Abstract
Context: Withania coagulans Dunal. (Solanaceae) has been shown to possess hypoglycemic, free radical scavenging and wound healing activity. Therefore, it may be worthwhile to study the effect of Withania coagulans in diabetic wound healing.
Objective: This study included determination of rate of wound contraction and estimation of various biochemical parameters such as collagen, hexosamine, total protein, total DNA, SOD and CAT levels in the granulation tissues.
Materials and methods: The hydroalcoholic fraction of the methanolic extract (standardized by withaferin-A using high performance thin layer chromatography (HPTLC) )of Withania coagulans in the form of 10% w/w ointment topically and at a dose of 500 mg/kg per oral (p.o.) was administered to streptozotocin-induced diabetic rats. The results obtained were compared with diabetic control and Aloe barbadensis Miller (syn. Aloe vera L.) (Liliaceae) was used as a reference drug.
Results: The amount of withaferin-A present in the methanolic extract was 3.67 mg/g of the extract. The hydroalcoholic fraction in both the forms, i.e., topical (10% w/w ointment) and oral (500 mg/kg, p.o.) showed a significant increase in the rate of wound contraction (83.02% topical and 65.14% oral) topical and 93.18% oral) when compared to diabetic control (66which was further justified with significant increase in the levels of collagen, protein, DNA, SOD, CAT, and decreased level of hexosamine.
Discussion and conclusion: The hydroalcoholic fraction of Withania coagulans in oral form is found to be more effective than the topical form. However, further studies are in progress to find the lead molecules responsible for the wound healing effect.
Introduction
Wound healing is a dynamic process which includes biochemical and physiological phenomena that behave in a harmonious manner involving progression of various sequential events (stages) in order to guarantee tissue restoration. Immediately after injury the inflammatory phase begins with vasoconstriction favoring homeostasis and releasing inflammation mediators. The proliferative phase is characterized by granulation tissue proliferation formed mainly by fibroblasts and the angiogenesis process. Reformulation and improvement in the collagen fiber components, which increases the tensile strength involves the remodeling phase (CitationMandelbaum et al., 2003). Alterations in any of these steps can lead to healing delay or impaired healing which may be due to consequence of pathological states associated with diabetes, immune disorders, ischemia, venous stasis, and in injuries such as burn, frostbite, and gunshot wounds. Diabetes mellitus is a condition which is known to be associated with a variety of connective tissue abnormalities. Abnormalities associated with diabetic wounds include prolonged inflammation, impaired neovascularization, decreased synthesis of collagen, increased levels of proteases, and defective macrophage function, therefore delaying wound healing processes (CitationGupta et al., 2008). Delayed wound healing in diabetes has become one of the greatest challenges for physicians and health care workers; an estimated 15% of diabetic patients will have a non-healing wound at some stage in their lives, resulting in poor quality of life, prolonged hospitalization, and possibly finally leading to amputation (CitationBoulton et al., 2005). It has been suggested from the ESR studies that patients with diabetes mellitus are susceptible to higher levels of oxidative stress (CitationDavison et al., 2002). This oxidative stress causes imbalance between the oxidant and antioxidant defense mechanisms resulting in lipid peroxidation (LPO), DNA damage, and enzyme inactivation, including free radical scavenger enzymes contributing to tissue damage, thus delaying the healing process (CitationSen et al., 2002; CitationShukla et al., 1997; CitationWiseman & Halliwell, 1996).
Although in recent times many plants have been tested for wound healing activity, very few plants, e.g., Aloe barbadensis Miller. (syn. Aloe vera L.) (Liliaceae) (CitationChithra et al., 1998), finger millet (Eleusine coracana (L.); CitationRajasekaran et al., 2004), Acalypha langiana Muell. (Euphorbiaceae) (CitationPerez et al., 2006) and Sparassis crispa Wulf. Fr. (Sparassidaceae) (CitationA.-Hon Kwon et al., 2009) have been evaluated successfully for diabetic wound healing. Among these, only Aloe vera have been clinically evaluated. Withania coagulans Dunal. (Solanaceae), commonly known as Indian cheese maker, is shown to have hypoglycemic, free radical scavenging activity (CitationHemalatha et al., 2004). Withanolides isolated from the aqueous extract of fruits of the plant have been proven to have good antihyperglycemic and antidyslipidemic activity in different animal models (CitationMaurya et al., 2008). It has also been shown to have wound healing activity (CitationHemalatha et al., 2008), anti-inflammatory (CitationBudhiraja et al., 1977), hepatoprotective (CitationBudhiraja et al., 1986), hypolipidemic (CitationHemalatha et al., 2006), antifungal (CitationChoudhary et al., 1995) and cardio tonic activities (CitationBudhiraja et al., 1983). As it is reported to have potent hypoglycemic, wound healing, and free radical scavenging activities, it is worthwhile to study the influence of topical and oral administration of Withania coagulans on an excision wound model in diabetic rats. Thus, with the help of the present study one can establish the role of various biochemical and antioxidant parameters in different phases of diabetic wound healing.
Materials and methods
Experimental animals
Healthy Charles Foster albino rats weighing between 150 ± 20 g of either sex were procured from the Central Animal House (Reg. No. 542/02/ab/CPCSEA), Institute of Medical Sciences, Banaras Hindu University, Varanasi. The animals were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycle at 25°C).They were fed commercially available rat feed (Hindustan Lever, Mumbai) and water ad libitum. All experimental protocols were conducted in accordance with accepted standard guidelines for care and use of laboratory animals.
Induction of diabetes
Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO) (70 mg/kg body weight) in 0.1 M freshly prepared citrate buffer, pH 4.5. Fasting blood glucose level was determined 3 days after streptozotocin injection by collecting blood from the retro-orbital plexus of the eye. Animals with blood glucose levels greater than 200 mg/dL were used for further study (CitationJunod et al., 1969).
Creation of excision wound
The back of each rat was shaved under Nembutal (35 mg/kg, i.p.) anesthesia and open circular wounds were produced on each rat by excising the skin. The wound area was measured immediately by placing transparent tracing paper over the wound and tracing it out. The wounded animals were housed separately in different cages (CitationMorton & Malone, 1972).
Preparation of drug
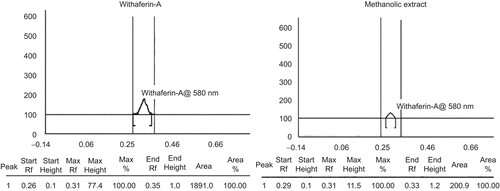
The fruits of Withania coagulans (with persistent calyx and pedicle) were purchased from the local market of Varanasi and were authenticated by V.K. Joshi from the Department of Ayurveda, Banaras Hindu University (Varanasi) and by Mr. D. Narayanappa, chief botanist at TAMPCOL (Chennai). The voucher specimen (SM/WC/02) has been deposited in the department. The coarsely powdered drug (500 g) was extracted with methanol (1.5 L) using a Soxhlet which was carried out until the whole drug was exhausted and concentrated using a Rota evaporator (to minimize the level of residual methanol up to negligible level). The obtained extract was then standardized for the presence of withaferin-A by HPTLC using standard withaferin-A (Ranbaxy, Gurgaon, Haryana, India). The quantification was executed by anchrom (Mumbai, Maharashtra, India) using a CAMAG automatic TLC applicator and a CAMAG TLC scanner with solvent system toluene:ethyl acetate:formic acid in the ratio of (5:5:1).
This was then made hydroalcoholic using distilled water (1:1) which was further fractionated with chloroform (2:1). Both the fractions i.e. the hydroalcoholic and chloroform fractions were concentrated and dried in a vacuum desiccator. After preliminary trials it was found that the hydroalcoholic fraction showed significant wound healing effect as compared to the chloroform fraction, thus it was considered for further study. For topical treatment a 10% w/w ointment of hydroalcoholic fraction was prepared as per the British Pharmacopoeia and a 10% w/w Aloe vera cream (Glenmark Pharm, Mumbai, Maharashtra, India) was taken as standard (CitationChithra et al., 1998; CitationSoulsby, 2006). In the case of oral treatment, the hydroalcoholic fraction, dissolved in distilled water at a dose of 500 mg/kg, was administered and compared with the Aloe vera solution, 100 mg/kg prepared from Aloe vera powder. The application of ointment and administration of the oral dose was fixed once a day from the starting day and was maintained up to day 16.
Grouping of animals
After the wound creation the animals were divided into two batches, i.e., topically treated and orally treated. These batches were further subdivided as:
Topically treated
The rats were subdivided into four groups: Group I: normal control treated with simple ointment, Group II: diabetic control treated with simple ointment, Group III: diabetic treated topically with Aloe vera, Group IV: diabetic treated topically with hydroalcoholic fraction of Withania coagulans (10% w/w).
Orally treated
In the case of oral treatment, Group I and Group II included normal and diabetic control rats treated with distilled water, whereas Group III and Group IV included diabetic rats treated with standard Aloe vera (100 mg/kg, p.o.) and the hydroalcoholic fraction of Withania coagulans (500 mg/kg, p.o.), respectively.
Rate of wound contraction
The rate of wound contraction was estimated by measuring the wound area, which was marked on tracing paper by placing the paper on the wound on respective days (4, 8, 12 and 16) after wound creation. The area of the traced wounds was calculated by the use of a planimeter and the percentage rate of wound contraction was calculated.
Biochemical analysis
The animals (6 animals in each group) were sacrificed on days 4, 8, 12 and 16 after wound creation and the granulation tissues from each wound were removed and stored at −70°C until analysis. For estimation of collagen and hexosamine, tissues were first defatted in acetone, dried and then hydrolyzed in 6 N HCL for 48 h in a boiling water bath. The collagen (hydroxyproline) was estimated by the method of CitationWoessner (1961) and hexosamine by CitationElson and Morgan’s (1933) method.
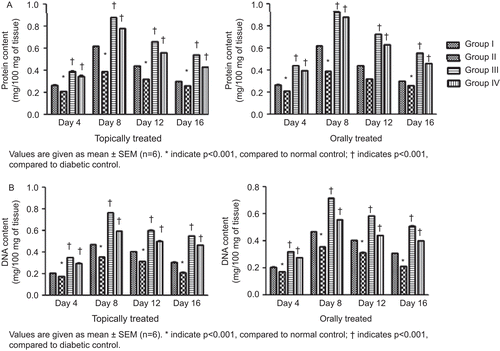
Total protein and DNA in wet granulation tissues were estimated by first homogenizing the tissue in 5% trichloroacetic acid (TCA) followed by centrifugation. Supernatant was used for the estimation of DNA content by the method of CitationBurton (1956). The precipitate was suspended in 0.1 M tris-HCL buffer pH 7.4 and protein content was estimated by the method of CitationLowry et al. (1951).
Estimation of antioxidant enzymes
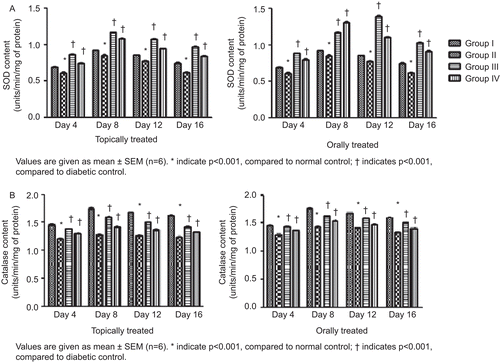
For estimation of SOD and CAT granulation, tissues were homogenized using 0.02 M potassium phosphate buffer pH 7.6 and centrifuged at 6000 rpm. The obtained clear supernatant was used for estimation of SOD by the method of CitationNishikimi et al. (1972) and CAT by the method of CitationChance and Maehly (1955).
Statistical analysis
Student-Newman-Keuls test was used to analyze the data (GraphPad InStat Version 3.06, La Jolla, CA).
Results
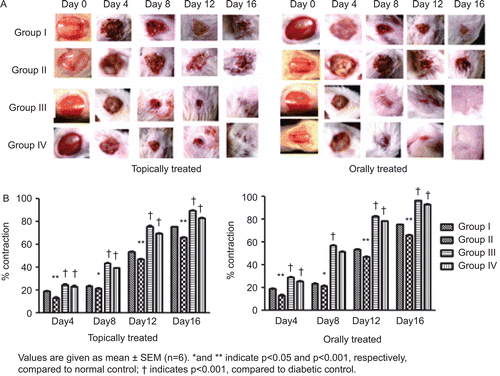
The amount of withaferin-A quantified by HPTLC in methanolic extract was found to be 3.67 mg/g of extract ( and ). The percentage rate of wound contraction on topical treatment was found to be 83.02% with the hydroalcoholic fraction treatment and 89.66% with Aloe vera treatment, compared to 66.71% in the diabetic control. In the case of the orally treated rats, the rate of wound contraction was found to be 93.18% with the hydroalcoholic fraction treatment and 96.46% with Aloe vera treatment, compared to 65.14% in the diabetic control ().
Table 1. Quantification of withaferin-A in methanolic extract of Withania coagulans by HPTLC.
Figure 2. Images of wounds on topical and oral treatment of hydroalcoholic fraction (A) and its effect on wound contraction (B). Group I: normal control, Group II: diabetic control, Group III: diabetic treated with Aloe vera and Group IV: diabetic treated with hydroalcoholic fraction of Withania coagulans.
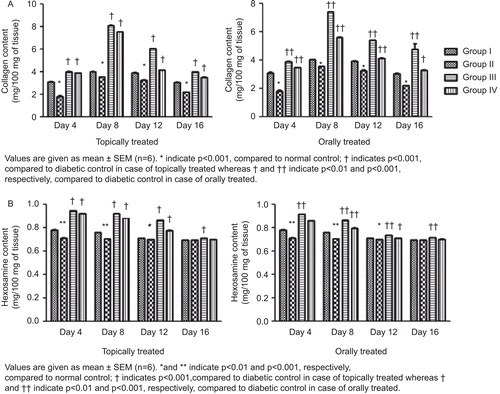
Both topically and orally treated groups showed increases in collagen content in the granulation tissues, which reached its maximum level on day 8. It was also observed that there was a steep rise in collagen content from day 4 to day 8 followed by a steep decline up to day 12, which tended to normalize on day 16. The maximum level of collagen in topically treated groups was found to be 7.55 mg/100 mg with the hydroalcoholic fraction treatment and 8.11 mg/100 mg with the Aloe vera treatment, whereas in the orally treated rats the maximum level of collagen was found to be 5.62 mg/100 mg (hydroalcoholic fraction treated) and 7.406 mg/100 mg (Aloe vera treated) of granulation tissues (). It was observed that the hexosamine level in the granulation tissues reached its maximum level on day 4 in both topically and orally treated rats and went on to normalize on day 16 (). The protein and DNA content of the granulation tissues showed significant increase up to day 8 of treatment in both topically and orally treated rats, followed by a slow reduction in their levels up to day 16 (). Estimation of antioxidants SOD and CAT in the granulation tissues showed a significant increase in their levels up to day 8 followed by a gradual reduction up to day 16 when compared to diabetic control ().
Figure 3. Effect of topical and oral treatment of hydroalcoholic fraction on collagen (A) and hexosamine (B) content.
Discussion
It has been observed that the rate of healing of wounds in diabetic patients is slow compared to healthy patients. Due to the unknown molecular mechanism involved and lack of successful evidence, treatment of diabetic wounds remains one of the greatest challenges for the clinician, making it important to understand the mechanism involved in the impaired skin wound healing in diabetes. Mounting evidence shows retardation in all stages of the healing events in diabetes, including inflammation, proliferation, angiogenesis, collagen deposition and modification are influenced, such as decreased chemotaxis and motility of inflammatory cells, diminished or unbalanced production of growth factors, poor collagen deposition and granulation tissue formation. The results obtained from the present study showed positive signs in potentiating rate of wound healing in diabetic rats when compared with untreated rats (CitationWang, et al., 2009).
The rate of wound contraction was found to be significant in both cases, i.e., topically and orally treated rats, but was considerably higher in orally treated rats. Increase in rate of wound contraction might be a result of the enhanced activity of fibroblasts mediated by specialized myofibroblasts found in the granulated tissues (CitationMoulin et al., 2000). Thus, rate of contraction determines the period of epithelialization which can be defined as the centripetal movement of the edges of a full-thickness wound to facilitate closure of the defect (CitationSuguna et al., 2002). The rate of contraction is directly associated with the amount of collagen deposited. The investigation showed a rapid increase in the collagen content of both topically and orally treated rats which reached their maximum level on day 8. Collagen plays an important role in providing strength and integrity to tissue matrix during the various phases of healing, i.e., epithelialization, remodeling (decrees in erythema and scar marks around the wound) and maintains homeostasis. Collagen is therefore, considered as the predominant extracellular protein synthesized in the rough endoplasmic reticulum of fibroblast cells. Hydroxylation of proline and lysine are important in collagen synthesis, and it occurs with the help of various co-factors such as iron, copper, vitamin C, etc. (CitationPeacock, 1984).
It was observed that treatment with Withania coagulans showed a reduction in hexosamine level. The decline was associated with a concomitant increase in collagen content which gets embedded on highly hydrated gel-like ground substances, i.e., glycosaminoglycans and proteoglycans (major components of hexosamine) synthesized by fibroblasts in the wound area (CitationDunphy & Udupa, 1955). As a result non-sulfated glycosaminoglycans and hyaluronic acid are replaced by more resilient proteoglycans such as chondroitin-4-sulfate. In addition, water is gradually reabsorbed from the scar, allowing collagen fibers and other matrix components to lie closer together and facilitate their cross-linking (CitationPeacock, 1984). Significant increases in the protein and DNA content of the granulation tissues of the treated groups suggest a higher rate of protein synthesis and cellular proliferation which resulted in better healing of diabetic wounds. SOD and CAT play a major role in detoxifying free radicals and other cytotoxic chemical species, which resulted in potentiation of healing processes. The results obtained from the present study showed increases in the levels of SOD and CAT up to day 8 of treatment, tending to normalize gradually on day 16. The levels of both enzymes were found to be higher in the orally treated rats compared to the topically treated rats. Diabetic control groups of both topically and orally treated rats showed decreased enzymatic activities which may be attributed to overproduction of reactive oxygen species (ROS), thus delayed healing response. It has also been observed that during the inflammation phase of wound healing, ROS such as superoxide anion radical (O−) and non-radical hydrogen peroxide (H2O2), are generated continuously. This H2O2 and other ROS at low concentration inhibit proliferation and migration of various cell types, like keratinocytes (CitationO’Toole et al., 1996). At high concentrations ROS can induce severe tissue damage and even lead to neoplastic transformation (CitationCerutti & Trump, 1991). Disease states such as diabetes and age associated biochemical phenomenon can retard this process by increasing the level of ROS which is due to the autoxidation of glucose, advanced glycation and abnormal mitochondrial function resulting in increased level of oxidative stress (CitationBaynes, 1991; CitationDavison et al., 2002; CitationIngold, 1993; CitationWolff, 1993). Endothelial cells and smooth muscle cells produce SOD and CAT which potentiate repairing activities and proliferation of new cells by regulating microvascular blood flow (CitationMarshall & Kontos, 1991).
The probable mechanism behind the wound healing activity of the hydroalcoholic fraction can be attributed to the increased level of collagen leading to proliferation of fibroblast cells fastening the epithelialization and remodeling phases, thus reducing erythema and scar marks. Higher levels of protein and DNA attribute to higher rate of cellular proliferation during all phases. Increased levels of antioxidants SOD and CAT accelerate vascular, inflammatory and other phases of healing processes by regulating vascular blood flow. Phenols present in the fraction (43.9 mg/g equivalent to gallic acid) may potentiate wound healing by quenching free radicals formed during the inflammatory phase. Tannins (32.6 mg/g equivalent to tannic acid) promote wound healing by chelation of free radicals, promoting contraction of the wound, increasing the formation of capillary vessels and fibroblasts, and it also induces keratinocytes proliferation (CitationDeters et al., 2001; CitationFernandez et al., 2002). Wound healing activity of the drug can also be attributed to its hypoglycemic activity and lowering of LPO level, thus protecting oxidative damage to the β cells of the pancreas, resulting in reduced blood glucose level (CitationHemalatha et al., 2004).
The present study concluded that the hydroalcoholic fraction of Withania coagulans accelerated wound healing in diabetic rats. However, further studies are required to identify the active constituent(s) responsible behind the therapeutic action and mechanism of action of that constituent(s).
Declaration of interest
The authors gratefully acknowledge the University Grants Commission (UGC), New Delhi, India for the financial support to Mr. S. K. Prasad.
References
- A.-Hon Kwon MD, Zeyu Qiu MD, Mamiko Hashimoto, Kyosuke Yamamoto, Takashi Kimura. (2009). Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. The Am J Surg, 197, 503–509.
- Baynes JW. (1991). Role of oxidative stress in the development of complications in diabetes. Diabetes, 40, 405–412.
- Boulton AJ, Vileikyte L, Ragnason-Gennvall, Alpelqvist J. (2005). The global burden of diabetic foot disease. Lancet, 306, 1719–1742.
- Budhiraja RD, Sudhir S, Craeg KN, Arora B. (1986). Protective effect of 3 beta-hydroxy-2,3 dihydro withanolide F against CCl4 induced hepatotoxicity Planta Med, 1, 28–29.
- Budhiraja RD, Sudhir S, Garg KN. (1983). Cardiovascular effects of a withanolide from Withania coagulans Dunal. fruits. Indian J Physiol Pharmacol, 27, 129–134.
- Budhiraja RD, Sudhir S, Garg KN. (1977). Pharmacological investigation on fruits of Withania coagulans Dunal. Planta Med, 32, 154–157.
- Burton K. (1956). A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J, 62, 315–323.
- Cerutti PA, Trump BF. (1991). Inflammation and oxidative stress in carcinogenesis, Cancer Cells, 3, 1–7.
- Chance B, Maehly AC. (1955). Assay of catalase and peroxidase. In: Colowick SP, Kaplan NO, ed. Methods in Enzymology. Academic Press: New York, 764–775.
- Chithra P, Sajithlal GB, Chandrakasan G. (1998). Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol, 59, 195–201.
- Choudhary MI, Shahwar, Dur-E, Zeba P, Jabbar A, Ali I, Rahman Atta-Ur. (1995). Antifungal steriodal lactones from Withania coagulans. Photochemistry, 40, 1243–1246.
- Davison GW, George L, Jackson SK, Young IS, Davies B, Bailey DM, Peters JR, Ashton T. (2002). Exercise, free radicals and lipid peroxidation in type 1 diabetes mellitus. Free Radic Biol Med, 33, 1543–1551.
- Deters A, Dauer A, Schnetz E, Fartasch M, Hensel A. (2001). High molecular weight compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: Influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Photochemistry, 58, 949–958.
- Dunphy JE, Udupa KN. (1955). Chemical and histochemical sequences in the normal healing wounds. New England J Med, 253, 847–851.
- Elson LA, Morgan WTJ. (1933). A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J, 27, 1824–1828.
- Fernandez O, Capdevila JZ, Dalla G, Melchor G. (2002). Efficacy of Rhizophora mangle aqueous bark extract in the open surgical wounds. Fitoterapia, 73, 564–568.
- Gupta A, Upadhyay NK, Sawhney RC, Kumar R. (2008). A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Rep Reg, 16, 784–790.
- Hemalatha S, Mishra N, Kumar M, Singh PN, Chansouria JPN, Mandal V. (2008). Wound healing activity of Withania coagulans fruits. Twelth Annual national Convention of Indian Society of Pharmacognosy at Moga, Punjab (India), 22nd Feb to 24th Feb, OP 13.
- Hemalatha S, Wahi AK, Singh PN, Chansouria JPN. (2004). Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J Ethnopharmacol, 93, 261–264.
- Hemalatha S, Wahi AK, Singh PN, Chansouria JPN. (2006). Hypolipidemic activity of aqueous extract of Withania coagulans Dunal in albino rats. Phytother Res, 20, 614–620.
- Ingold WM. (1993). Wound therapy: Growth factors as agents to promote healing. Trends Biotechnol, 11, 387–392.
- Junod A, Lambert AE, Stauffacher W, Renold AE. (1969). Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J Clin Invest, 48, 2129–2130.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RT. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–276.
- Mandelbaum SH, Di Santis EP, Mandelbaum MHSA. (2003). Cicatrization: Current concepts and auxiliary resources Part I. An Bras Dermatol, 72, 393410.
- Marshall JJ, Kontos HA. (1991). Endothelium and cerebral vascular diseases. In: GM Rubanyi (Ed.), Cardiovascular Significance of Endothelium-Derived Vasoactive Factors. New York: Futura, 125–145.
- Maurya R, Akankshaa, Jayendraa, Singh AB, Srivastava AK (2008): Coagulanolide, a withanolide from Withania coagulans fruits and antihyperglycemic activity. Bioorg Medicinal Chem Lett, 18, 6534–6537.
- Morton JJ, Malone MH. (1972). Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther, 196, 117–126.
- Moulin V, Auger FA, Garel D, Germain L. (2000). Role of wound healing myofibroblasts on re-epithelization of human skin. Burns, 26, 3–12.
- Nishikimi M, Rao A, Yagi K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun, 46, 849–854.
- O’Toole EA, Goel M, Woodley DT. (1996). Hydrogen peroxide inhibits human keratinocyte migration. Dermatol Surg, 22, 525–529.
- Peacock EE. (1984). Wound Repair, third edition. Philadelphia: WB Saunders.
- Perez Gutierrez RM, Vargas RS. (2006). Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia, 77, 286–289.
- Rajasekaran NS, Nithyac M, Rosec C, Chandr TS. (2004). The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim Biophys Acta, 1689, 190–201.
- Sen CK, Khanna S, Gordillo G, Bagchi D, Bagchi M, Roy S. (2002). Oxygen, oxidants, and antioxidants in wound healing: An emerging paradigm Ann NY Acad Sci, 957, 239–249.
- Shukla A, Rasik AM, Patnaik GK. (1997). Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defense enzymes in healing cutaneous wounds. Free Radic Res, 26, 93–101.
- Soulsby J. (2006). The new ointment bases of the British Pharmacopoeia 1948. British J. Dermatol, 61, 206–210.
- Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. (2002). Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res, 16, 223–227.
- Wang Z, Li L. (2009). The plasmid encoding Hsp47 enhances collagen expression and promotes skin wound healing in an alloxan-induced diabetic model. Cell Biol Int, 20, 1–6.
- Wiseman H, Halliwell B. (1996). Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem J, 313, 17–29.
- Woessner JR. (1961). The determination of hydroxyproline in tissues and protein samples containing small proportions of this amino acid. Arch Biochem Biophys, 93, 440–447.
- Wolff SP. (1993). Diabetes mellitus and free radicals. Br Med Bull, 49, 642–652.