Abstract
Context: Leaves of Murraya exotica L. (Rutaceae) are used for the treatment of various disorders such as cough, fever, and infectious wounds, as well as alleviating pains in folk medicine in southern China.
Objective: The objectives of this study were to investigate the in vivo antinociceptive and anti-inflammatory activities of ethanol (70%) extracts and isolated compounds obtained from the dried leaves of M. exotica.
Materials and methods: The antinociceptive activities were evaluated with the methods of acetic acid-induced writhing response and hot-plate latent pain response test. Carrageenan induced hind paw edema, xylene induced ear edema, and a rat knee osteoarthritis model were employed to measure the anti-inflammatory activities. The compounds were isolated using column chromatography and thin-layer chromatography, and the structures identified by 1H NMR, 13C NMR, MS, and IR.
Results: The ethanol (70%) extracts significantly decreased in the acetic acid-induced writhing response; increased in hot-plate latency; suppressed xylene induced ear swelling and the carrageenan-induced paw edema effectively. In the rat knee osteoarthritis model, the treatment of the ethanol (70%) extracts resulted in a significant increase in the activity of superoxide dismutase, an inhibition on inducible nitric oxide synthase activity, and a decrease in the contents of interleukin-1β and tumor necrosis factor-α of the rat serum. Following this, we explored the components of the ethanol (70%) extracts and isolated six known coumarins, including murracarpin, which exhibited the most potential in antinociceptive and anti-inflammatory activities.
Discussion and conclusion: M. exotica displayed remarkable antinociceptive and anti-inflammatory activities.
Introduction
Murraya exotica L. (Rutaceae) (common names: jiulixiang, qianlixiang) grows widely in southern China and is known as an ornamental and hedge plant for its pleasant smell and beauty. However, the leaves and roots of the plant have been traditionally used as a medicine to treat stomachalgia, rheumatalgia, toothache, and body pains from injury or trauma. This is well documented in the Pharmacopoeia of the People’s Republic of China (CitationChina Pharmacopaeia Committee, 2005). The indigenous population of southern China apply it to cure cough, fever, some infectious wounds such as furuncle and carbuncle, and eliminate pain. It is known to be used in India for treatment of diarrhea and dysentery (CitationChopra et al., 1956). There is evidence from the CitationNational Traditional Chinese Medicines Compilation (1996) that M. exotica was a common anesthetic drug for tonsillectomy, an effective drug for antifertility in local hospitals in Fujian Province, for treatment of epidemic encephalitis type B and tetanus in Guangdong Province, and for acute or chronic nephritis in Yunnan Province. A past report revealed that M. exotica possessed multi-pharmaceutical functions such as antibiotic activity against Mycococcus pyogenes and Escherichia coli (CitationLily, 1980). The essential oil from M. exotica exhibited strong antifungal activity against Candida albicans and exhibited a modest antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Sarchina lutea (CitationEI-Sakhawy et al., 1998).
Phytochemical studies have been carried out revealing coumarins and flavanoids are the two main components in the leaves of M. exotica. The coumarins included murrangatin, osthole, meranzin, meranzin hydrate, phebalosin, murrangatin acetate, isomurralonginol, isomeranzin, umbelliferone, and scopoletin (CitationIto & Furukawa, 1987); the flavanoids included 3,3′,4′,5,5′,6,7-heptamethoxyflavone, bannamurpanisin, exoticin, methyl N-methyl anthranylate, gardenin A, gardenin C, and gardenin E (CitationSaied, 2005). Information available primarily focuses on the extracts, fractions and essential oils of the aerial parts and flowers of the plant (CitationRahman et al., 1997; CitationRout et al., 2007). The pharmacological activities of the compounds are relatively unknown. However, umbelliferone was reported to show significant anti-inflammatory and analgesic activity at 10 mg/kg, and osthole was revealed to have anti-inflammatory activity (CitationChen et al., 1995). Moreover, umbelliferone was reported to have antihyperlipidemic and antidiabetic activities (CitationRamesh & Pugalendi, 2005) and antioxidant effects (CitationRamesh & Pugalendi, 2006). Scopoletin could induce apoptosis in HL-60 cells by activating NF-κB and caspase-3 (CitationKim et al., 2005). Scopoletin was found to exhibit dual effects on both concanavalin A-stimulated murine T cell proliferations by interacting with PKC and antiproliferative activity in a lymphoma cell line (CitationManuele et al., 2006).
Until now, there has been no systemic data to elucidate the biological activities of M. exotica. To provide scientific evidence for further investigation, this paper will investigate and evaluate the antinociceptive and anti-inflammatory activities of the ethanol extracts using in vivo animal models. In the course of our continuing studies, we carried out primary screening in vivo of six coumarins isolated from the dried leaves of M. exotica.
Experimental methods
Animals
Kunming strains of male mice (CitationLu et al., 2005) weighing 20 ± 2 g were obtained from the animal house of Fujian Medical University. They were kept under controlled standard environment conditions and not fed on the day before the experiment, but allowed free access to water. The animals were randomly distributed in control and in test groups of six. Efforts were made to minimize animal suffering and reduce the numbers used. All experimental procedures were approved by the Institutional Animal Ethical Committee of HuaQiao University.
Plant material and solvent for extraction
The M. exotica leaves used for study were collected from Zhangzhou (Fujian, China) in 2008. The plants were identified by Jinghong Zhang. The voucher specimen (ID: M20060081) was deposited in the herbarium of the Institute of Molecular Medicine, HuaQiao University. The leaves were harvested, air-dried and then ground into powder with a laboratory scale mill. Petroleum, diethyl ether, ethyl acetate, chloroform, ethanol (70%) and boiled water were taken into reflux for extraction from the powder (every solvent for 50 g powder) to test its antinociceptive activity by the writhing test mouse model.
Extracts and isolation
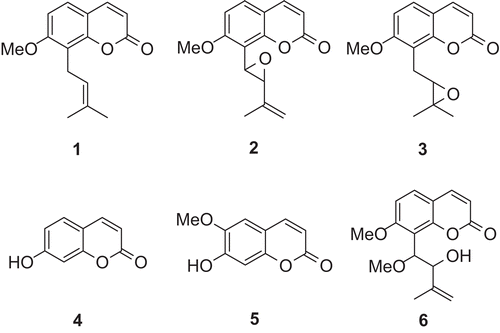
The powder (1 kg) was extracted (by maceration) with 10 L of ethanol (70%) for 48 h at room temperature. The extract was evaporated in vacuum to give a crude ethanol extract (19.26%, w/w). Part of the crude extract (100 g) was subjected to column chromatography (CC) on silica gel (200–300 mesh), eluted with petroleum and ethyl acetate under increasing polarity (1:1–1:4, v/v), and were combined according to analytic thin layer chromatography (TLC) to give seven fractions. Fraction 2 (petroleum-ethyl acetate 4/1; 17 g) was subjected to CC on silica gel (200–300 mesh) (eluted with petroleum-acetone-chloroform (10:0.2:0.1, v/v/v)) giving colorless needles, osthole (1; 56 mg; melting point (m.p.) 79°–81°C) (CitationIto & Furukawa, 1987). Fraction 4 (petroleum-ethyl acetate 2/1; 31 g) was subjected to CC on silica gel (200–300 mesh) (eluted with petroleum-acetone-chloroform (10:1:0.5, v/v/v)) giving colorless needles, phebalosin (2; 203 mg; m.p. 118°–120°C) (CitationIto & Furukawa, 1987), and meranzin (3; 256 mg; m.p. 90°–92°C) (CitationIto & Furukawa, 1987). Fraction 5 (petroleum-ethyl acetate 4/3; 39 g) was subjected to CC on silica gel (200–300 mesh) (eluted with petroleum-acetone-chloroform (10:2:1, v/v/v)) giving light yellow needles, umbelliferone or 7-hydroxycoumarin (7-HC) (4; 89 mg; m.p. 230°–232°C) (CitationBulut & Erk, 1996); colorless needles, scopoletin (5; 76 mg; m.p. 202°–203°C) (CitationIto & Furukawa, 1987); colorless needles, (±)-murracarpin (6; 27 mg; m.p. 153°–154°C) (CitationWu et al., 1989). The chemical structures of the isolated compounds are illustrated in .
Figure 1. Chemical structures of compounds isolated from M. exotica. They were confirmed by 1H NMR, 13C NMR, MS and IR spectra and comparing with reference data from available literature (CitationIto et al., 1987; CitationBulut et al., 1996; CitationWu et al., 1989).
Pre-coated silica gel GF254 plates (Qingdao Marine Chemicals, Qingdao, China) were used for TLC, eluted with mixtures of solvents such as petroleum/ethyl acetate (4:1); petroleum/acetone/chloroform (10:1:0.5) and the spots were visualized using ultraviolet light (254 and 365 nm). NMR spectra were recorded on a Bruker Avance-400 (Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan, China) at both 400 MHz (1H) and 100 MHz (13C), with the residual solvent peaks used as internal references. MS and IR were also taken into consideration. The structures of the compounds were confirmed by comparing with reference data from available literature.
General procedure
The crude extracts by different solvents were suspended in 0.8% sodium carboxymethyl cellulose (CMC) with distilled water and administrated at the dose of 5 mg powder/mouse, which is the dose recommended in the Pharmacopoeia of the People’s Republic of China (CitationChina Pharmacopoeia Committee, 2005).
Each mouse was intragastrically treated with the ethanol extracts dissolved in 0.8% sodium CMC in 50, 100 and 200 mg/kg doses (100 mg/kg was the recommended dose in PPRC). The isolated compounds were dissolved in 1% Tween-80 to give a final concentration of 10, 5, and 2.5 mg/kg for intraperitoneal injection for each mouse. The control group animals received the same experimental handling as those of the test groups except that the drug treatment was replaced with appropriate volumes of the dosing vehicle. Either indomethacin (10 mg/kg) or hexadecadrol (0.5 mg/kg) was used as reference.
The kits of superoxide dismutase (SOD) and inducible nitric oxide synthase (iNOS) were purchased from Nanjing Jiancheng Bio-engineering Institute; the kits of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were purchased from Nanjing Jiancheng Bio-engineering Institute (Nanjing, China).
Acetic acid-induced writhing response in mice
The writhing test was carried out as described by CitationNakamura et al. (1986). Each mouse was treated with a test drug or vehicle 1 h before intraperitoneal injection of 0.6% acetic acid at 0.1 mL/10 g body weight. The number of stretchings or writhings was recorded between 5 and 20 min after acetic acid injection. The analgesic effects were calculated by comparing the number of abdominal writhes of the test group with that of the control group. Indomethacin (10.0 mg/kg) was used orally as the reference drug.
Hot-plate latent pain response test in mice
The hot-plate test was carried out according to the method of CitationEddy and Leimback (1953). Each male mouse was placed on a 55° ± 1°C hot-plate to observe its pain responses (hind-paw-licking or jumping). The latent time before the occurrence of the pain response was recorded as an analgesic parameter. Untreated mice with a background latent response time shorter than 5 s or longer than 30 s were excluded from the study. Each mouse was given a single dose of drug or vehicle then the hot-plate latent response time of each animal was recorded. Indomethacin (10 mg/kg) was orally used as the reference drug.
Carrageenan-induced hind paw edema in rats
Paw edema was induced in rats by carrageenan injection (CitationWinter et al., 1962). Each rat was administered a single dose of drug or vehicle 1 h prior to carrageenan injection. To induce paw edema, 0.1 mL of carrageenan (1% in normal saline solution) was injected intradermally on the plantar side of the right hind paw. Edema was evaluated by the paw volume before and 1, 2, 3 or 4 h after carrageenan injection. Hexadecadrol (0.5 mg/kg) was used intraperitoneally as the reference drug.
Xylene-induced ear edema in mice
We followed the procedure as outlined from past methods (CitationChen, 1993). Each mouse was given a single dose of a test drug or vehicle 1 h before the induction of ear edema by topical application of 0.02 mL xylene on the right ear. The left ear served as a control. Mice were sacrificed by cervical dislocation 1 h after xylene application. Ear disks of 8 mm in diameter were punched out and weighed. The extent of edema was evaluated by the weight difference between the right and the left ear disks of the same animal. Hexadecadrol (0.5 mg/kg) was intraperitoneally used as reference drug.
Rat knee osteoarthritis model
Rat osteoarthritis (OA) model was established by Hulth’s method (CitationRogart et al., 1999). The procedure was as follows: the rats were anesthetized with intravenous injection of 3% pentobarbitone (30 mg/kg) and after routine disinfection, a 1-cm longitudinal incision was made at the medial parapatella separating and cutting off the tibial collateral ligament, the articular cavity was opened and the cruciate ligament of the knee was cut off, the medial meniscus was excised and the articular cavity rinsed and sutured layer by layer, then the rats underwent penicillin treatment for one week for prevention against infection. After 8 weeks establishment of the model the rats were sacrificed and 5 mL blood was taken from the heart, then the serum was separated and kept at −20°C for determination of SOD, iNOS, IL-1β and TNF-α. The contents of these cytokines were determined with the methods recommended by kit’s manual (CitationHe et al., 2006; CitationQi et al., 2009). Other segments of the synovial membrane and cartilage were taken and fixed in 10% formalin for gross observation and histomorphological examination under light microscope.
Statistical analysis
Results were expressed as mean ± SD. Statistical analyses were performed with one-way ANOVA followed by Student’s t-test where P < 0.05 was considered statistically significant.
Results
The structures of the isolated compounds were established using spectroscopic analysis and direct comparison with published information, and with authentic specimens obtained by our research group for some cases. The compounds isolated from the leaves of M. exotica were osthole (1), phebalosin (2), meranzin (3), umbelliferone or 7-hydroxycoumarin (7-HC) (4), scopoletin (5), and (±)-murracarpin (6).
Extraction from different solvents for antinociceptive activities
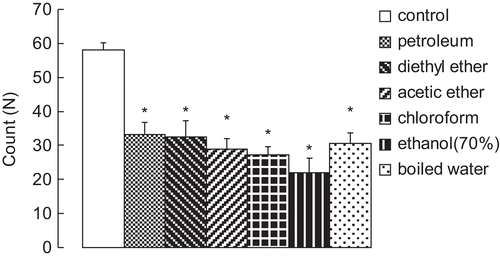
After evaporation of the solvents, the extracting efficacies by petroleum, diethyl ether, ethyl acetate, chloroform, ethanol (70%) and boiled water were 6.43, 9.08, 11.39, 12.99, 22.36 and 23.50% (w/w), respectively.
Results in showed that the ethanol (70%) extraction decreased the writhing responses most to 22 ± 4.2 (P < 0.05) compared with the control group, which had 58 ± 2.3 writhes within 15 min after administration of acetic acid (0.6%, v/v, 0.1 mL/10 g, i.p.). Extraction from boiled water followed. However, extraction from petroleum and diethyl ether seemed to show no obvious difference on decreasing the writhing responses in the mice.
Acetic acid induced writhing response in mice
As shown in , acetic acid (0.6%, v/v, 0.1 mL/10 g, i.p.) could significantly induce the writhing responses. Indomethacin at 10 mg/kg markedly decreased the writhing response to 8.3 ± 0.7 (P < 0.01). The ethanol extracts decreased the writhing response in a dose-dependent manner. At doses of 50, 100 and 200 mg/kg, the counts of the writhings were 25.6 ± 2.7 (P < 0.05), 15.4 ± 1.2 (P < 0.05) and 11.8 ± 1.9 (P < 0.05), respectively.
Figure 3. (A) Effect of the ethanol extracts on acetic acid-induced writhing response in mice. (B) Effect of the isolated compounds on acetic acid-induced writhing response in mice. Results are expressed as mean ± SD (n = 6), *P < 0.05 and **P < 0.01 compared with control.
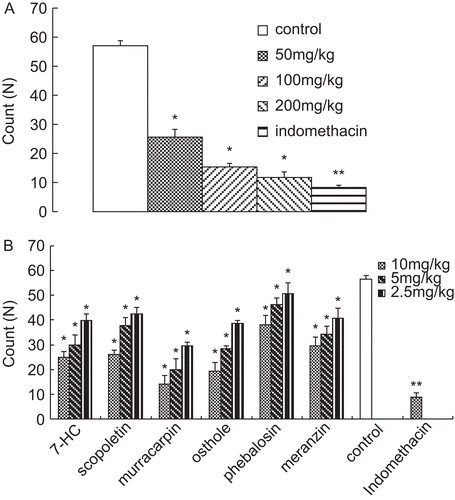
In , among the isolated compounds, murracarpin showed most potential in decreasing the writhing response (14.2 ± 3.3 at the dose of 10 mg/kg, P < 0.05) in a dose-dependent manner, albeit less effective than that in the indomethacin group (8.9 ± 1.6, P < 0.01). In contrast, phebalosin exhibited little effect on decreasing the writhing response.
Hot-plate latent pain response test in mice
Results in indicate that the positive control drug indomethacin (10 mg/kg) significantly increased the pain threshold of mice (39.6 ± 1.2 s, P < 0.05 in 60 min). The ethanol extracts greatly increased hot-plate latency in a dose-dependent manner. The maximum effect of the ethanol extracts was observed at 1 h after drug administration, when the latency time was up to 32.4 ± 0.5 s. (P < 0.05) at the dose of 200 mg/kg.
Figure 4. Effect of the ethanol extracts on hot-plate test in mice. Results are expressed as mean ± SD (n = 6), *P < 0.05 and **P < 0.01 compared with control.
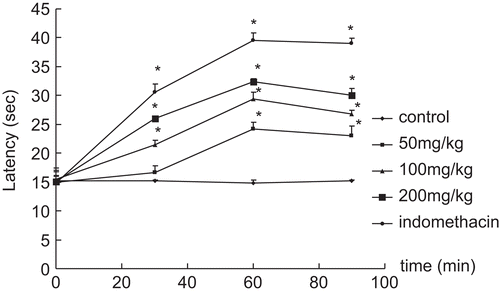
In , it indicated that murracarpin, among the isolated compounds, exhibited the most potential in increasing latency in a dose-dependent manner, which were similar to that in writhing test, murracarpin also exhibited the most potential in increasing latency in a dose-dependent manner. The maximum effect of murracarpin (43.6 ± 4.1 s, P < 0.05) was observed at 1 h after administration, which was much better than that of indomethacin (39.7 ± 5.1 s). In addition, osthole showed approximately the same effect as that of indomethacin at 1 h, but this began to decrease 90 min after administration. Phebalosin and meranzin were the least effective in increasing latency.
Table 1. Effect of the isolated compounds on hot-plate test in mice.
Carrageenan-induced hind paw edema in rats
As shown in , carrageenan (1% w/v, 0.1 mL/paw, s.c.) could significantly induce edema at 1, 2, 3, and 4 h after injection in the control group in rats. The ethanol extract at an oral dose of 200 mg/kg at 3 h (35.2 ± 2.4%, P < 0.05) after injection of carrageenan significantly inhibited the paw edema compared with the control. Its activity was less than hexadecadrol (0.5 mg/kg) at 3 h (25.3 ± 3.4%, P < 0.05) under the same conditions.
Figure 5. (A) Inhibitory action of the ethanol extracts on carrageenan- induced paw edema in rats. (B) Inhibitory action of the isolated compounds on carrageenan-induced paw edema in rat. Results are expressed as mean ± SD, (n = 6), *P < 0.05 and **P < 0.01 compared with control.
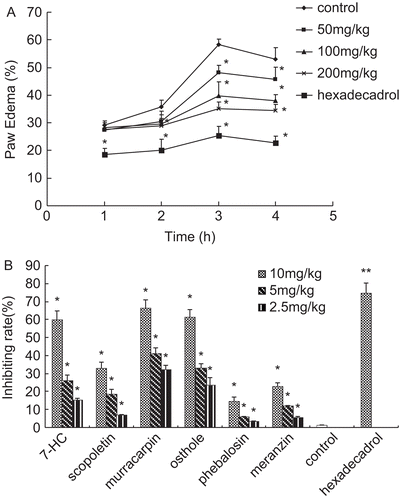
The ethanol extract showed its greatest effect at 3 h. To evaluate the inhibiting effect on paw edema induced by carrageenan, the isolated compounds from the dried leaves were as important and effective as the compounds in the ethanol extract, which exhibited similar properties. Thus, we evaluated their inhibiting rates at 3 h. Results in indicated that murracarpin, among the isolated compounds, exhibited the most potent effect in inhibiting carrageenan-induced paw edema. Its inhibiting rate was 66.39 ± 4.7% (P < 0.05), at the dose of 10 mg/kg, still less than that of hexadecadrol (0.5 mg/kg). It was observed that at the dose of 2.5 mg/kg, phebalosin and meranzin did not exhibit obvious effect on inhibiting carrageenan- induced paw edema in rat.
Xylene-induced ear edema in mice
The results presented in showed that the ethanol extract of M. exotica leaves suppressed xylene- induced ear swelling in the mice 41.4 ± 0.3% (P < 0.05) inhibition rate at the dose of 200 mg/kg and worked dose-dependently. Hexadecadrol at the dose of 0.5 mg/kg also exerted significant inhibitory effect, which increased to 58.8 ± 1.2% (P<0.01).
Figure 6. (A) Effect of ethanol extract on xylene-induced ear edema in mice. (B) Effect of the isolated compounds on xylene-induced ear edema in mice. Results are expressed as mean ± SD, (n = 6),*P < 0.05 and **P < 0.01 compared with control.
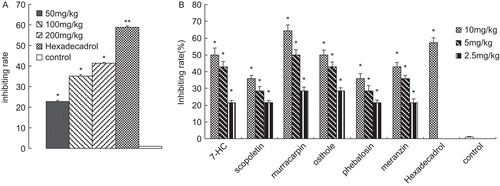
As shown in , murracarpin showed much more potent activity (64.3 ± 3.4%, P < 0.05) than that of hexadecadrol. In addition, osthole and 7-HC also exhibited high effect on inhibiting the ear edema in a dose-dependent manner. Their inhibiting activities were 50 ± 2.8% (P < 0.05), 50 ± 4.2% (P < 0.05) at the dose of 10 mg/kg.
Rat knee osteoarthritis model
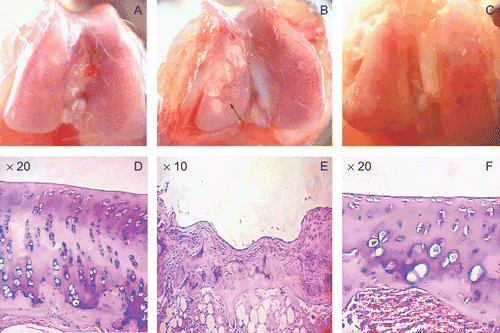
There were no adverse reactions to the ethanol extracts noted in any of the treated animals. All the surviving animals developed knee OA eight weeks after the operation in the model group. Gross observation and histomorphological examination () found some degrees of hyperplasia, hypertrophy, and congestion in femoral condyle and the rough surface of cartilage with erosion and even ulcer in the model group, indicating the successful establishment of knee OA model. No obvious damage was found from gross observation in the ethanol extract group, except a little congestion, exhibiting significant effect of preventing knee osteoarthritis by the ethanol extract. In contrast, the model group showed the hyperplasia of synovium in the surface of cartilage and the disappearance of other layers below it in the ulcer area. The two histological examinations were for the purpose of basic comparison, data of the dose-dependent pathological changes are not provided. However, the contents of biochemical cytokines occurred to be affected when administrated in different dose.
Figure 7. The gross observation and the histomorphology of femoral condyle in knee joint. (A) and (D) are the control groups; (B) and (E) are the model groups, which demonstrated the severe ulcer (see the arrow) in femoral condyle. In this ulcer area, hyperplasia was seen in the synovium and the other cell layers were unclear. (C) and (F) are the ethanol extract (200 mg/kg) groups. There were no obvious differences when compared with the control groups, indicating regular surface and good arrangement of all cell layers. However, there was a little congestion in (C) and hypertrophy and a little shorter height in cartilage in (F).
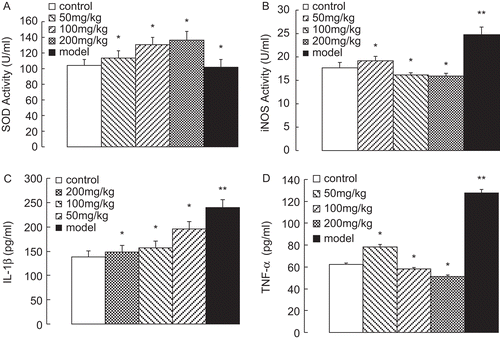
As shown in , the ethanol extract could significantly elevate SOD activities dose-dependently in 136.76 ± 10.7 U/mL (P < 0.05) at the dose of 200 mg/kg, after an eight week establishment of the model. In contrast, the model group showed SOD activity as 101.57 ± 10.20 U/mL (P < 0.05), which was slightly lower than that of the control group at 104.19 ± 7.3 U/mL. In the activity of iNOS was significantly induced up to 24.76 ± 1.6 U/mL (P < 0.01) in the model group. However, the ethanol extracts could effectively inhibit the production of iNOS in a dose-dependent manner. The contents of IL-1β and TNF-α in the serum were determined by ELISA assay. As shown in , the contents of IL-1β and TNF-α were significantly decreased by the ethanol extract dose-dependently, and down to 148.25 ± 14.10 pg/mL (P < 0.05) and 51.47 ± 1.10 pg/mL, respectively, at the dose of 200 mg/kg.
Discussion
In this study, we first investigated the antinociceptive and anti-inflammatory response of the ethanol extracts and the isolated compounds from M. exotica in experimental animal models.
Two animal models were employed to investigate the potential antinociceptive activity of the ethanol extracts and the isolated compounds. The methods for antinociception were selected such that both centrally and peripherally mediated effects were investigated. Acetic acid induced writhing response is believed to show the involvement of peripheral mechanisms, whereas the hot-plate test is believed to show that of central mechanisms (CitationPaulino et al., 2003). The evident antinociceptive activity in the models demonstrated that the ethanol extract and the isolated compounds possess both central and peripherally mediated activities. In the writhing response it has been suggested that acetic acid acts by releasing endogenous mediators that stimulate the nociceptive neurons (CitationCollier et al., 1968). It was postulated that the writhing response is induced by local peritoneal receptor activation and involved prostaglandin (PG) E2, PGF2 (CitationDerart et al., 1980), and lipooxygenase (CitationDhara et al., 2000) as well. The results of the present study showed that indomethacin, which inhibits cyclooxygenase, caused a significant inhibition of acetic acid induced pain. The hot-plate test is considered a central model that has selectivity for opioid-derived analgesics (CitationAbbott & Melzack, 1982; CitationAbbott & Franklin, 1986; CitationNavarro et al., 2002). Administration of the ethanol extracts and the isolated compounds exerted potent antinociceptive action confirming the central activity. Whether it involved the mediation of opioid receptors is still a question to be further investigated. It is necessary that other mechanisms responsible for the analgesic effects of the ethanol extracts and the isolated compounds need to be understood.
The carrageenan-induced paw edema as an in vivo model of acute inflammation has been frequently used to assess the anti-inflammatory effect of natural products. The acute inflammatory responses induced by carrageenan involved phases of chemical mediator release (CitationVinegar et al., 1969). The early phase (1–2 h) involves the release of serotonin and histamine; kinins play a role in the middle phase (CitationDi & Sorrentino, 1968), while PGs appear to be the most important mediator in the final phase (3–5 h) of the post-carrageenan response (CitationDi et al., 1971; CitationDi, 1972). Xylene-induced ear edema is another acute inflammation model. Ear edema may involve inflammatory mediators such as histamine, serotonin, bradykinin and PGs, which induced ear edema by promoting vasodilation and increasing vascular permeability (CitationCarlson et al., 1985). In the present study the two models of evaluating anti-inflammatory activity of the ethanol extracts and the isolated compounds suggested that their mechanisms might involve multiple anti-inflammatory factors and mediators.
OA is a well-known disease that is part of the aging process and one of the most common diseases among mammals. There is now strong evidence that the structural changes globally observed in OA are due to a combination of factors, ranging from mechanical to biochemical (CitationNuki, 1999; CitationPelletier et al., 2000). It is increasingly apparent that chondrocytes have the capacity to produce a variety of cytokines and mediators associated with inflammation such as nitric oxide (NO), PGs, IL-1β and TNF-α. Proinflammatory cytokines have been demonstrated to play a pivotal role in the development of this disease process. In particular, IL-1β and TNF-α seem prominent to cartilage destruction (CitationCaron et al., 1996; CitationVan de Loo et al., 1995). The iNOS could cause excessive production of NO, which is responsible for inducing an inflammatory reaction, tissue destruction, as well as cell death in OA model, although NO is suggested to possess destructive and protective functions in other cells. The selective inhibition of iNOS has proven to exert positive effects on the progression of lesions in an experimental canine OA model (CitationPelletier et al., 1998). NO and its reactive oxygen species derivatives could produce peroxynitrite which contributes to a number of destructive events in cartilage (CitationDel Carlo & Loeser, 2002). Joint fluid analysis in patients with OA had lower concentrations of the superoxide scavenger enzyme extracellular SOD, suggesting an increase in oxidative derivatives may contribute damage (CitationRegan et al., 2008). Our study showed that the ethanol extracts could significantly decrease iNOS activity, increase SOD activity, and so too decrease the products of NO and its reactive oxygen species. Following this, it protected cartilage and chondrocytes from destruction. It is widely known, IL-1β and TNF-α are considered to be master cytokines in chronic destructive arthritis. Though, IL-1β is much more potent than TNF-α in inducing cartilage destruction in vivo (CitationVan de Loo & van den Berg, 1990). IL-1β and TNF-α can stimulate their own production and induce chondrocytes and synovial cells to produce other cytokines, such as IL-8, IL-6 and leukocyte inhibitory factor, as well as PGE2 (CitationBertolini et al., 1986). TNF-α appears to be an important mediator of matrix degradation and a pivotal cytokine in inducing synovial membrane inflammation. Therefore, these proinflammatory cytokines are considered potential contributing factors in the pathogenesis of this disease. Our results showed that the ethanol extracts could significantly decrease the contents of both proinflammatory cytokines, IL-1β and TNF-α. From the histomorphological examination, it shows that the ethanol extract could be effective in maintaining the normal function of cartilage in femoral condyle and the arrangement of different layers of cartilage cells.
Coumarins and flavanoids are the two major components in the leaves of M. exotica, and the ethanol extracts have significant anti-inflammatory and antinociceptive activities. It can be deduced that coumarins and flavanoids were the compounds responsible for such activities. In this paper, we isolated six compounds, which are simple coumarins, from the leaves of M. exotica. Among these compounds, murracarpin showed the most potential in antinociceptive and anti-inflammatory activity.
Conclusion
For the first time, the antinociceptive and anti-inflammatory activities of the extract of M. exotica leaves are reported. Of the isolated compounds, murracarpin exhibited the most potent activity in antinociceptive and anti-inflammatory actions. On the basis of this evidence, it could explain and support, in part, the folk use of M. exotica in Chinese traditional medicine. However, more research is required to supplement these findings.
Acknowledgements
We would like to thank Professor Ruian Xu and Xiao Wang for their assistance in experiments and Dr. Jinghong Zhang for her kind help on identification of the herb.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbott FV, Melzack R (1982): Brainstem lesions dissociated neural mechanisms of morphine analgesia in different kinds of pain. Brain Res 251: 149–155.
- Abbott FV, Franklin KBJ (1986): Noncompetitive antagonism of morphine analgesia by diazepan in the formalin test. Pharmacol Biochem Behav 24: 319–321.
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR (1986): Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319: 516–518.
- Bulut M, Erk C (1996): Improved synthesis of some hydroxycoumarins. Dyes and Pigments 30: 99–104.
- Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ (1985): Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 17: 197–204.
- Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier JP (1996): Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis: Suppression of collagenase-1 expression. Arthritis Rheum 39: 1535–1544.
- Chen Q (1993): Pharmacological Research Methods in Traditional Chinese Medicine. Beijing, People’s Medical Publishing House, p. 305.
- Chen YF, Tsai HY, Wu TS (1995): Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med 61: 2–8.
- China Pharmacopoeia Committee (2005): Pharmacopoeia of the People’s Republic of China. Beijing, Chemical Industry Press, p. 9.
- Chopra RN, Nayar SL, Chopra IC (1956): Glossary of Indian Medicinal Plant. New Delhi, Council of Scientific and Industrial Research, p. 171.
- Collier HOJ, Dinneen LC, Johnson CA, Schneider C (1968): The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol 32: 295–310.
- Del Carlo M Jr, Loeser RF (2002): Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum 46: 394–403.
- Derart R, Jouaney S, Delevalcee F, Falhout M (1980): Release of prostaglandins E and F in algogenic reaction and its inhibition. Eur J Pharmacol 51: 17–24.
- Dhara AK, Suba V, Sen T, Pal S, Nagchaudhuri AK (2000): Preliminary studies on the anti-inflammatory and analgesic activity of the methanolic fraction of the root extract of Tragia involucrate. J Ethnopharmacol 72: 265–268.
- Di RM, Sorrentino L (1968): The mechanism of the inflammatory effect of carrageenan. Eur J Pharmacol 4: 340–342.
- Di RM, Giroud JP,Willoughby DA (1971): Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104: 15–29.
- Di RM (1972): Biological properties of carrageenan. J Pharm Pharmacol 24: 89–102.
- Eddy NB, Leimback D (1953): Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 107: 385–393.
- EI-Sakhawy FS, EI-Tantawy ME, Ross SA, and EI-Sohly MA (1998): Composition and antimicrobial activity of the essential oil of Murraya exotica L. Flavour Frag J 13: 59–62.
- He H, Dai D, Dai Y (2006): CPU0213, a novel endothelin receptor antagonist, ameliorates septic renal lesion by suppressing ET system and NF-κB in rats. Acta Pharmacol Sin 27: 1213–1221.
- Ito C, Furukawa H (1987): Constituents of Murraya exotica L. structure elucidation of new coumarins. Chem Pharm Bull 35: 4277–4285.
- Kim EK, Kwon KB, Shin BC, Seo EA, Lee YR, Kim JS, Park JW, Park BH, Ryu DG (2005): Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor κB and caspase-3. Life Sci 77: 824–836.
- Lily EM (1980): Medicinal Plants of East and Southeast Asia. Cambridge, MA, MIT Press, p. 367.
- Lu B, Wu X, Tie X, Zhang Y, Zhang Ying (2005): Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol 43: 783–792.
- Manuele MG, Ferraro G, Barreiro Arcos ML, Lopez P, Cremaschi G, Anesini C (2006): Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci 79: 2043–2048.
- Nakamura H, Shimoda A, Ishii K, Kadokawa T (1986): Central and peripheral analgesic action of non-acidic non-steroidal anti-inflammatory drugs in mice and rats. Arch Int Pharmacody Ther 282: 16–25.
- National Traditional Chinese Medicines Compilation (1996): The National Traditional Chinese Medicines Compilation (Second edition). Beijing, People’s Medical Publishing, p. 19.
- Navarro DF, Souza MM, Neto RA, Golin V, Niero R, Yunes RA, Monache FD, Filho VC (2002): Phytochemical analysis and analgesic properties of Curcuma zedoaria grown in Brazil. Phytomedicine 9: 427–432.
- Nuki G (1999): Role of mechanical factors in the aetiology, pathogenesis and progression of osteoarthritis, in: Reginster JY, Pelletier JP, Martel-Pelletier J, Henrotin Y, eds., Osteoarthritis: Clinical and Experimental Aspects. Berlin, Springer, p. 101–114.
- Paulino N, Dantas AP, Bankova V, Longhi DT, Scremin A, Decastro SL, Calixto JB (2003): Bulgarian propolis induces analgesics and anti-inflammatory effects in mice and inhibits in-vitro contraction of airway smooth muscle. J Pharmacol Sci 93: 307–313.
- Pelletier JP, Martel-Pelletier J, Howell DS (2000): Etiopathogenesis of osteoarthritis, in: Koopman WJ, editor. Arthritis & Allied Conditions: A Textbook of Rheumatology. fourteenth edition. Baltimore, Lippincott Williams & Wilkins, pp. 2195–2245.
- Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, Di Battista JA, Martel-Pelletier J (1998): Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum 41: 1275–1286.
- Qi J, Li Y, Zhang H, Cheng Y, Sun Y, Cao J, Zhao Y, Wang F (2009): A novel conjugate of low-molecular-weight heparin and Cu, Zn-superoxide dismutase: Study on its mechanism in preventing brain reperfusion injury after ischemia in gerbils. Brain Res 1260: 76–78.
- Rahman AU, Shabbir M, Sultani SZ, Jabbar A, Choudhary MI (1997): Cinnatates and coumarins from the leaves of Murraya paniculata. Phytochemistry 44: 683–685.
- Ramesh B, Pugalendi KV (2005): Antihyperlipidemic and antidiabetic effects of umbelliferone in streptozotocin diabetic rats. Yale J Biol Med 78: 187–194.
- Ramesh B, Pugalendi KV (2006): Antioxidant role of umbelliferone in STZ-diabetic rats. Life Sci 79: 306–310.
- Regan EA, Bowler RP, Crapo JD (2008): Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage 16: 515–521.
- Rogart JN, Barrach HJ, Chichester CO (1999): Articular collagen degradation in the Hulth-Telhag model of osteoarthritis. Osteoarthr Cartil 7: 539–547.
- Rout PK, Rao YR, Sree A, Naik SN (2007): Composition of essential oil, concrete, absolute, wax and headspace volatiles of Murraya paniculata (L.) Jack flowers. Flavour Fragr J 22: 352–357.
- Saied S (2005): Studies in the chemical constituents of Murraya paniculata and Ipomoea hederacea. Ph.D. Thesis, University of Karachi.
- Van de Loo AAJ, van den Berg WB (1990): Effects of murine recombinant IL-1 on synovial joints in mice: Measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis 49: 238–245.
- Van de Loo FAJ, Joosten LAB, van Lent PLEM, Arntz OJ, van den Berg WB (1995): Role of interleukin-1, tumor necrosis factor α, and interleukin-6 in cartilage proteoglycan metabolism and destruction: Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum 38: 164–172.
- Vinegar R, SchreiberW Hugo, R (1969): Biphasic development of carrageenan oedema in rats. J Pharmacol Exp Ther 166: 96–103.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drug. Pro Soc Exp Biol Med 111: 544–547.
- Wu T-S, Liou M-J, Kuoh C-S (1989): Coumarins of the flowers of Murraya paniculata. Phytochemistry 28: 293–294.