2005). GMDZ decoction significantly decreased the UCMS induced increased glutamate level in hippocampus and frontal cortex, suggesting that the antidepressant effect of GMDZ decoction may be partly due to the decrease in glutamate concentration.
Alterations in NMDA receptor expression could be a consequence of altered glutamate levels in brains of depressed subjects. Abnormalities in the NMDA receptor system have been previously observed in brains of major depression and depressive-like animals (CitationPadovan & Guimaraes, 2004). Now we demonstrate that NR2A and NR2B proteins are reduced in the hippocampus and frontal cortex in depressive-like rats. In line with our present study is the recent observation by (CitationFeyissa et al., 2009) who reported reduced expression of the NR2A and NR2B in the prefrontal cortex in depression. Additionally, a reduction in expression of both NR2A and NR2B transcripts in the perirhinal cortex was also observed in depression (CitationBeneyto et al., 2007). Moreover, a single dose of the NMDA antagonist ketamine induces a rapid antidepressant effect (CitationRowland et al., 2005; CitationZarate et al., 2006), suggesting that the dysfunction of NMDA receptors is involved in depression. Taken together, these studies support the hypothesis that NMDA receptor signaling is altered in depression.
Functional NMDA receptors are obligate heterotetramers, composing primarily of NR1 and NR2 subunits. The NR1 subunit has one gene product but many splice variants, which are expressed ubiquitously (CitationLaurie et al., 1995). In contrast, expression of the four NR2 subunit gene products (NR2A–NR2D) shows tight temporal and spatial regulation. The incorporation of different NR2 subunits has a major impact on the functional properties of the NMDA receptor, critically influencing agonist and antagonist affinity, receptor deactivation kinetics, channel conductance and interactions with intracellular proteins. For example, NR2A or NR2B-containing receptors exhibit higher responsiveness to glutamate and higher fractional Ca2+ current than do heteromers containing the NR2C or NR2D subunits (CitationYamakura & Shimoji, 1999). Differential NR2 subunit expression also occurs across brain regions. In adulthood the majority of the NMDA receptors in the hippocampus and frontal cortex are expressed as NR2A and NR2B in heteromeric complexes composed of NR1/NR2A, NR1/NR2B, or NR1/NR2A/NR2B subunits (CitationFeyissa et al., 2009). NR1 subunits are expressed in large excess, and are rapidly degraded when unassembled with NR2 partners. Hence the expression of NR2 subunits may control the number of functional NMDA receptors. NR2 subunits define the functional properties of the NMDA receptor, and NR2 subunits are more finely regulated than NR1 subunits with respect to regional and developmental expression (CitationHuh & Wenthold, 1999). Lower levels of NR2A and NR2B protein could be translated into reduced numbers of functional receptors, suggesting that depression is associated with hypofunction of the NMDA receptor in the frontal cortex and hippocampus.
GMDZ decoction treatment significantly increased the UCMS induced lower levels in NMDA subunit NR2A and NR2B, which would be a compensatory adaptation of the NMDA receptor system. Since NMDA receptor antagonists are capable of increasing NMDA receptor levels (CitationWang et al., 2000), the GMDZ decoction may have certain antagonism effect on NMDA subunit NR2A and NR2B. Fluoxetine also decreased the glutamate level and mildly increased the expression of NR2A and NR2B in the hippocampal and frontal cortex of stressed rats. There is a growing amount of evidence showing that glutamatergic and serotonergic systems interact in the mechanism of action of antidepressants (CitationPittaluga et al., 2007). Indeed, serotonin can inhibit glutamate release (CitationMaura et al., 2000). Moreover, a recent study has shown that fluoxetine inhibits NMDA receptors in the clinically relevant concentration range (CitationSzasz et al., 2007). It is plausible that the influence on glutamate, NR2A and NR2B might contribute to the intervention of glutamate system by fluoxetine observed in the UCMS treated rats.
The present study also explored NR1 protein levels in the hippocampal and frontal cortex and found no changes in depressive-like rat relative to controls. This is congruent with previous reports that no changes in the expression of NR1 subunit were observed in depression (CitationKarolewicz et al., 2005). As the specific properties of NMDA receptors such as binding affinities for agonists and antagonists and differences in conductance properties are shaped by the combination of NR1 with NR2 subunits (CitationPrybylowski et al., 2002). Normal levels of NR1 apparent in the present study does not rule out the possibility of more subtle disturbances in the trafficking, assembly or modulation of NMDA receptor localized at the hippocampus and frontal cortex excitatory synapses in depression.
Taken together, the excess excitatory amino acids could lead to the down-regulation of NMDA subunit NR2A and NR2B in the hippocampus and frontal cortex. High levels of glutamate were found in hippocampus and frontal cortex in UCMS rats, suggesting that the dysfunction of the glutamatergic system may be involved in the psychopathology of depression. GMDZ decoction decreased the level of glutamate and increased the expression of NMDA subunit NR2A and NR2B at these two brain regions, which may represent a protection against glutamate-induced neurotoxicity. Therefore, the actions of the glutamatergic system may partly account for the therapeutic effects of GMDZ.
In conclusion, the present study indicates that GMDZ has antidepressant properties. The dysfunction of the glutamate neurotransmitter system and behavior changes in UCMS rats were largely reversed by administration of the GMDZ. The findings in this study suggest that the therapeutic effects of the decoction may be due to modulation of glutamate and subsequent expression of NR2A and NR2B in hippocampus and frontal cortex. In addition, this study also helps to provide new evidence for the clinical use of the GMDZ.
Declaration of interest
This work was supported by Key Laboratory of Mental Health, Chinese Academy of Sciences, Young Scientist project from IPCAS (08CX043004), NNSF grant (30800301), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-254, KKCX1-YW-05) and Ph.D. Programs Foundation of National New Drug Research (96-901-06-75).
References
- Begley DJ, Reichel A, Ermisch A (1994): Simple high-performance liquid chromatographic analysis of free primary amino acid concentrations in rat plasma and cisternal cerebrospinal fluid. J Chromatogr B Biomed Appl 657: 185–191.
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007): Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32: 1888–1902.
- Cassano P, Fava M (2004): Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry 16: 15–25.
- Cunha RA, Ferré S, Vaugeois JM, Chen JF (2008): Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 14: 1512–1524.
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009): Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33: 70–75.
- Fornal CA, Metzler CW, Mirescu C, Stein SK, Jacobs BL (2001): Effects of standardized extracts of St. John’s wort on the single-unit activity of serotonergic dorsal Raphe neurons in awake cats: Comparisons with fluoxetine and sertraline. Neuropsychopharmacology 25: 858–870.
- Greene JG, Greenamyre JT (1996): Bioenergetics and glutamate excitotoxicity. Prog Neurobiol, 48, 613–634.
- Guo JY, Huo HR, Li LF, Guo SY, Jiang TL (2009): Sini tang prevents depression-like behavior in rats exposed to chronic unpredictable stress. Am J Chin Med 37: 261–272.
- Hashimoto K, Sawa A, Iyo M (2007): Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 62: 1310–1316.
- He ZS, Yin YL, Hu YL, Huang RL, Kong XR, Li TJ, Li FW (2008): Effects of glycyrrhetinic acid on the growth, plasma hormone and amino acid level and hematology in milk-fed piglets. J Nanjing Agric Univ, 31, 111–115.
- Huh KH, Wenthold RJ (1999): Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem 274: 151–157.
- Karolewicz B, Stockmeier CA, Ordway GA (2005): Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology 30: 1557–1567.
- Laurie DJ, Putzke J, Zieglgänsberger W, Seeburg PH, Tölle TR (1995): The distribution of splice variants of the NMDAR1 subunit mRNA in adult rat brain. Brain Res Mol Brain Res 32: 94–108.
- Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpé S (1998): Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: Modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand 97: 302–308.
- Maura G, Marcoli M, Pepicelli O, Rosu C, Viola C, Raiteri M (2000): Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in human neocortex slices: Involvement of 5-HT(2C) and 5-HT(1A) receptors. Br J Pharmacol 130: 1853–1858.
- National Pharmacopoeia Committe (2005) The People’s Republic of China Pharmacopoeia. Beijing, China: Medical Science Publishing House.
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press.
- Neyton J, Paoletti P (2006) Relating NMDA receptor function to receptor subunit composition: Limitations of the pharmacological approach. J Neurosci 26: 1331–1333.
- Padovan CM, Guimaraes FS (2004): Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats. Pharmacol Biochem Behav 77: 15–19.
- Pittaluga A, Raiteri L, Longordo F, Luccini E, Barbiero VS, Racagni G, Popoli M, Raiteri M (2007): Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacology 53: 27–36.
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, Vicini S (2002): Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci 22: 8902–8910.
- Rosenberg DR, MacMaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Villafuerte RA, Moore GJ, Renshaw P (2005): Reduced anterior cingulate glutamate in pediatric major depression: A magnetic resonance spectroscopy study. Bio Psychiatry 58:700–704.
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM (2005): Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: A 4-T proton MRS study. Am J Psychiatry 162: 394–396.
- Schildkraut JJ (1965): The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry 122: 509–522.
- Shen YH, Zhou Y, Zhang C, Liu RH, Su J, Liu XH, Zhang WD (2009): Antidepressant effects of methanol extract and fractions of Bacopa monnieri. Pharm Biol 47: 340–343.
- Shuang J, De L, Ma QZ, Ba Y, NIu XL (2001): Influence of licorice root extract on pig’s fattening and content of muscular free amino acids in compound additive. J Inner Mongolia Agric Anim Husb 22: 75–79.
- Suen PC, Wu K, Xu JL, Lin SY, Levine ES, Black IB (1998): NMDA receptor subunits in the postsynaptic density of rat brain: Expression and phosphorylation by endogenous protein kinases. Brain Res Mol Brain Res 59: 215–228.
- Szasz BK, Mike A, Karoly R, Gerevich Z, Illes P, Vizi ES, Kiss JP (2007): Direct inhibitory effect of fluoxetine on N-methyl-D-aspartate receptors in the central nervous system. Biol Psychiatry 62: 1303–1309.
- Wang C, Wang Y, Zhao Z (2000): Peripheral NMDA and non-NMDA receptors contribute to nociception: An electrophysiological study. Brain Res Bull 52: 31–34.
- Willner P (2005): Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52: 90–110.
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987): Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364.
- Wu J (2002): Antidepressant effect of gan-mai-da-zao tang on depression compared with fluoxetine. J Chinese Physician 30: 18–19.
- Yamakura T, Shimoji K (1999): Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol 59: 279–298.
- Yang FE, Qiao QF, Gong YP (2009): Antidepressant effect of gan-mai-da-zao tang on 30 women with postpartum depression. Shanxi J TCM 30: 851–852.
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006): A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864.
