Abstract
Context: Blumea balsamifera DC (Compositae) leaves have been recommended for use as a folk medicine in the treatment of various diseases related to urolithiasis in southeast Asia. Phytochemical studies of this plant revealed it contains four classes of flavonoids (e.g., flavonols, flavones, flavanones, and dihydroflavonol derivatives).
Objective: In view of the broad pharmacological activity of flavonoids, this study was carried out to determine the xanthine oxidase (XO) inhibitory and enzymatically produced superoxide radical scavenging activity of different organic extracts and that of the isolated flavonoids from B. balsamifera leaves.
Materials and methods: The inhibitory activity of XO was assayed spectrophotometrically at 295 nm. The superoxide radicals scavenging activity was assessed by NBT reduction method, spectrophotometrically at 560 nm. A dose response curve was plotted for determining IC50 values.
Results: The methanol extract (IC50 = 0.111 mg/mL) showed higher XO inhibitory activity than the chloroform (0.138 mg/mL) and pet-ether extracts (0.516 mg/mL). IC50 values of scavenging of superoxide radicals for extracts decreased in the order of: methanol (0.063 mg/mL) > chloroform (0.092 mg/mL) > pet-ether (0.321 mg/mL). The XO inhibitory activity of the isolated flavonoids and reference compounds tested decreased in the order of: allopurinol > luteolin > quercetin > tamarixetin > 5,7,3′,5′-tetrahydroxyflavanone > rhamnetin > luteolin-7-methyl ether > blumeatin > dihydroquercetin-4′-methyl ether > dihydroquercetin-7,4′-dimethyl ether > l-ascorbic acid.
Discussion and conclusion: The results indicated that the flavone derivatives were more active than the flavonol derivatives. The flavanone derivatives were moderately active and the dihydroflavonol derivatives were the least. The higher flavonoid content of extracts contributed to their higher XO inhibitory activity.
Introduction
Medicinal plants are widely consumed as home remedies and raw materials for the pharmaceutical industries. The past decade has seen a significant increase in the use of herbal medicine. Blumea balsamifera DC (Compositae), a medicinal herb, has been traditionally used in southeast Asia in treating diseases in the human body related to kidney disorders. The leaves of this plant are also used in folk medicine as a stomachic, expectorant, antispasmodic and diaphoretic, and in fever, lumbago, to increase appetite, in skin diseases, to treat wounds and liver cirrhosis (CitationIsmail et al., 1999). The leaves of this plant are also found in the preparation of Vietnamese traditional medicine to treat gout (CitationNguyen et al., 2004). B. balsamifera is a shrubby plant 2–3 m tall, widely distributed in Malaysia, the Moluccas, the Philippines, and in the tropical Himalayas. Several studies on the chemical constituents of B. balsamifera have been reported and a number of flavonoids, monoterpenes, sesquiterpenes, and triterpenes have been isolated (CitationNessa et al., 2004a, Citation2004b; CitationOsaki et al., 2005). Flavonoids are a ubiquitous group of polyphenolic substances which are present in most plants. The increasing interest in flavonoids is due to the appreciation of their broad pharmacological activities such as antioxidant (CitationRice-Evans, 1995; CitationMiddleton, 1998; CitationNijveldt et al., 2001; CitationNessa et al., 2004b), antiviral (CitationMiddleton, 1998; CitationSelway, 1986), anticancer (CitationAmes et al., 1995; CitationIto et al., 1999), etc. Experimental evidence suggests that flavonoids can contribute to a decrease in oxidative stress via inhibition of key regulating enzymes such as XO (CitationBeiler & Martin, 1951; CitationNoro et al., 1983; CitationBindoli et al., 1985; CitationAdkins & Taylor, 1990; CitationHatano et al., 1991; CitationNielsen et al., 1996; CitationMatsumura et al., 1998; CitationCos et al. 1998; CitationNagao et al., 1999). The enzyme XO catalyses the oxidation of hypoxanthine and xanthine to uric acid, which plays a crucial role in gout (CitationNugent et al., 1962; CitationHall et al., 1967). During reoxidation of XO, molecular oxygen acts as an electron acceptor, producing superoxide radical (O2•-) and hydrogen peroxide (H2O2) (CitationFridovich, 1970; CitationCos et al., 1998). Consequently, XO is considered to be an important biological source of superoxide radicals. Moreover, the excessive production of uric acid crystals leads to deposits in joints and surrounding tissues, causing pain, inflammation, and swelling of the joints, which has long been considered the most important risk factor for the onset of gout (CitationNugent et al., 1962; CitationHall et al., 1967). In extreme cases, uric acid crystals can enter the kidney tubules causing blockage of the tubules, which can lead to kidney failure (CitationCoe et al., 2005). Moreover, superoxide anion radicals generated by XO are involved in various pathological states such as hepatitis, inflammation, ischemia reperfusion, carcinogenesis, and aging (CitationCos et al., 1998). Allopurinol and oxypurinol are clinically used XO inhibitors in the treatment of gout, but these drugs can cause side effects such as hepatitis, nephropathy, and allergic reactions (CitationOkamoto et al., 2003). Thus, the search for novel XO inhibitors would be beneficial to combat various other diseases. In our earlier work we have reported the free radical and non-enzymetically produced superoxide radical scavenging properties of extracts and flavonoids of B. balsamifera (CitationNessa et al., 2004b; CitationFazilatun et al., 2004). In view of the broad pharmacological activity of flavonoids and our continuing research for other beneficial properties of this plant, the present work was undertaken to evaluate the XO inhibitory activity of different organic extracts and that of the isolated flavonoids of B. balsamifera leaves () in relation to a known natural antioxidant (ascorbic acid) and a potent XO inhibitor (allopurinol).
Materials and methods
General experimental methods
Melting points were determined on a Gallenkamp (Leicestershire, UK) and are uncorrected. FTIR spectra (Bomem Hartmann and Brau, MB-Series, Quebec, Canada) were recorded on a Bomem Hartmann and Braun, MB-Series; UV spectra were recorded on a Hitachi U-2000 spectrophotometer (Tokyo, Japan). MS (ESI/EI) were measured on a Finnigan LC-Q Classic, Ion Trap spectrometer (MD, USA) and Hewlett Packard mass spectrometer (model no. 5989A) (CA, USA). 1H NMR (DMSO-d6) was measured on a Bruker Avance 300 MHz and 400 MHz spectrometers (NMR, Zurich, Switzerland). Chemical shifts were recorded in δ (ppm) relative to that of TMS (δ = 0.0 ppm).
Chemicals and enzymes
Allopurinol, l-ascorbic acid, xanthine, xanthine oxidase (EC 1.1.3.22) (from buttermilk, 0.46 units/mg protein), superoxide dismutase (SOD; EC 1.15.1.1) (from bovine erythrocytes, 5100 U/mg protein), nitroblue tetrazolium (NBT, approx. 98%), methanol (spectroscopic grade), sodium phosphate dibasic 12 hydrate (99%), potassium phosphate monobasic (anhydrous, 99%), Folin-Ciocalteu reagent, anhydrous sodium carbonate, were all purchased from Sigma (St Louis, MO, USA). Chromatography of samples was carried out on Merck silica gel or Sephadex LH-20. AR grade (Merck, Darmstadt, Germany) solvents were used in the extraction and chromatographic analysis.
Extraction and isolation
The leaves of B. balsamifera (6.5 kg) (herbarium voucher specimen, FRI 57083, Botany unit of the Forest Research Institute of Malaysia) were oven dried at 40°C for 6 days. They were then crushed into powder and extracted with pet-ether (60-80°C), chloroform and methanol, subsequently. After removal of the solvent by evaporation under reduced pressure, the yield of residue from pet-ether, chloroform and methanol extracts was about 4, 2 and 5%, respectively. The crude pet-ether extracts of B. balsamifera (PEB) (15 g) were subjected to vacuum liquid chromatography (VLC) (silica gel 60 GF254, E. Merck, 100 g) with petroleum ether:ethyl acetate (2:8) as the eluent to give 1 (107.7 mg). The crude chloroform extracts of B. balsamifera (CEB) [10 g] were subjected to VLC (silica gel 60 GF254, E. Merck, 100 g) with petroleum ether:ethyl acetate (1:1) as the eluent to give 2 (207.7 mg). The crude methanol extracts of B. balsamifera (MEB) (35 g) were suspended in water (500 mL) and filtered. The resulting residues [15 g] were repeatedly subjected to VLC (silica gel 60 GF254, 100 g) with petroleum ether:ethyl acetate:methanol (8:1:1) as the eluent, and repeated chromatograph on Sephadex LH-20 with chloroform: methanol (19:1; 9:1 and 8:2) as eluents, to give 3 (38.9 mg), 4 (12 mg), 5 (50.8 mg), 6 (102.8 mg), 7 (19.5 mg), 8 (155 mg), and 9 (233 mg).
Determination of total polyphenols
The total polyphenol content of B. balsamifera leaf extract was determined according to the method described by CitationScalbert et al. (1989). The Folin-Ciocalteu reagent was diluted 1:10 before use. All the extracts (PEB, CEB and MEB) were dissolved in water, sonicated and then centrifuged at 10,000 g for 10 min. The supernatant portion was used for total phenolics assay. Samples (100 μL, three replicates) were introduced into the tubes, and then 2 mL Folin-Ciocalteu reagent and 2 mL sodium carbonate (7.5%) were added. The tubes were then kept in a water bath at 30°C for 1.5 h before measuring the absorbance at 760 nm. Calibration was achieved with aqueous catechin solutions (1-100 μg/ mL). Correlation coefficient (r) of the regression equation (y = 0.0011x − 0.0076) was 0.9987. Total polyphenol values were expressed as mg of catechin equivalent (CE) per gram of dried extract.
Determination of xanthine oxidase inhibitory activity
The xanthine oxidase (XO) inhibitory activities with xanthine as the substrate were measured spectrophotometrically with the method described by CitationNoro et al. (1983) with slight modification. The buffer used was 0.1 M phosphate buffer, pH 7.5. The substrate solution, 100 μM xanthines in water, and enzyme solution containing about 0.04 unit/mL in phosphate buffer, was prepared immediately before use.
The assay mixture consisted of 1 mL of test solution, 1.9 mL phosphate buffer (pH 7.5) and 0.1 mL of enzyme solution (0.04 units/mL) was pre-incubated at 25°C for 5 min. An aqueous solution of xanthine (1 mL) was added to the mixture and the resulting solution was incubated for 10 min at 25°C. The enzyme reaction was stopped with 1 M HCl (1 mL) and the absorbance at 295 nm of the reaction mixture was measured spectrophotometrically. The spectroscopic measurements were carried out with a Hitachi U-2000 UV-VIS spectrophotometer. A blank was prepared in the same way, but the enzyme solution was added to the assay mixture after adding 1 M HCl. XO inhibitory activity was expressed as the percentage inhibition of XO in the above assay system, and was calculated as follows:
where AS is the absorbance of the enzyme with test material, and AB is the absorbance of the enzyme without test material. Different concentrations of flavonoids were analyzed, and then the half-maximal inhibitory concentration (IC50, the concentration of the test samples required to inhibit 50% of the enzyme XO = 50% decrease in uric acid production) was calculated by regression analysis.
Determination of superoxide radicals scavenging activity (enzymatic)
The influence of extracts, flavonoids, and reference compounds on the generation of superoxide radicals was measured by means of spectrophotometric measurement of the product on reduction of nitro blue tetrazolium (CitationRobak & Gryglewski, 1988). NBT is a reagent which is frequently used to detect the occurrence of the superoxide anion radical in an enzymatic reaction (CitationAuclair et al., 1978; CitationKirby & Fridovich, 1982). Superoxide anions are generated in an enzymatic (xanthine-xanthine oxidase, X/XO) system, quenched by NBT (yellow), then reduced to diformazan (purplish-blue), which is insoluble in water. If radical scavenging compounds are present in the solution, less formazan blue will be formed, thus decreasing the absorbance at 560 nm.
The reaction mixture comprised 1 mL of test solution, 0.9 mL phosphate buffer (0.1 M, pH 7.5) 0.1 mL of XO (0.04 units/mL), 1 mL of 100 μM of xanthine, 1 mL of 600 μM NBT in 0.1 M phosphate buffer pH 7.5, was incubated at 25°C for 10 min and the absorbance was read with a Hitachi U-2000 UV-VIS spectrophotometer at 560 nm against blank solutions which did not contain the enzyme. The percentage of scavenging activities (%) was calculated as follows:
Different concentrations of extracts, flavonoids and reference compounds were added to samples and their effect on the generation of superoxide radicals was used to calculate regression lines and IC50 values. The experiments were run in triplicate.
Statistical analysis
All the results are expressed as mean ± standard deviation (SD). The data were analyzed using a one-way analysis of variance. When a statistically significant difference was obtained, Tukey’s test (p < 0.05) was then performed for multiple comparisons using Jandel SigmaStat for Windows Statistical Software (Version 2.0, San rafael, CA, USA) to isolate the group or groups that differ from the others.
Results and discussion
The chemical structures of the compounds 1-9 () were elucidated by means of different analytical methods such as UV, IR, NMR, MS, elemental analyses and comparison with literature value as dihydroquercetin-7,4′-dimethyl ether (1) (CitationRuangrungsi et al., 1981; CitationNessa et al., 2004b), blumeatin (2) (CitationLin et al., 1988; CitationNessa et al., 2004b), tamarixetin (3), rhamnetin (4) luteolin-7-methyl ether (5) luteolin (6), quercetin (7), 5,7,3′,5′-tetrahydroxyflavanone (8) (CitationAnthoni et al., 1998; CitationNessa et al., 2004b), and dihydroquercetin-4′-methyl ether (9) (CitationRuangrungsi et al., 1981; CitationNessa et al., 2004b).
Crude extracts of B. balsamifera as XO inhibitors and superoxide radical scavengers (enzymatic)
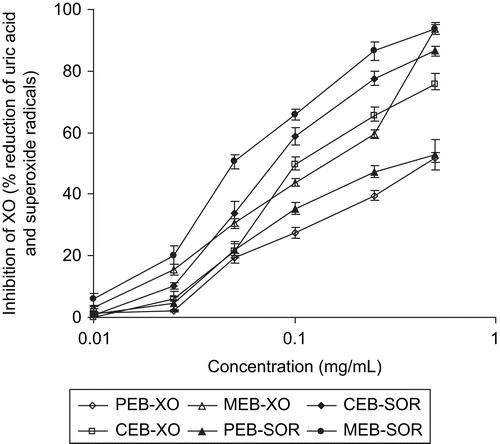
The activities of three different organic solvent extracts against inhibition of XO and scavenging of superoxide radicals are presented in . The results were expressed as IC50 values [the concentration of the test samples required to inhibit 50% of the enzyme XO (= 50% decrease in uric acid production) and to reduce 50% production of superoxide anions].
Table 1. The IC50 values of extracts of the leaves of Blumea balsamifera for inhibition of xanthine oxidase and reduction of superoxide level.
The XO inhibitory activity as assessed by the xanthine-xanthine oxidase (X/XO) system was decreased in the order of: MEB (0.111 ± 0.002 mg/mL) > CEB (0.138 ± 0.004 mg/mL) > PEB (0.516 ± 0.003 mg/mL). The X/XO system also generated superoxide anions (O2•-) and the scavenging activity of these anions was measured by the reduction of NBT. MEB (0.063 ± 0.001 mg/mL) and CEB (0.092 ± 0.001 mg/mL) exhibited potent superoxide scavenging activities and the activity decreased in a similar fashion as it was observed for inhibitory activities on XO. The results are presented in . There were statistically significant differences (p < 0.05) in IC50 values of each extracts. It was also observed that the IC50 value of extracts for inhibition of xanthine oxidase was higher than the scavenging of superoxide radicals ().
Figure 2. The concentration of different solvent extracts of Blumea balsamifera leaves versus the inhibition of xanthine oxidase and scavenging of enzymatically generated superoxide radicals. Results are mean ± SD (n = 3).
The total polyphenol content of the extracts was determined. The result was expressed as mg of catechin equivalent (CE) per gram of dried extract. The highest polyphenol content was found in MEB (294.13 mg ± 2.509) then CEB (198.61 mg ± 1.891). The lowest concentration was recorded in PEB (8.33 mg ± 0.603). There was a statistically significant difference (p < 0.05) in polyphenolic contents between the extracts. The phytochemical studies on the leaves of B. balsamifera revealed the presence of hydrocarbons, sterols (sitosterol, stigmasterol) flavone and dihydroflavonol derivatives in PEB, flavone, flavonol, and flavanone derivatives in CEB and flavone, flavonol, flavanone, and dihydroflavonol derivatives in MEB (CitationNessa et al., 2004a, Citation2004b). With regard to the inhibition of XO enzyme by extracts of B. balsamifera, the data showed the existence of a modest correlation between the flavonoid content of extracts of B. balsamifera and XO inhibitory activity for the extracts examined. It could be postulated that the high flavonoid content of MEB contributed for higher inhibitory activity on XO and scavenging of superoxide anions produced by X/XO system.
Flavonoids of B. balsamifera as XO inhibitors
Nine isolated flavonoids from the leaves of B. balsamifera () were studied for their XO inhibitory activity and scavenging of enzymatically produced superoxide radicals. represents the effect of flavonoids, l-ascorbic acid and allopurinol on XO. All the tested flavonoid derivatives showed inhibition on XO. The half-maximal inhibitory concentrations of the flavonoids and reference compounds are listed in . The results were decreased in the order of: allopurinol > luteolin (6) > quercetin (7) > tamarixetin (3) > 5,7,3′,5′-tetrahydroxyflavanone (8) > rhamnetin (4) > luteolin-7-methyl ether (5) > blumeatin (2) > dihydroquercetin-4′-methyl ether (9) > dihydroquercetin-7,4′-dimethyl ether (1) > l-ascorbic acid. Allopurinol and l-ascorbic acid were used as reference compounds. Allopurinol, a potent XO inhibitor, exhibited lowest IC50 values among the tested compounds, and ascorbic acid, a well-known antioxidant, was inactive at the 1-100 μM concentration level in this assay system.
Table 2. The IC50 values of flavonoids of the leaves of Blumea balsamifera for inhibition of xanthine oxidase.
Figure 3. The concentration of flavonoids (2–9) and reference compounds (allopurinol and ascorbic acid) versus inhibition of xanthine oxidase, the enzyme that produces uric acid and superoxide radicals. Results are mean ± SD (n = 3).
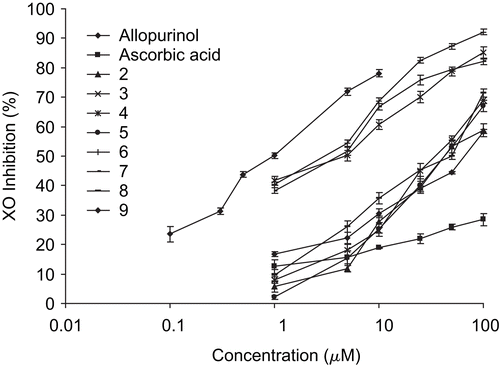
As per the ranking of XO inhibitory activity of the tested flavonoids, it can be seen that luteolin bearing four hydroxyl groups exhibited lower IC50 value than quercetin, bearing five hydroxyls. The presence of the methyl group at C-7 position markedly reduced the inhibitory activity on XO as it was observed as follows: luteolin > luteolin-7-methyl ether; 5,7,3′,5′-tetrahydroxyflavanone > blumeatin; quercetin > rhamnetin and dihydroquercetin-4′-methyl ether > dihydroquercetin-7,4′-dimethyl ether. The result agreed with CitationCos et al. (1998). These observations illustrated the importance of the C-5 and C-7 hydroxyl groups in the inhibition of XO. Comparing the inhibition of XO by flavones with the corresponding flavonols, it can be concluded that the absence of the hydroxyl group at C-3 enhances slightly the inhibitory effect on xanthine oxidase as it was observed in the case of luteolin and quercetin.
It appears that the hydroxyl groups at C-5 and C-7 and the double bond between C-2 and C-3 are important in the inhibition of XO by flavonoids. The presence of the hydroxyl group at C-3 slightly decreased the inhibitory activity (CitationCos et al., 1998). The structure of flavanone and dihydroflavonol differs from flavones and flavonols by the presence of a single bond between C-2 and C-3 in the former and a double bond in the latter. Apparently, this structural difference influences the inhibitory effect on xanthine oxidase. With a double bond between C-2 and C-3, ring B will be coplanar with rings A and C due to the conjugation. Saturation of this double bond will destroy the conjugation and coplanarity. This suggested that a planar flavonoid structure is important for inhibition of XO (CitationCos et al., 1998).
Although 5,7,3′,5′-tetrahydroxyflavanone is a flavanone derivative, it showed good inhibitory activity on XO. The presence of methoxyl group at C-7 position in B ring of blumeatin lowered the inhibitory activity on XO. It was also observed in the case of dihydroquercetin-4′-methyl ether and dihydroquercetin-7,4′-dimethyl ether. The former compound was more active than the latter in the inhibition of XO.
The XO inhibitory effects of all the tested flavonoids were compared with that of reference compounds such as allopurinol and ascorbic acid. All the flavonoids showed significantly (p < 0.05) higher activity on XO inhibition than ascorbic acid but lower activity than allopurinol. However, there were no statistically significant differences (p < 0.05) observed in IC50 values between luteolin and quercetin; quercetin and tamarixetin; luteolin and tamarixetin, and luteolin and allopurinol.
Flavonoids of B. balsamifera as superoxide radical scavengers (enzymatic)
The superoxide scavenging activities of flavonoids measured using the NBT reduction method is presented in . The half-maximal inhibitory concentrations of the flavonoids and reference compounds are listed in . The results were decreased in the order of: allopurinol > luteolin (6) > quercetin (7) > tamarixetin (3) > 5,7,3′,5′-tetrahydroxyflavanone (8) > rhamnetin (4) > blumeatin (2) > l-ascorbic acid > luteolin-7-methyl ether (5) > dihydroquercetin-4′-methyl ether (9) > dihydroquercetin-7,4′-dimethyl ether (1). Allopurinol and l-ascorbic acid were used as reference compounds.
Table 3. The IC50 values of flavonoids of the leaves of Blumea balsamifera for reduction of enzymatically (xanthine/xanthine oxidase) generated superoxide radicals.
Figure 4. The concentration of flavonoids (2–9) and reference compounds (allopurinol and ascorbic acid) versus scavenging of enzymatically (xanthine/xanthine oxidase system) generated superoxide anions. Results are mean ± SD (n = 3).
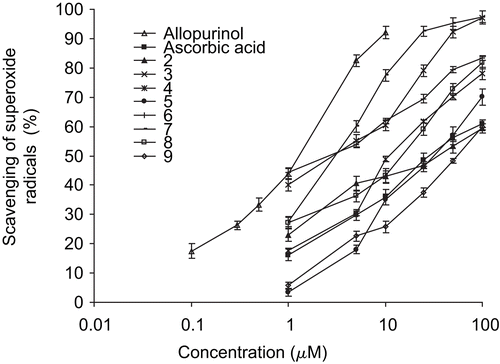
Luteolin showed lower IC50 values than quercetin and it indicated that the 3-OH group had no effect on superoxide scavenging as it was also observed between flavanone and dihydroflavonol derivatives. The flavanone derivative exhibited comparatively higher scavenging activity than the dihydroflavonol derivatives (5,7,3′,5′-tetrahydroxyflavanone > blumeatin > dihydroquercetin-4′-methyl ether > dihydroquercetin-7,4′-dimethyl ether). It appeared that the saturation of the C2-C3 bond with the presence of the 3-OH group markedly reduced the scavenging activity. But with the saturation of the C2-C3 bond without the 3-OH group, it had little effect on scavenging activity such as 5,7,3′,5′-tetrahydroxyflavanone had lower IC50 value than rhamnetin.
Methoxyl substitution at C-7 position in A ring lowered the superoxide scavenging activity (CitationCos et al., 1998) as it was also observed in this experiment as follows: luteolin > luteolin-7-methyl ether; dihydroquercetin-4′-methyl ether > dihydroquercetin-7,4′-dimethyl ether; tamarixetin > rhamnetin and 5,7,3′,5′-tetrahydroxyflavanone > blumeatin.
The IC50 values of all test flavonoids were compared with allopurinol and ascorbic acid. Allopurinol showed significantly higher (p < 0.05) scavenging activity than the test flavonoids. Although ascorbic acid was not a potent XO inhibitor, it exhibited higher (p < 0.05) scavenging activity than luteolin-7-methyl ether and dihydroflavonol derivatives. It appears that ascorbic acid scavenges superoxide radical potentially rather than inhibition of XO, as it was inactive as an XO inhibitor at the 1-100 μM concentration level. The other test flavonoids exhibited higher (p < 0.05) scavenging activity than ascorbic acid.
In this experiment, all the test flavonoids showed dual activity, that is, both were active as superoxide scavengers and as XO inhibitors in a dose-dependent manner except dihydroquercetin-7,4′-dimethyl ether, a dihydroflavonol was inactive at the 1-100 μM concentration level. It has been reported that antioxidant enzyme superoxide dismutase (SOD) scavenged superoxide anion radicals in a dose-dependent manner (CitationRobak & Gryglewski, 1988), as it was also observed in the experiment. The IC50 value for SOD was 606.71 mU/mL ± 2.24 ().
Conclusion
The results of this study provide evidence that B. balsamifera leaf extract and its flavonoids are potent inhibitors of XO, an enzyme involved in uric acid and superoxide radical production. A reduction in uric acid production results automatically in an equivalent reduction in superoxide radical (CitationFridovich, 1970). This means that the rate of uric acid reduction equals the rate of superoxide reduction. Moreover, the test extracts and their flavonoids were also potent scavengers of enzymatically generated superoxide radicals. Accordingly, these XO inhibitors and superoxide radical scavengers can be useful in the search for better traditional drugs for human gout, kidney stone, and ischemia. In addition, it justifies the further investigation of its other beneficial biological properties.
Acknowledgement
The first author is very much thankful to Prof. Dr. Zhari Ismail of School of Pharmaceutical Sciences, Universiti Sains Malaysia, for providing all kinds of laboratory facilities for carrying out this research.
Declaration of interest
The authors declare no conflict of interest.
References
- Adkins WK, Taylor AE. (1990). Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J Appl Physiology, 69, 2012–2018.
- Ames BN, Gold LS, Willett WC. (1995). The causes and prevention of cancer. Proc Nat Acad Sci USA, 92, 5258–5265.
- Anthoni U, Rosalba ED, Nielsen PH, Carsten C. (1998). Huazhongilexone is not 3′,5,5′,7-tetrahydroxyflavanone. Preparation of 3′,5′-dimethoxy-5,7-dihydroxyflavanone. Acta Chem Scand, 52, 1243–1246.
- Auclair C, Torres M, Hakim J. (1978). Superoxide anion involvement in NBT reduction by NADPH-cytochrome P-450 reductase. FEBS Lett, 89, 26–28.
- Beiler JM, Martin GJ. (1951). The inhibition of xanthine oxidase by flavonoids and related compounds. J Biol Chem, 192, 831–834.
- Bindoli A, Valente M, Cavallini L. (1985). Inhibitory action of quercetin on xanthine oxidase and xanthine dehydrogenase activity. Pharm Res Commun, 7, 831–839.
- Coe FL, Evan A, Worcester E. (2005). Kidney stone disease. J Clin Invest, 115, 2598–2608.
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Poel BV, Pieters L, Vlietinck AJ, Berghe DV. (1998). Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod, 61, 71–76.
- Fazilatun N, Nornisah M, Zhari I. (2004). Superoxide radical scavenging properties of extracts and flavonoids isolated from the leaves of Blumea balsamifera. Pharm Biol, 42, 404–408.
- Fridovich I. (1970). Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem, 245, 4053–4057.
- Hall AP, Barry PE, Dawber TR, McNamara PM. (1967). Epidemiology of gout and hyperuricemia: A long-term population study. Am J Med, 42, 27–37.
- Hatano T, Yasuhara T, Yoshihara R, Ikegami Y, Matsuda M, Yazaki K, Agata I, Nishibe S, Noro T, Yoshizaki M, Okuda T. (1991). Inhibitory effects of galloylated flavonoids on xanthine oxidase. Planta Med, 57, 83–84.
- Ismail Z, Ismail N, Jaafar L. (1999). Malaysian Herbal Monograph, Vol. 1, 9-15, Kuala Lumpur: Malaysian Monograph Committee.
- Ito H, Miyake M, Nishitani E, Mori K, Hatano T, Okuda T, Takasaki M, Nishino H, Yoshida T. (1999). Anti-tumor promoting activity of polyphenols from Cowania mexicana and Coleogyne ramosissima. Cancer Lett, 143, 5–13.
- Kirby TW, Fridovich I. (1982). A picomolar spectrophotometric assay for superoxide radical. Anal Biochem, 127, 435–440.
- Lin Y, Long K, Deng Y. (1988). Studies on the chemical constituents of the Chinese medicinal plant Blumea balsamifera. Acta Sci Nat Univ Sunyatseni, 2, 77–80.
- Matsumura F, Yamaguchi Y, Goto M, Ichiguchi O, Akizuki E, Matsuda T, Okabe K, Liang J, Ohshiro H, Iwamoto T, Yamada S, Mori K, Ogawa M. (1998). Xanthine oxidase inhibition attenuates Kupffer cell production of neutrophil chemoattractant following ischemia-reperfusion in rat liver. Hepatology, 28, 1578–1587.
- Middleton E Jr. (1998). Effect of plant flavonoids on immune and inflammatory cell function. Adv Expt Med Bio, 439, 175–182.
- Nagao A, Seki M, Kobayshi H. (1999). Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem, 63, 1787–1790.
- Nessa F, Ismail Z, Mohamed N. (2004a). Isolation of sterols and hydrocarbons from the leaves of Blumea balsamifera DC. ACGC Chem Res Commun, 17, 9–15.
- Nessa F, Ismail Z, Mohamed N, Mas Haris MRH. (2004b). Free radical scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem, 88, 243–252.
- Nielsen VG, Tan S, Weinbroum A, McCammon AT, Samuelson PN, Gelman S, Parks DA. (1996). Lung injury after hepatoenteric ischemia-reperfusion: Role of xanthine oxidase. Am J Resp Crit Care Med, 154, 1364–1377.
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. (2001): Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutri, 74, 418–425.
- Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. (1983). Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull, 31, 3984–3987.
- Nugent CA, MacDiarmid WD, Tyler FH. (1962). Renal excretion of uric acid in leukemia and gout. Arch Intern Med, 109, 540–544.
- Nguyen MTT, Awale S, Tezuka Y, Tran QL, Watanabe H. (2004). Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull, 27, 1414–1421.
- Okamoto K, Eger BT, Nishino T, Kondo S, Pai EF, Nishino T. (2003). An extremely potent inhibitor of xanthine oxidoreductase: Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem, 278, 1848–1855.
- Osaki N, Koyano T, Kowithayakorn T, Hayashi M, Komiyama K, Ishibashi M. (2005). Sesquiterpenoids and plasmin-inhibitory flavonoids from Blumea balsamifer. J Nat Prod, 68, 447–449.
- Robak J, Gryglewski RJ. (1988). Flavonoids are scavengers of superoxide anions. Biochem Pharmacol, 36, 837–841.
- Rice-Evans C. (1995). Plant polyphenols: Free radical scavengers or chain-breaking antioxidants? Bioche Soc Symp, 61, 103–116.
- Ruangrungsi N, Tappayuthpijaran P, Tantivatana P. (1981). Traditional medicinal plants of Thailand. I. Isolation and structure elucidation of two new flavonoids, (2R, 3R)-dihydroquercetin-4′-methyl ether and (2R, 3R)-dihydroquercetin-4′,7-dimethyl ether from Blumea balsamifera. J Nat Prod, 44, 541–545.
- Scalbert A, Monties B, Janin G. (1989). Tannins in wood: Comparison of different estimation models. J Agri Food Chem, 37, 1324–1329.
- Selway JW. (1986): Antiviral activity of flavones and flavans. Prog Clin Biol Res, 213, 521–536.