Abstract
Objective: To evaluate the aqueous extract from aerial parts of Artemisia copa Phil. (Asteraceae) administered orallyfor its psychopharmacological activities in several experimental models
Methods: The extract was administered p.o. in Swiss albino mice and tested on pentobarbital-induced hypnosis, locomotor activity, exploration in the hole-board, anxiolytic like profile evaluated in the marble-burying test and anticonvulsant activity on convulsions induced by pentylenetetrazol.
Results: Artemisia copa at doses up to 1.5 g/kg produced a dose-dependent sleep induction and potentiation of sub-hypnotic and hypnotic doses of pentobarbital. The extract also produced a dose-dependent increase and decrease in the spontaneous motor activity (0.5–1.5 g/kg, respectively), no disruption or a decrease on exploratory (hole-board) behavioral profiles (0.5–1.5 g/kg respectively) and a dose-related anxiolytic-like activity as indicated by increases in the percentage of marbles they left uncovered in the marble-burying test at doses (0.5 g/kg) that do not disrupt the motor activity. In addition, the extract (1.5 g/kg) produced a significant increase in the latency time and a decrease in the duration of seizures and mortality induced by PTZ 75 mg/kg in mice.
Conclusion: These results suggest that the aqueous extract of Artemisia copa may contain sedative principles with potential anxiolytic and anticonvulsant activities.
Introduction
Artemisia copa Phil. (Asteraceae), commonly known as “copa-copa”, is a small and very branched bush with a height of 30–60 cm that grows over 3000 m in the northwest of Argentina and in the north of Chile. The plant is regularly sold in local markets and herb health stores, and infusions of the aerial parts are used in popular medicine as antitussive, digestive, for lowering fever, for pulmonary diseases and hypertension (CitationGiberti, 1983). The leaves, macerated in alcohol, are also used locally to rub off rheumatic pains (CitationRatera & Ratera, 1980).
In a previous phytochemical study, four flavonoids (jaceidin, jaceidin-7-methyl-ether, luteolin and kaempferol-6-methyl-ether 3-rhamnoglucoside) were isolated from Artemisia copa (CitationQuarenghi et al., 1991). Luteolin and its derivatives have been described to exert several interesting pharmacological activities, namely antitumoral, antioxidant, anti-inflammatory, analgesic (CitationBlock et al., 1998; CitationKotanidou et al., 2002) and anxiolytic (CitationColeta et al., 2008). Pharmacological studies of the plant revealed that the aqueous extract of aerial parts of A. copa possess analgesic and anti-inflammatory activity (CitationAcevedo et al., 2005).
In this paper we studied the anticonvulsant activity of A. copa, considering that several genera of the Compositae family, including the genus Artemisia, have been used in traditional medicine to treat epilepsy in Brazil and other countries (de Lima et al., 1992).
Taking into account all that information, this study was to carry out a psychopharmacological screening of Artemisia copa in different experimental models in mice.
Materials and methods
Plant collection
The aerial parts of Artemisia copa Phil. (Asteraceae), were collected and identified by Gustavo Giberti in Antofagasta de la Sierra, Catamarca Province, Argentina, in November 2008. A voucher specimen has been deposited at the Museo de Farmacobotánica Juan A. Domínguez, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Argentina, under BAF 10313.
Preparation of A. copa aqueous extract
The plant material was dried under airflow in an oven at 45 ± 1°C and powdered mechanically. The aqueous extract was prepared by macerating 50 g of powdered plant material for 20 min using 500 mL of boiling water. The extract was filtered and freeze-dried. The yield of the infusion was 12.2% w/v. The lyophilized aqueous extract of A. copa was dissolved in physiological saline on the day of experiment.
Drugs
The following drugs were used: Diazepam (DZ) (Hoffmann-La Roche), pentobarbital sodium (PB), pentylenetetrazole (PTZ) (Sigma, Saint Louis, MO).
Experimental animals and treatments
Female albino Swiss mice (25–30 g) were used. They were housed in groups of 10/cage for a minimum of 3 days prior to pharmacological studies. They were kept in a room maintained at 25 ± 1°C with free access to standard laboratory food and tap water under a 12 h light/dark cycle (light from 8.00 a.m. to 8.00 p.m.) and used taking into account international guiding principles and local regulations concerning the care and use of laboratory animals for biomedical research (CitationANMAT, 1996). All animals were fasted 8 h with tap water ad libitum prior to experiment. A minimum of 10 animals per group was used in all tests.
Different doses of the lyophilized aqueous extract of A. copa between 0.2 and 1.5 g/kg were administered p.o. in a volume of 0.1 mL/10 g body weight 60 min before tests. Control mice (saline 10 mL/kg) were tested in parallel with those animals receiving drug treatment.
Evaluation of pharmacological activities
Psychopharmacological assays
Hypnogenic activity. Vehicle or A. copa aqueous extract (0.2, 0.4, 0.8 and 1.5 g/kg) were administered p.o. to mice 60 min before i.p. injection of sub-hypnotic or hypnotic doses of PB (30 and 40 mg/kg, respectively). In both experiments and for each mouse, the time spent between loss and recovering of the righting reflex on each treatment was recorded.
Studies on spontaneous motor activity. Spontaneous locomotor activity (SMA) was recorded in a 5 min period with a photocell activity meter apparatus (Animex, LKB, Farad, Sweden). DZ was used as a reference drug and administered at different doses of 0.3, 0.5, 1 and 3 mg/kg, i.p., 30 min before the test. Two doses of A. copa aqueous extract (0.5 and 1.5 g/ kg) were tested.
Studies on exploratory behavior. The hole-board test (CitationPerez et al., 1998) was used for this study. The animals were singly placed in a hole-board apparatus (Ugo Basile, Italy) and the number of times mice dipped their heads into the holes during a 5-min trial was recorded for control and treated groups (A. copa 0.3, 0.5 and 1.5 g/kg). A decrease in the head-dips, compared to controls, reveals a sedative behavior.
Marble-burying test. The marble-burying test (MBT) consisted of a Plexiglas cage 23 × 17 × 14 cm with a smooth lid punctured by small ventilation holes. The floor was covered with a 5 cm layer of sawdust and 24 glass marbles 1.5 cm diameter, evenly spaced against the walls (4 × 8 × 4 × 8) of the cage (CitationYoung et al., 2006). The mouse was placed in the cage for 30 min after which it was removed and the burying response quantified by counting the number of marbles that were more than two thirds covered with sawdust. An anxiolytic-like effect is assumed from drug-induced decreases in marbles buried (or the reciprocal measure of increases in marbles left uncovered). Drug treatments and doses were as follows: DZ 0.3, 0.5, 1 and 3 mg/kg, i.p. administered 30 min before the test, and A. copa aqueous extract 0.5 and 1.5 g/kg, p.o., 60 min before.
Pentylenetetrazol-induced seizures. The mice were treated with pentylenetetrazol (75 mg/kg, i.p.,) 60 min before A. copa aqueous extract (1.5 g/kg, p.o.,), and the latencies (s) to onset of the first convulsion, duration (s) and lethality were recorded. Animals devoid of seizures were considered protected.
Statistical analysis
All data were expressed as mean ± SEM (standard error of mean). The statistical tests used were one-way analysis of variance (ANOVA) followed by Dunnett’s t-test and the non parametric chi2 test, when correspond. A value of P < 0.05 was considered significant.
Results
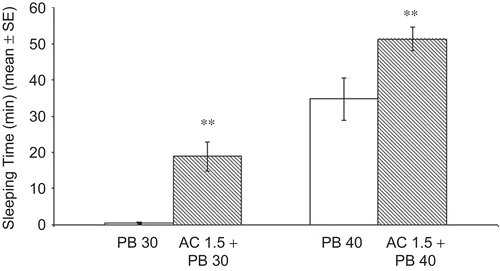
The A. copa aqueous extract at doses of 0.2, 0.4 and 0.8 g/ kg does not induce or potentiate sleeping time produced by sub-hypnotic (30 mg/kg) or hypnotic doses of PB (40 mg/kg), but given at the dose of 1.5 g/kg produced a significant increment of the sleep in both treatments, (P < 0.01) ().
Figure 1. Effect of AC on the sleeping time in Swiss mice of subhypnotic and hypnotic doses of pentobarbital. Mice were given A. copa aqueous extract at doses of 0.2, 0.4, 0.8 and 1.5 g/kg, p.o., 60 min before PB. Doses up to 0.8 g/kg do not induce or potentiate sleeping time produced by sub-hypnotic (30 mg/kg) or hypnotic doses of PB (40 mg/kg). Each bar represents the mean ± SEM of the sleeping time (min) of ten mice. **P < 0.01 versus control (ANOVA followed by Dunnett’s test).
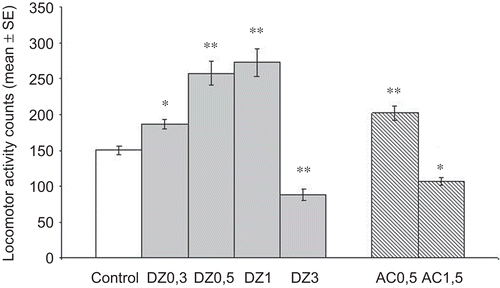
The overall effects of the p.o. administration of the plant extract on SMA are shown in . A. copa produced a dose-dependent and significant increase and decrease in the SMA at doses of 0.5 and 1.5 g/kg (P <0.01 and P <0.05, respectively). DZ also showed a significant increase on the SMA (0.3, 0.5 and 1 mg/kg) and a decrease at 3 mg/kg.
Figure 2. Effect of A. copa on the spontaneous motor activity during a 5-min test session. AC (0.5 and 1.5 g/kg, p.o.) was administered 60 min before test and DZ at doses between 0.3 and 3 mg/kg, i.p., 30 min before. Each bar represents the mean ± SEM of the SMA of ten mice. *P < 0.05; **P < 0.01 versus control (ANOVA followed by Dunnett’s test).
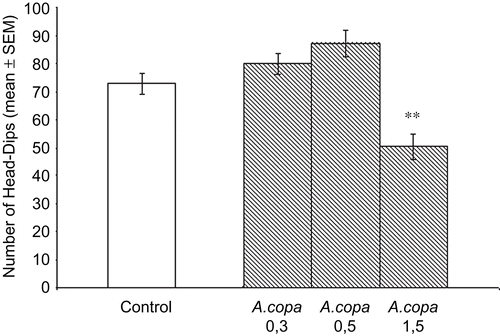
In the hole-board test, the A. copa aqueous extract (1.5 g/kg) showed a significant decrease in the number of nose-pokes (P < 0.01) without effects at doses of 0.3 and 0.5 g/kg, compared to control. This decline reveals a sedative effect at high doses ().
Figure 3. Exploratory behavior in the hole-board. Effects of A. copa aqueous extract administered 60 min before the test during 5 min in mice. A. copa (0.3, 0.5 and 1.5 g/kg, p.o.) was administered 60 min before the test. Each bar represents the mean ± SEM of the nose-pokes of ten mice. *P < 0.01 (ANOVA followed by Dunnett’s test).
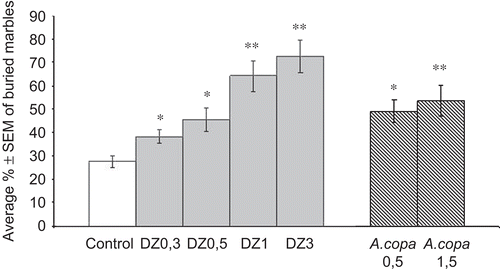
indicates that low doses of DZ between 0.3 and 1 mg/kg and A. copa aqueous extract at the dose of 0.5 g/kg, displayed a significant anxiolytic-like activity indicated by increases in the percentage of marbles they left uncovered on top of their bedding material. Animals with doses greater than 3 mg/kg for DZ and 1.5 g/kg for A. copa also increased the percentage of marbles they left uncovered (P <0.01) but in this case as a result of sedative activity.
Figure 4. Effects of A. copa on the behavior of mice in the marble burying test. Mice were given A. copa aqueous extract at doses of 0.5 and 1.5 g/kg, p.o., 60 min before the test and DZ at several doses (0.3, 0.5, 1 and 3 mg/kg, i.p.) 30 min before. Each bar represents the average % (mean ± SEM) of marbles that remained uncovered for ten mice. *P < 0.05; **P < 0.01 versus control (ANOVA followed by Dunnett’s test).
A. copa aqueous extract produces a significant increase in the latency (P< 0.05) and a decrease in the duration of seizures and lethality (P<0.01 and 0.05, respectively) induced by PTZ () at doses which also affected locomotor activity (1.5 g/kg).
Table 1. Influence of acute pretreatment of mice with AC aqueous extract p.o. on latency and duration to convulsions induced by PTZ (75 mg/kg, i.p.). Data are expressed as means ± SEM. *P <0.05; **P <0.01 (ANOVA followed by Dunnett’s test) and ***P<0.05 (chi2).
Discussion
The results of the present study demonstrate that the p.o. administration of increasing doses of an aqueous extract of A. copa, causes several effects including a dose related increase and decrease of SMA, an induction and potentiation of sub-hypnotic and hypnotic doses of PB, a dose related anxiolytic-like activity in the marble burying assay and a protective action against convulsions induced by PTZ.
Animals, such as mice and rats, bury objects in the bedding material of their cage. One example of this type of behavior occurs when mice are placed in an environment which contains glass marbles as unfamiliar objects. An anxiolytic-like effect is assumed from pretreatment with drugs that induced reduction in number of marbles buried (or the reciprocal measure of increases in marbles left uncovered) without concomitant impairments in tests that are typically used as potential indicators of side-effect liability such as locomotor and exploratory screen assays (CitationMalick, 1987).
The administration of diazepam inhibits marble-burying behavior at doses that not disrupt the motor activity (CitationYoung et al., 2006). In a similar way, the A. copa aqueous extract-like low doses of DZ present an anxiolytic effect at doses (0.5 g/kg) that do not produce a decrease in the activities evaluated in the SMA and hole-board tests. The increase in locomotor activity is a consequence of the anxiolytic effect produced by low doses of A.copa and diazepam (CitationHiller & Zetler, 1996).
It is known that the efficacy of the γ-aminobutyric acid (GABA) system plays an inhibitory role on activity of the central nervous system (CNS). In this study, A. copa was effective against PTZ-induced seizures, a receptor antagonist that reduces the activity of GABAA (CitationKasture et al., 2000). This may suggest that the anticonvulsant action of A. copa is mediated by a modulatory action on the channel of GABAA/benzodiazepine receptor complex, increasing the concentration of GABA in brain.
Since the discovery that certain flavonoids (namely flavones) specifically recognise the central BDZ receptors, several efforts have been made to identify naturally occurring GABA(A) receptor benzodiazepine binding site ligands. Flavonoid derivatives with a flavone-like structure such as apigenin and chrysin have been reported for their anxiolytic-like activity in different animal models of anxiety. One of the constituents present in the aqueous extract of A. copa, luteolin (3′,4′,5,7-tetrahydroxyflavone) (Quarenghi et al., 1991), is a widespread flavonoid aglycon and has CNS activity with anxiolytic-like effects despite the low affinity for the shown in vitro. Our findings suggest a possible interaction with other neurotransmitter systems but we cannot rule out the possibility that luteolin’s metabolites might show a higher affinity for the BDZ-R in vivo, thus eliciting the evident anxiolytic-like effects through a GABAergic mechanism (CitationColeta et al., 2008).
These results suggest that the aqueous extract of Artemisia copa, contains sedative principles with potential anxiolytic and anticonvulsant activities. However, further studies including toxicological evaluations and the use of all-purified fractions are necessary to confirm and extend the present findings.
Declaration of interest
This work was supported by Secretaría de Ciencia y Técnica de la UBA (UBACYT), Universidad de Buenos Aires.
References
- Acevedo C, Miño J, Moscatelli V, Hnatyszyn O, Gorzalczany S, Ferraro G. (2005). Antinociceptive and antiinflammatory activities of Artemisia copa. Fitoterapia, 5, 137–139.
- ANMAT. (1996). Regulations for animal facilities of Laboratories of Medicinal Specialities and / or Laboratories to conduct quality control testing of medicinal specialities own and / or others who use experimental animals. Disposition N° 6344. National Administration of Medicines. Foods and Medical Tecnology. Buenos Aires. Argentina.
- Block LC, Santos ARS, de Souza MM de, Scheidt C, Yunes RA, Alves Santos M, Delle Monache F, Cechinel Filho V. (1998). Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J Ethnopharmacol, 61, 85–89.
- Coleta M, Graca Campos M, Cotrim MD, de Lima TCM, da Cunha AP. (2008). Assessment of luteolin (3',4',5,7-tetrahydroxyflavone) neuropharmacological activity. Behav Brain Res, 189, 75–82.
- de Lima TCM, Morato G, Takahashi R. (1993). Evaluation of the central properties of Artemisia verlotorum. Planta Med, 59, 326–329.
- Giberti G. (1983). Herbal folk medicine in northwestern Argentina: Compositae. J. Ethnopharmacol, 7, 321–341.
- Hiller KO, Zetler G. (1996). Neuropharmacological studies on ethanol extracts of Valeriana officinalis L.: Behavioural and anticonvulsant properties. Phytother Res Arch, 10, 145–151.
- Kasture VS, Chopde CT, Deshmukh VK. (2000). Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosasinesis and Butea monosperma in experimental animals. J Ethopharmacol, 71, 65–75.
- Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotsis T, Papapetropoulos A, Roussos C. (2002). Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Respir Crit Care Med, 165, 818–823.
- Malick JB. (1987). Neuropsychopharmacological drug development. In: Willams M, Malick JB. eds. Drug Discovery and Development. Clifton, NJ: Humana Press, 115–149.
- Perez RMG, Perez JAL, Garcia LMD, Sossa HM. (1998). Neuropharmacological activity of Solanum nigrum fruit. J Ethnopharmacol, 61, 143–152.
- Quarenghi de Riera MV, Catalán CN, Israilev LRA de, Seeligmann P. (1991). Flavonoides foliares de Artemisia copa (Compositae). Bol Soc Argent Bot, 27, 253–255.
- Ratera EL, Ratera MO. (1980). Plants of the Flora Argentina used in folk medicine. Ed. Hemisferio Sur S.A. Buenos Aires, pp. 102, 144, 171.
- Young R, Batkai S, Dukat M, Glennon R. (2006). TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo-4,5-g]isoquinoline) exhibits anxiolytic-like activity in a marble-burying assay in mice. Pharmacol Biochem Behav, 84, 62–73.
