Abstract
Context: Gmelina arborea Roxb. (Verbenaceae) is an important medicinal plant in the traditional system of medicine of India. The plant is used in the treatment of snake-bites, fever, piles, and diabetes. However, there is little toxicological information available regarding its safety after exposure. The present study was designed to evaluate acute and repeated dose toxicity of the aqueous extract of Gmelina arborea stem bark.
Materials and methods: In the acute toxicity test, Swiss albino mice were treated with aqueous extract (300, 2000, and 5000 mg/kg), orally. Animals were observed periodically during the first 24 h after administration of the extract, and daily thereafter for 14 days. In the repeated dose toxicity study, the aqueous extract of Gmelina arborea (300, 1000, and 2000 mg/kg per day) was administered orally for a period of 28 days in Wistar rats. The effects on body weight, food and water consumption, organ weight, hematology, clinical biochemistry, as well as histology, were studied.
Results and conclusion: Aqueous extract did not produce mortality, changes in behavior or any other physiological activities in mice, for any of the selected doses. There were no significant differences in the body weight, organ weights and feeding habits between control and treated animals. Hematological and biochemical analysis showed no marked differences in any of the parameters examined in either the control or treated groups. Pathologically, neither gross abnormalities nor histopathological changes were observed. The aqueous extract of Gmelina arborea was found safe in acute and repeated dose toxicity studies when tested in rodents.
Introduction
Gmelina arborea Roxb. (Verbenaceae) is an important medicinal plant in the Indian Ayurvedic system of medicine. An unarmed tree is about 60 ft high, has a clear bole up to 20-30 ft and a girth of 5-7 ft. It is found scattered in deciduous forests throughout the greater part of India up to an altitude of 5000 ft (CitationCSIR, 2003). The drupes, leaves, flowers, roots, and bark are used in medicine. The plant is used in treatment of snake-bites, scorpion sting (CitationNadkarni, 2000), and diabetes (CitationKhan & Khanum, 2005). The plant is anthelmintic and used for treating hallucinations, excess thirst, piles, abdominal pains, burning sensations, and fever (CitationKirtikar & Basu, 1999).
Its chemical constituents have been examined, and the isolation of luteolin (CitationRao et al., 1967) and indole alkaloids (CitationBhattacharjee & Das, 1969) from the leaves has been reported. The occurrence of hentriacontanol (CitationJoshi et al., 1971) and lignans such as arboreol, isoarboreol, methyl arboreol, arborone, gmelanone, gummadiol, and 7-oxodihydrogmelinol (CitationGovindachari et al., 1972; CitationAnjaneyulu et al., 1975; CitationSatyanarayana et al., 1986) in the heartwood has also been noted. Some coumarin glycosides (CitationSatyanarayana et al., 1985) and iridoid glycosides (CitationHosny & Rosaazza, 1998) have been isolated from roots and leaves, respectively.
Pharmacological activities of crude extracts of Gmelina arborea have been investigated using different animal models. Different extracts of the plant are reported to have wound-healing properties (CitationShirwaikar et al., 2003), antidiarrheal activity (CitationAgunua et al., 2005), and antioxidant activity (CitationSinha et al., 2006).
Despite the wide use of Gmelina arborea in traditional medicine, no study has been published regarding its toxicological profile. The present study was designed to determine the acute and repeated dose oral toxicity of the aqueous extract of stem bark of Gmelina arborea in experimental animals as it is mainly the stem bark that is used in the Ayurvedic system of medicine.
Materials and methods
Plant material
The stem bark of the Gmelina arborea was collected in November 2006 from Jawhar (Thane District), Maharashtra, India. It was identified and authenticated by P.S.N. Rao of the Botanical Survey of India, Pune, India. A voucher specimen (b-03) of the stem bark is deposited in the department for future reference. The plant material was air-dried at room temperature and ground into a fine powder. The powdered bark was used to prepare the aqueous extract.
Preparation of aqueous extract
The aqueous extract of bark of Gmelina arborea (AE) was prepared by maceration. Powdered bark (500 g) was macerated with 5 L of distilled water for seven days, with frequent shaking. After seven days the aqueous extract was filtered and the marc was again subjected to maceration with distilled water for complete extraction. After filtration the aqueous extracts were combined and concentrated with the help of a rotary vacuum evaporator under reduced pressure. The aqueous extract was dried in vacuum dryer and stored in refrigerator. The yield of the aqueous extract was found to be 20% w/w, with respect to powdered bark.
Preliminary phytochemical screening of the aqueous extract was done for identifying the presence of different phytoconstituents by standard methods (CitationHarborne, 1998). The estimation of total saponins was carried out by the method given by CitationRajpal (2002), while estimation of total phenolics was carried out by the method given by CitationSadashivam and Manickam (1997). The aqueous extract indicated presence of saponins (1.67%) and phenolics (2.31%). The extract was prepared in double distilled water at the time of administration. The volume of extract administered to the experimental animals was no greater than 2 mL/100 g.
Experimental animals
Adult Wistar albino rats and Swiss albino mice were used in the present investigation. The animals were maintained in polypropylene cages in the Departmental Animal House Facility with 12 h light: 12 h dark cycle. All the animals were kept under laboratory conditions (temperature 25°C ± 2°C, relative humidity 75% ± 5%) for an acclimatization period of 7 days before carrying out the experiments. Normal pellet diet (Amrut Laboratory Animal Feed, Maharashtra, India) and filtered water was provided ad libitum. All the experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) and complied with the NIH guidelines on handling of experimental animals.
Acute toxicity study
The acute toxicity of the aqueous extract of Gmelina arborea (AE) was evaluated in mice using the OECD Guidelines 423 (CitationOECD, 2001). Three groups containing three female mice (weight: 25-35 g, age: 6-8 weeks) received Gmelina arborea aqueous extract at doses of 300, 2000, and 5000 mg/kg body weight, orally by gavage while the control group received distilled water. Animals were observed individually after dosing once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h and daily thereafter, for a total of 14 days.
Cage side observations
Observations included changes in skin and fur, eyes and mucous membranes, and also respiratory, circulatory, autonomic and central nervous systems, somatomotor activity and behavior pattern. Special attention was directed to observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma.
Body weight, food and water intake
Body weight was recorded at two-day intervals. The animals were placed in metabolic cages with measured quantities of food and water. The remaining quantities of food and water were measured the next day. The food intake and water intake were recorded at two-day intervals.
Pathology
Surviving animals were fasted overnight then weighed and humanely killed on day 15 using anesthetic ether. All test animals were subjected to gross necropsy.
Repeated dose toxicity study
Repeated toxicity studies were conducted on four groups of rats (0 mg/kg control; 300 mg/kg low dose; 1000 mg/kg medium dose; 2000 mg/kg high dose), each containing five males and five females. Whilst the extract was orally administered using gavage to the test groups, distilled water was administered to the control group for 28 days. All animals were supplied with standard food and water ad libitum during the testing periods. All rats were observed daily for toxic manifestations and mortality.
Body weight, food and water intake
Body weight, water and food intake were measured once a week.
Hematology
Hematological analysis was performed using an automatic hematological analyzer (Sysmex, Japan). Hemoglobin, hematocrit, total red blood corpuscles (RBC), total white blood corpuscles (WBC), platelets and red cell indices including mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) of blood samples were recorded.
Clinical biochemistry
Cholesterol, high density lipoproteins (HDL), triglycerides (TGL), bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, albumin, blood urea nitrogen (BUN), creatinine, glucose and important ions like sodium, potassium, calcium, phosphate and chloride were recorded, using an autoanalyzer (Erba Chem 7, Germany).
Pathological examination
Gross necropsy
All animals in the study were subjected to a full, detailed gross necropsy which included careful examination of the external surface of the body, all orifices, and the cranial, thoracic and abdominal cavities and their contents. The liver, lungs, kidneys, adrenals, gonads, spleen, heart and brain of all animals were removed and their wet weights were taken immediately after dissection to avoid drying.
Histopathology
Liver, kidney, stomach, intestine, spleen, pancreas, adrenal, lungs, heart, brain, and gonads were fixed immediately in 10% formalin for routine histopathological examination. The tissues were embedded in paraffin and then sectioned, stained with hematoxylin and eosin and were examined under light microscope. Histopathological evaluations were performed by a pathologist. Photomicrographs of the microscopic sections were taken with the help of a photomicroscope (Motic, Canada) provided with Motic Images Plus 2.0 software.
Statistical analysis
The differences among experimental and control groups were determined using the statistical software Sigmastat Version 2.03 for Windows. Comparisons among different groups were performed by using one way analysis of variance (ANOVA) followed by Dunnett’s test. Significant differences between control and experimental groups were assessed by Student’s t-test. All data are expressed as mean ± standard error of mean (SEM); p values less than 0.05 was considered to be significant.
Results
Acute toxicity study
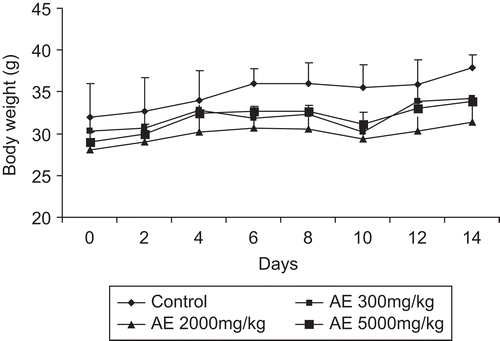
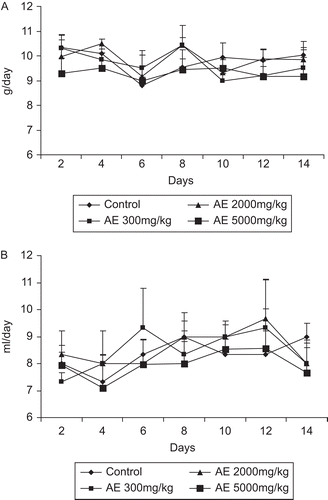
There was no mortality or morbidity observed in animals through the 14-day period following single oral administration at all selected dose levels of the water extract of Gmelina arborea (). The maximally tolerated dose (MTD) for oral administration of Gmelina arborea extract is greater than 5000 mg/kg body weight. The animals did not show any changes in general appearance during the observation period. Morphological characteristics (fur, skin, eyes and nose) appeared normal. No tremors, convulsion, salivation, diarrhea, lethargy or unusual behaviors such as self mutilation, walking backward and so forth were observed; gait and posture, reactivity to handling or sensory stimuli, grip strength were all normal. There was no toxicological significant difference in body weights between control and treatment groups (). Food and water intake showed daily fluctuations within the range of control animals ( and ).
Table 1. Sign of toxicity, mortality results of acute toxicity study of Gmelina arborea extract in mice.
Repeated dose toxicity study
Body weight, food and water intake
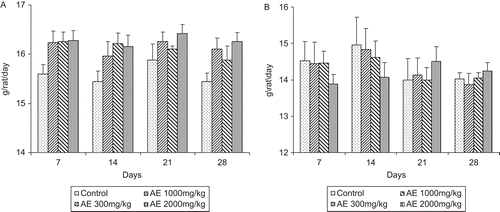
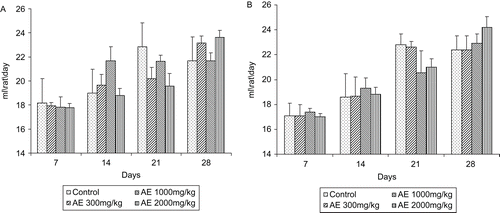
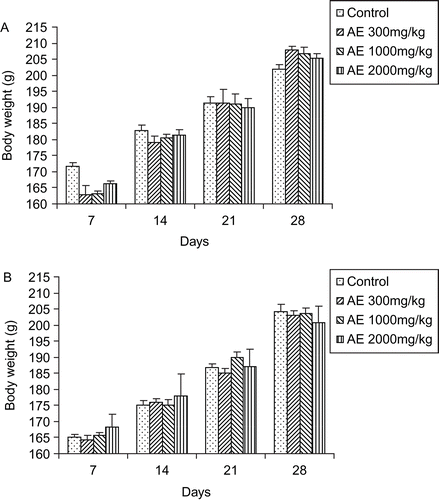
There was no significant difference in body weights between control and treatment groups ( and ) of either sex. There was no significant difference between food and water consumption of treated animals compared with control (, , , ).
Figure 3. Effect of aqueous extract of Gmelina arborea (AE) on body weight changes in male rats (A) and female rats (B). Data are expressed as mean ± SEM (n = 5).
Hematology and clinical biochemistry
The effect of repeated dose oral administration of aqueous extract (AE) on hematological parameters is presented in . No statistically significant changes were observed in blood analysis of hemoglobin, hematocrit, red blood cells and white blood cells in male and female treatment groups compared to the control group. The platelet count was slightly decreased in female rats treated with high doses of AE. The effect of repeated dose oral administration of AE on biochemical parameters is presented in . There were no toxicologically significant effects observed in any of the biochemical parameters examined in either the control or treated groups of the male and female rats.
Table 2. Effect of aqueous extract of Gmelina arborea (AE) on hematological values in repeated dose toxicity.
Table 3. Effect of aqueous extract of Gmelina arborea (AE) on blood chemistry values in repeated dose toxicity.
Pathological examination
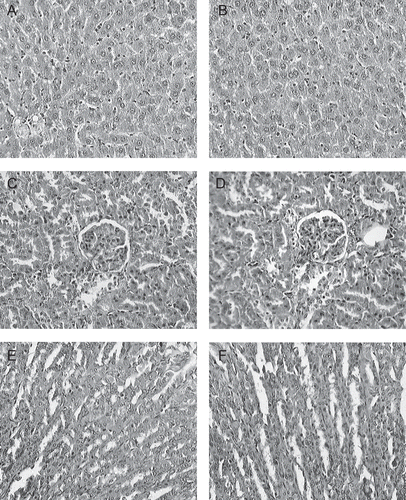
There were no significant differences between the control and treated groups in the organ weights of male and female rats (). No alterations were detected in pathological examinations of the tissues during the microscopic examination of the internal organs (). The findings were generally consistent with the expected pattern for Wistar rats of this particular age.
Table 4. Effect of aqueous extract of Gmelina arborea (AE) on organ weights (g) in rats in repeated dose toxicity.
Figure 6. Histopathological analysis (400×) of organs treated with aqueous extract AE (repeated dose toxicity study): (A) liver from control group; (B) liver from AE 2000 mg/kg/bw treated group; (C) kidney from control group; (D) kidney from AE 2000 mg/kg/bw treated group; (E) intestine from control; (F) intestine from AE 2000 mg/kg/bw treated group.
Discussion
To determine the safety of drugs and plant products for human use, toxicological evaluation is carried out in various experimental animals to predict toxicity and to provide guidelines for selecting a safe dose in humans. A Word Health Organization survey indicated that about 70–80% of the world’s populations rely on non-conventional medicine, mainly of herbal source, in their primary healthcare. This is especially the case in developing countries where the cost of consulting a western style doctor and the price of medication are beyond the means of most people (CitationMukinda & Syce, 2007). Although medicinal plants may produce several biological activities in humans, generally very little is known about their toxicity and the same applies for Gmelina arborea.
Usually, acute (single dose) toxicity studies are carried out on laboratory animals by using high doses (sufficient to produce death or morbidity) of the substance in question and/or based on previous reports of its toxicity or toxicity of structurally related compounds. As there was no previous report on the toxicity of Gmelina arborea, as per OECD guidelines, three dose levels starting at 300, 2000 and limit dose 5000 mg/kg were selected for acute toxicity study. No mortality was observed in either control or in groups of all selected dose levels. Animals in all groups did not exhibit any signs of adverse effect and thus the no observed adverse effect level (NOAEL) of the extract is greater than 5000 mg/kg.
In the repeated dose toxicity study, the extract was given orally at doses up to 2000 mg/kg in rats. There was no change in animal behavior and the changes in body weight were not significantly different in treated rats as compared to controls. Since changes in body weight have been used as an indicator of adverse effects of drugs and chemicals (CitationTofovic & Jackson, 1999; CitationRaza et al. 2002; CitationTeo et al., 2002), the present results suggest that at the dose levels administered, the aqueous extract is non-toxic in rats.
In addition, determination of food consumption is important in the study of the safety of a product with therapeutic purpose, as proper intake of nutrients is essential to the physiological status of the animals and to the accomplishment of the proper response to the drug tested, instead of a false response due to improper nutritional conditions (CitationRamesh et al., 2007). In the present studies, Gmelina arborea extract-treated rats did not show significant differences in food consumption or in water consumption when compared with rats from control groups.
Analysis of blood parameters is relevant to risk evaluation, as the changes in the hematological system have a higher predictive value for human toxicity when data are translated from animal studies (CitationOlson et al., 2000). After 28 days of treatment, there were no significant changes in the hematological parameters between control and treated groups.
Levels of white blood cells, red blood cells, red blood cell indices, hematocrit, and hemoglobin were not significantly different between control and test groups following repeated administration of the extract, although platelet count (high dose group females) was found to be different. Platelet alone, however, cannot be taken as indicative of hematotoxicity, as a decrease in platelets at the high dose was not biologically significant as it did not show a dose–response relationship. The results indicate that the Gmelina arborea extract was not toxic to the circulating red cells, white blood cells and platelets, nor did it interfere with their production. Hematopoiesis and leucopoiesis were also not affected even though the haematopoietic system is one of the most sensitive targets for toxic compounds and an important index of physiological and pathological status in man and animals (CitationAdeneye et al., 2006) Therefore, it plausible to assume the extract is not hematotoxic.
Significant changes in enzymes such as ALP, AST and ALT represent liver impairment, since these are important indices of liver toxicity (CitationHayes, 1989). Serum cholesterol and proteins are mainly regulated via synthesis in the liver and increase or decrease in serum concentrations of constituents suggests liver toxicity. These enzymes and biochemical parameters were not altered by administration of Gmelina arborea extract after administration for 28 days, indicating that there was no severe liver damage.
Parameters related to kidney such as urea and creatinine plasma levels (CitationGanong, 1977; CitationJesse, 1982) remained normal after administration of Gmelina arborea extract at all selected dose levels. Thus it can be stated that the extract does not show any renal toxicity.
The histopathological examination of important organs such as kidney, liver, stomach and intestine indicated no severe damage; these observations further support the safety of aqueous extract of Gmelina arborea in rats.
Conclusion
In conclusion, at the oral doses tested, the extract was well tolerated and neither produced overt signs of clinical toxicity nor any signs of hepato-, nephro- or hematotoxicity when studied in rodents. Thus, the aqueous extract of Gmelina arborea was found to be non-toxic when oral acute and repeated dose toxicities were performed. Overall, this study provides valuable data on the toxicity profile of Gmelina arborea that should be useful for the planning of future preclinical and clinical studies of the medicinal plant.
Acknowledgments
The authors are thankful to Laxman Doke and Bhau Jadhav, Jawhar, Dist-Thane for their help during collection and processing of plant material.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. (2006). Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol, 105, 374–379.
- Agunua A, Yusuf S, Onyiloyi Andrew G, Abdulkadir Umar Zezi Abdurahman, EM. (2005). Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol, 101, 27–30.
- Anjaneyulu ASR, Jaganmohan RK, Kameswara RV, Ramachandra RL, Subrahmanyam C, Pelter A, Ward RS. (1975). The structures of lignans from Gmelina arborea. Tetrahedron, 31, 1277–1285.
- Bhattacharjee AK, Das AK. (1969). Phytochemical survey of a few Mysore plants. Econ Bot, 23, 274–276.
- CSIR. (2003). The Wealth of India. New Delhi, Publication and Information Directorate, Council for Scientific and Industrial Research.
- Ganong WF. (1977). Review of Medical Physiology. CA, Lange Medical.
- Govindachari TR, Parthasarathy PC, Dasai HK. (1972). Arboreol, a new lignan from Gmelina arborea. Ind J Chem, 10, 1120–1122.
- Harborne JB. (1998). Phytochemical Methods. London: Chapman and Hall.
- Hayes AW. (1989). Guidelines for acute oral toxicity testing, in: Principles and Methods of Toxicity. New York: Raven, 184.
- Hosny M, Rosaazza JPN. (1998). Gmelinosides A–L, twelve acylated iridoid glycosides from Gmelina arborea. J Nat Prod, 61, 734–742.
- Jesse B. (1982). Animal Anatomy and Physiology. Reston, VA: Reston.
- Joshi KC, Prakash L, Singh LB. (1971). Extractives from heartwoods: Isolation of hentriacontanol from Gmelina arborea. J Ind Chem Soc, 48, 1175–1176.
- Khan IA, Khanum A. (2005). Herbal Therapy for Diabetes. Hyderabad: Ukaaz.
- Kirtikar KR, Basu BD. (1999). Indian Medicinal Plants. Dehradun: International Book Distributors.
- Mukinda JT, Syce JA. (2007). Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J Ethnopharmacol, 112, 138–144.
- Nadkarni KM. (2000). Indian Materia Medica. Mumbai: Popular Prakashan.
- OECD. (2001). The OECD Guidelines for Testing of Chemicals, 423. Acute oral toxicity test. Paris: Organization of Economic Co-operation Development.
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. (2000). Concordance of toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol, 32, 56–67.
- Rajpal V. (2002). Standardisation of Botanicals. New Delhi: Eastern.
- Ramesh T, Lee K, Lee HW, Kim SJ. (2007). Acute oral toxicity study of Asiasari radix extract in mice. Int J Toxicol, 26, 247–251.
- Rao DV, Rao EV, Viswanathan N. (1967). Occurrence of luteolin in the leaves of Gmelina arborea Linn. Curr Sci, 3, 71–74.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. (2002). Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharmaceutic, 70, 135–145.
- Sadashivam S, Manickam A. (1997). Biochemical Methods. New Delhi: New Age International.
- Satyanarayana P, Koteswara R, Ward RS, Pelter A. (1986). Arborone and 7-oxo-dihydrogmelinol: Two new keto-lignans from Gmelina arborea. J Nat Prod, 49, 1061–1064.
- Satyanarayana P, Subrahmanyam P, Kasai R, Tanka O. (1985). An apiose-containing coumarin glycoside from Gmelina arborea root. Phytochemistry, 24,1862–1863.
- Shirwaikar A, Ghosh S, Padma GM Rao. (2003). Effects of Gmelina arborea Roxb. leaves on wound healing in rats. J Nat Rem, 3, 45–48.
- Sinha S, Dixit P, Bhargava S, Devasagayam TPA, Ghaskadbi S. (2006). Bark and fruit extracts of Gmelina arborea protect liver cells from oxidative stress. Pharm Biol, 44, 237–243.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. (2002). A 90-day oral gavage toxicity study of d-methylphenidate and d-, l-methylphenidate in Sprague-Dawley rats. Toxicology, 79, 183–196.
- Tofovic SP, Jackson EK. (1999). Effect of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J Cardioavasc Pharmacol, 33, 360–366.