Abstract
Context: Nauclea latifolia Smith (Rubiaceae) is a small tree found in tropical areas in Africa. It is used in traditional medicine to treat malaria, epilepsy, anxiety, pain, fever, etc.
Objective: The aim of this study was to investigate the effects of Nauclea latifolia roots decoction on the peripheral and central nervous systems and its possible mechanisms of action.
Materials and methods: The analgesic investigation was carried out against acetic acid-induced writhing, formalin-induced pain, hot-plate and tail immersion tests. The antipyretic activity was studied in Brewer’s yeast-induced pyrexia in mice. Rota-rod test and bicuculline-induced hyperactivity were used for the assessment of locomotor activity.
Results: Nauclea latifolia induced hypothermia and had antipyretic effects in mice. The plant decoction produced significant antinociceptive activity in all analgesia animal models used. The antinociceptive effect exhibited by the decoction in the formalin test was reversed by the systemic administration of naloxone, Nω-l-nitro-arginine methyl ester or glibenclamide. In contrast, theophylline did not reverse this effect. Nauclea latifolia (antinociceptive doses) did not exhibit a significant effect on motor coordination of the mice in Rota-rod performance. Nauclea latifolia protected mice against bicuculline-induced behavioral excitation.
Discussion and conclusion: Overall, these results demonstrate that the central and peripheral effects of Nauclea latifolia root decoction might partially or wholly be due to the stimulation of peripheric opioid receptors through the action of the nitric oxide/cyclic monophosphate guanosin/triphosphate adenosine (NO/cGMP/ATP)-sensitive- K+ channel pathway and/or facilitation of the GABAergic transmission.
Introduction
Nauclea latifolia Smith (Rubiaceae) is a shrub or small spreading tree that is a widely distributed plant that grows in north Cameroon and other African countries (CitationArbonnier, 2000). It is found in the forest and fringe tropical forests. In Tupuri language, in Cameroon it is known as “koumkouma”. Nauclea latifolia root decoction is a herbal preparation that has been used traditionally for treating different disease conditions. Medicinal uses vary from one traditional setting to another, and include: fever, pain, dental caries, septic mouth, malaria, dysentery, diarrhea, and diseases of the central nervous system such as epilepsy (CitationArbonnier, 2000, CitationAmos et al., 2005; CitationNgo Bum et al., 2009; CitationAbbah et al., 2009). The root of Nauclea latifolia is the preferred part of the plant used in Cameroonian traditional medicine for treating pain and fever. This part is usually harvested, sun dried and pulverized to obtain powder. About 100 g of the powdered material is macerated in 500 mL of water and boiled. The decoction obtained is administered orally at the dose range of 80–160 mg/kg.
The aqueous extract of leaves of the plant has been used as a remedy for diabetes in northern Nigeria (CitationGidado et al., 2005). The plant also has been reported to have antihypertensive and laxative activities (CitationAkpanabiantu et al., 2005). Previous works have shown that the aqueous extract of the bark of Nauclea latifolia (freeze-dried extract) attenuated writhing episodes induced by acetic acid and increased the threshold for pain perception in the hot-plate test in mice. The extract remarkably decreased both the acute and delayed phases of formalin-induced pain in rats and also caused a significant reduction in both yeast-induced pyrexia and egg albumin-induced edema in rats (CitationAbbah et al., 2009). In the course of pharmacological studies, anticonvulsant, anxiolytic and sedative properties of Nauclea latifolia root decoction (CitationNgo Bum et al., 2009) have already been reported from this laboratory. Phytochemical investigations of the bark and wood of Nauclea latifolia have reported the presence of naucleamides A-E, new monoterpene indole alkaloids from Nauclea latifolia (CitationShigemori et al., 2002). Unfortunately, none of these compounds have been tested for their pharmacological activities.
The present study was therefore carried out to confirm the veracity of the aforementioned traditional claims of Nauclea latifolia root decoction usefulness. Thus, we investigated the effects of Nauclea latifolia roots decoction on the peripheral and central nervous systems by using several experimental models of fever and pain in mice. To examine the possible mechanisms of the decoction in the antinociception effects we used naloxone (a non-selective opioid receptor antagonist), theophylline (a non selective adenosine receptor antagonist), glibenclamide (an ATP-sensitive K+ channel inhibitor) and Nω-l-nitro-arginine methyl ester (l-NAME), a NO synthase inhibitor. Preliminary acute toxicity and phytochemical tests were also carried out to evaluate the secondary metabolites present and the safety of this widely used plant.
Materials and methods
Plant material
The roots of Nauclea latifolia used in this study were collected in the dry season (March 2008) from the National Park of Benoué (north Cameroon). Dr. Vroumsia Tchinaye at the National Herbarium, Yaoundé in Cameroon identified the plant material. Voucher herbarium specimen No 20144/SRF/Cam has been deposited at the Yaoundé herbarium.
Preparation of the extracts
The bark of dried roots of Nauclea latifolia was ground. The powder (1000 g) was macerated in 5000 mL of distilled water for 1 h. This mixture was boiled for 20 min. After it cooled, the supernatant (decoction) was collected and filtered with Whatman No. 1 filter paper. After filtration, water was evaporated in a drying oven at 45°C and 81 g of a dark brown solid was obtained. The yield of the extraction was 8.1%. The plant extracts (8 g) were dissolved in 250 mL distilled water. This was the stock solution for the pharmacological tests. The decoction, prepared 30 min to 1 h before its administration in mice, was administered orally (p.o.) 1 h before the pharmacological test. The following doses were used: 16, 40, 80, 160, and 320 mg/kg.
Other doses were prepared and administered either orally or intraperitoneally in acute toxicity study. For each other dose used, we calculated the volume to be follows:
where D is dose used (g/kg body weight), P is body weight (kg), C is concentration of the extract (g/mL) and V is volume of extract (mL).
Drugs and chemicals
Acetylsalicylic acid (Aspirin®, Laboratoires 3M, Pithiviers, France), glibenclamide (Doanil®, was from Sanofi-Avensis, Guildford, UK), formalin (BDH, Poole, UK), theophylline (Xanthium®, SMB, Boulogne-Billancourt, France), morphine sulfate, naloxone hydrochloride, Nω-l-nitro-arginine methyl ester, brewer’s yeast (Arkopharma, Carros, France), acetic acid, bicuculline (Sigma Aldrich, St Louis, MO), indomethacin (Indocid®, MerckSharp-DolmeChibret, Thimister-Clermont, France) and diazepam (Valium®, was from Roche, France). Chemicals were prepared in the form of suspensions using a few drops of Tween 80 and diluted with distilled water. All treatments were administered in a volume of 10 mL/kg mice body weight.
Animals
Adult male and female mice (Mus musculus Swiss; 20–25 g) were used throughout these studies. The animals were housed in standard cages at 25°C on a 12 h light-dark cycle. They were supplied with food and water ad libitum. The experiments were carried out in accordance with national (reg. no. FWA-IRB00001954) and international (CitationEEC, 1987; CitationUSNRC, 1996) ethical committee guidelines for the care and used of laboratory animals. All efforts were made to minimize both the suffering and number of animals used.
Preliminary phytochemical test
Preliminary phytochemical properties of the decoction of the roots of Nauclea latifolia were tested using the following chemicals and reagents: flavonoids (NaCl and HCl), alkaloids with Mayer and Dragendorff’s reagents, saponins (frothing test), tannins (FeCl3), glycosides (NaCl3 and Fehling’s solution A and B), cardiac glycosides (Salkowski test), anthraquinones (Borntrager’s reaction), phenols [FeCl3 and K3Fe(CN6)], and lipids (filter paper) (CitationTrease & Evans, 1983).
Acute toxicity studies
Mice of either sex were divided in groups of 10 animals. All animals had free access to tap water and food, except for a short fasting period before oral administration of single doses of the decoction of the roots of Nauclea latifolia. The extract was administered by gavage at the doses of 1000, 2000, 4000, 8000, 10,000, 12,000 and 14,000 mg/kg or by the intraperitoneal route at the doses of 100, 250, 500, 1000, 2000, 4000 and 8000 mg/kg. The general behavior of mice was observed continuously for 1 h after the treatment and then intermittently for 4 h, and thereafter over a period of 24 h (CitationTwaij et al., 1983). The mice were further observed for up to 14 days following treatment for any signs of toxicity and death, and the latency of death. Any adverse effects, such as hypoactivity, piloerection, salivation, and syncope, were evaluated immediately after administration of Nauclea latifolia extract. Also, anorexia and weight loss were observed and noted. The LD50 value was determined according to the method of Litchfield and Wilcoxon (1949). A confirmatory test was carried out and the LD50 was calculated from the graph of percentage (%) of mortality (converted to probit) against log-dose of the extract.
Antipyretic activity
Body temperature
Four groups of mice received orally various doses of the N. latifolia roots decoction (16, 40, 80 and 160 mg/kg, p.o.) and one group received distilled water. Rectal temperature was recorded with an electronic thermometer at predetermined times in groups of mice before and after (0, 0.5, 1, 2, 3 and 24 h) the administration of either distilled water or N. latifolia decoction. Pre-drug recording served as the reference point for the determination of the temperature change (CitationPal & Nag, 1999).
Antipyretic test
An initial rectal temperature was recorded by insertion of an electronic thermometer 2 cm deep into the rectum. This was recorded again 30 min after and an average taken. A 15% suspension of Brewer’s yeast in 0.9% saline solution was prepared. Pyrexia was induced by injecting 20 mg/kg of Brewer’s yeast suspension subcutaneously in the back behind the nape of the neck. Following the injection, the site was massaged in order to spread the suspension beneath the skin. Room temperature was kept at 30°C. The animals were starved for 18 h but water made available ad libitum. The rectal temperature measurement was done again 18 h post-injection to record its rise. Only mice with body temperatures greater than 36.5°C were taken into the test. The animals received the N. latifolia decoction (16, 40, 80 and 160 mg/kg, p.o.) and standard drug (acetylsalicylic acid 150 mg/kg, p.o.) orally and measurements were taken 0.5-6 h post-dosing (CitationBrune & Alpermann, 1983).
Antinociceptive activity
Acetic acid-induced abdominal constriction
The plant decoction (16, 40, 80 and 160 mg/kg, p.o.), acetylsalicylic acid (150 mg/kg, p.o.), morphine (5 mg/kg, s.c.), naloxone + N. latifolia decoction (1 mg/kg, i.p. + 160 mg/kg, p.o.) and distilled water (p.o.) were administered 1 h prior to acetic acid treatment. One hour after oral administration of these substances, each animal was injected intraperitoneally with 0.6% acetic acid in a volume of 10 mL/kg body weight. After acetic acid injection, the number of stretching or writhing responses per animal was recorded during a subsequent 30 min after a latency period of 5 min and permitted to express the percentage of inhibition (CitationAsongalem et al., 2004).
Formalin-induced nociception
The formalin test was carried out as described by CitationHunskaar and Hole (1987) with a few modifications. The negative control was treated with distilled water. The positive control received indomethacin (10 mg/kg, p.o.) and morphine (5 mg/kg, s.c.) two reference analgesic compound. Four groups of mice were treated with the extract of the plant (16, 40, 80 and 160 mg/kg, p.o.). Pain was induced by injecting 0.05 mL of 2.5% formalin (40% formaldehyde) in distilled water in the subplantar of the right hind paw. Mice were given N. latifolia decoction (16, 40, 80, 160 mg/kg, p.o.), indomethacin (10 mg/kg), morphine (5 mg/kg, s.c.), or distilled water (p.o.) 1 h after injecting formalin. These mice were individually placed in transparent Plexiglas (15 × 15 × 15 cm) observation chambers. The amount of time spent licking and biting the injected paw was indicative of pain and was recorded in 0–5 min (first phase) and 15-30 min (second phase). The four other groups were pretreated with: naloxone (2 mg/kg, i.p.), theophylline (5 mg/kg, i.p.), glibenclamide (8 mg/kg, p.o.) or l-NAME (10 mg/kg, i.p.). After 15 min (pretreatment with naloxone, theophylline and glibenclamide) and 30 min (pretreatment with l-NAME), the mice received the N. latifolia decoction at the dose of 160 mg/kg. The nociceptive response to the formalin intraplantar injection was recorded 1 h after administration of extract.
Hot-plate
The apparatus consisted of a metallic cylinder (diameter 14 cm and 10 cm high) placed in a water bath with the temperature set at 55 ± 0.5°C (CitationLanhers et al. 1991), onto which the mice were placed. Each mouse (six per group) acted as its own control. Prior to treatment, the reaction time of each mouse (licking of the forepaws or jumping response) was assessed at 0 and 10 min intervals. The average of the two readings was obtained as the initial reaction time. The reaction time following the administration of the decoction (16, 40, 80 and 160 mg/kg, p.o.), acetylsalicylic acid (150 mg/kg, p.o.), morphine (5 mg/kg, s.c.), naloxone + N. latifolia decoction (1 mg/kg, i.p. + 160 mg/kg, p.o.) or distilled water (p.o.), was measured at 0.5, 1, 2, 3, 4, 5 and 6 h after a latency period of 30 min (CitationAsongalem et al., 2004).
Tail immersion
The tail immersion test was carried out according to the method described by CitationViswanatha et al. (2006). This involved immersing the extreme 3 cm of the mouse’s tail in a water bath containing water at a temperature of 55 ± 0.5°C. Within a few seconds, the mouse reacted by withdrawing the tail. The reaction time was recorded with a stopwatch. The mice were treated with N. latifolia decoction (16, 40, 80 and 160 mg/kg, p.o.), naloxone + Nauclea latifolia decoction (1 mg/kg, i.p. + 160 mg/kg, p.o.), a standard drugs, acetylsalicylic acid (150 mg/kg, p.o.), morphine (5 mg/kg, s.c.) or distilled water (p.o.). The reaction time of the mice was tested at intervals 0, 15, 30 and 60 min after a latency period of 1 h following the administration of the decoction and drugs.
Studies of motor coordination (Rota-rod test)
Motor performance was assessed as previously reported by CitationPieretti et al. (1999) with a Rota-rod apparatus (Ugo Basile 7560; Milano, Italy) consisting of a bar with a diameter of 3 cm, subdivided into five compartments by a disk 240 mm in diameter. The bar rotated at a constant speed of 16 revolutions per min. A preliminary selection of mice was made on the day of the experiment excluding those that did not remain on the Rota-rod bar for two consecutive periods of 45 s each. The integrity of motor coordination was assessed on the basis of the number of falls from the Rota-rod in 180 s. Selected animals were tested immediately at 0, 30, 60, 90 and 120 min after administration of N. latifolia decoction (16, 40, 80, 160 and 320 mg/kg, p.o.), diazepam (1 mg/kg, i.p.) or distilled water (10 mL/kg, p.o.).
Antagonism to bicuculline-induced behavioral excitation
The method has been described previously (CitationNgo Bum et al., 2002). In brief, mice were injected i.p. with bicuculline (2 mg/kg), 1 h after the oral administration of N. latifolia root decoction (16, 40, 80 and 160 mg/kg, p.o.), diazepam (3 mg/kg, i.p.) or distilled water (p.o.). They were placed one by one in the center of an open field. The open field used was a wooden square box (40 cm × 40 cm × 45 cm) divided in lines on the floor into 16 smaller squares of equal dimensions (10 cm × 10 cm). The mice were observed for 1 h. Rearing, grooming, immobility and sedation times were recorded and the number of line crossings was counted.
Statistical analysis
Data were expressed as mean ± SEM per group. Statistical differences between control and treated groups were tested by two-way repeated measures analysis of variance (ANOVA), followed by Tukey’s post hoc test. The differences were considered significant at P <0.05. The statistical package used for the analysis was XLSTAT 2009.
Results
Preliminary phytochemical test
Our phytochemical studies () indicate that the decoction of the roots of N. latifolia contains flavonoids, phenols, anthraquinones, tannins, glycosides, cardiac glycosides, alkaloids and saponins. Bufadienolides and lipids were absent (CitationNgo Bum et al., 2009).
Table 1. Phytochemical constituents of the decoction of the roots of Nauclea latifolia.
Acute toxicity studies
There were no deaths or any signs of toxicity observed after oral administration of single doses of the decoction of the roots of N. latifolia at any dose level up to the highest dose tested (14 g/kg). N. latifolia decoction did not produce any significant change in behavior, breathing, cutaneous effects, sensory nervous system responses and gastrointestinal effects in male or female mice. These results showed that, at single dose, there are no adverse effects of N. latifolia decoction. These data indicate that the median lethal dose (LD50) should be higher than 14 g/kg for male and female mice. In contrast, the mortality rate, as well as the acute toxicity, of the intraperitoneally administrated Nauclea latifolia decoction increased progressively with increasing doses. The mortality rate of 0% at a dose of 100 mg/kg gradually rose to 100% at 8000 mg/kg, the highest dose studied. The no-observed-adverse-effect level for the intraperitoneal dose was 500 mg/kg, while the lowest-observed-effects level was 750 mg/kg (CitationAlexeeff et al., 2002). Some adverse effects, such as hypoactivity and salivation, were seen immediately after intraperitoneal injection, while others (e.g., anorexia and weight loss) were observed later, and were more pronounced at the highest doses and persisted until death (). The acute intraperitoneal toxicity (LD50) of the decoction of the roots of N. latifolia in mice was 2197.85 mg/kg.
Table 2. Acute toxicity of the decoction of the roots of Nauclea latifolia administered by intraperitoneal injection to mice.
Antipyretic activity
Body temperature
Administration of N. latifolia decoction produces an alteration of body temperature in mice. In the control group, no significant variations of rectal temperature were detected. Pretreatment with N. latifolia decoction at doses of 80 and 160 mg/kg produced a significant fall of body temperature at 1 h (F(4,23) = 19.76, P <0.01), 2 h (F(4,23) = 62.8, P <0.01), and 3 h (F(4,23) = 14.2, P <0.01) after treatment of animals. At 1 h and 2 h time intervals the hypothermic effect was significant only at doses of 40, 80 and 160 mg/kg. At 3 h hypothermia was observed with all doses of N. latifolia decoction. The body temperature returned toward basal values after 24 h ().
Table 3. Influence of the decoction of the roots of Nauclea latifolia on body temperature.
Antipyretic test
The data revealed that 160 mg/kg of N. latifolia decoction caused a significant reduction (F(5,28) = 21.71, P <0.05) of body temperature up to 1 h after administration. However, the effect increases very significantly for 40, 80 and 160 mg/kg N. latifolia decoction until 3 h after administration. Acetylsalicylic acid and 160 mg/kg of the extract reduced the fever after 1 h by 0.8° and 0.5°C, respectively. Whereas the plant showed effective antipyretic activity 2 h post dosing at 16, 40 and 80 mg/kg, all doses of the decoction of the roots of N. latifolia effectively reduced the fever within 6 h. The antipyretic effect was compared with that of standard acetylsalicylic acid ().
Table 4. Influence of the decoction of the roots of Nauclea latifolia on Brewer’s yeast-induced pyrexia.
Nociceptive activity
Acetic acid-induced abdominal constriction
presents the pain behavior of writhing response, which was presented as cumulative abdominal stretching response. The protective effect of N. latifolia decoction was dose-dependent with 27.3% (F(7,38) = 124.5, P<0.05) reduction observed for 16 mg/kg and 40.8% (F(7,38) = 124.5, P <0.05) seen for 40 mg/kg. The protection offered by 160 mg/kg, 61.7% (F(7,38) = 124.5, P < 0.001) was comparable to that of morphine (a centrally acting analgesic), 66.2% (F(7,38) = 124.5, P <0.001). Acetylsalicylic acid had only 54.3% (F(7,38) = 124.5, P < 0.01) inhibition. Naloxone (1 mg/kg, i.p., a non-selective opioid receptor antagonist) significantly reversed (from 61.7% to 19.6%) the antinociceptive effect of Nauclea latifolia decoction. Pretreatment with naloxone did not block effectively the protective actions of the extract.
Table 5. Influence of the decoction of the roots of Nauclea latifolia on acetic acid-induced writhing.
Formalin-induced nociception
Formalin administration produced a typical pattern of flinching and licking behavior. Treating the mice with N. latifolia decoction produced a marked and dose-related inhibition of formalin-induced biphasic pain responses in mice. The analgesic effect of this extract at the dose of 160 mg/kg occurred predominantly during the early (F(6,33) = 227.2, P <0.01) and late (F(6,33) = 239, P <0.001) phases. The positive control drug, morphine (5 mg/kg), significantly attenuated both the neurogenic (F(6,33) = 227.2, P <0.001) and the inflammatory (F(6,33) = 239, P <0.001) pain phase, whereas indomethacin (10 mg/kg) was efficient in late phase (67.1% inhibition). Pretreatment of mice with naloxone (2 mg/kg, i.p.), a non-selective opioid receptor antagonist, completely and significantly reversed the antinociceptive effect of N. latifolia decoction (160 mg/kg) in both phases of formalin test. Systemic pretreatment of mice with l-NAME, a NO synthase inhibitor (10 mg/kg, i.p.), also prevented the antinociception produced by the oral administration of N. latifolia decoction (160 mg/kg) in the formalin test. Pretreatment of mice with an ATP-sensitive K+ channel inhibitor, glibenclamide (8 mg/kg, p.o.), significantly prevented the antinociceptive effect induced by the oral administration of N. latifolia decoction (160 mg/kg) in both phases of formalin test. The adenosine antagonist theophylline (5 mg/kg), however, did not have any significant effect of the antinociceptive effect of N. latifolia decoction in both phases of formalin test ().
Table 6. Influence of the decoction of the roots of Nauclea latifolia on formalin-induced pain.
Tail immersion
As shown in , all doses of N. latifolia decoction, 30 and 60 min after administration caused an increase in tail withdrawal latency. There was a significant reduction of pain sensation induced by tail immersion in warm water. N. latifolia decoction at 160 mg/kg produced a significant increase in the withdrawal latencies of the tail as depicted in the time-course curve (F(7,38) = 52.7, P <0.001). The effect was dose-dependent. At 60 min, N. latifolia increased the withdrawal latency by 8.0 ± 0.3 min, (83.3%). Similarly, morphine (5 mg/kg) produced a significant anti-nociceptive effect by increasing the withdrawal latencies of animals (F(7,38) = 52.7, P <0.001) by an average of 8.1 ± 0.5 min, (98.2%). Acetylsalicylic acid had no effect on this test. The anti-nociceptive activities of N. latifolia decoction was not effectively blocked by naloxone.
Table 7. Influence of the decoction of the roots of Nauclea latifolia on tail flick response in mice after immersion in a water bath at 55°C.
Hot-plate
Data indicate that the mean reaction time was highest 1 h after administration of the decoction with all doses used compared to those at 0 and 0.5 h. This reaction was somewhat dose-dependent. Augmentation in reaction time reached 56.1 ± 3.9 min, (230.5%) (F(7,38) = 94.21, P <0.0001) with 160 mg/kg at 3 h. Pretreatment with naloxone did not effectively reduce the antinociceptive potential of the extract. Acetylsalicylic acid at 150 mg/kg did not offer any protection against heat-induced pain. Morphine sulfate at 5 mg/kg showed a maximal protective effect of 62.7 ± 1.9 min, (235.3%) (F(7,38) = 117.4, P <0.0001) after 4 h compared to 56.1 ± 3.9 min, (230.5%) (F(7,38) = 117.4, P <0.0001) for 160 mg/kg of the extract after 3 h ().
Table 8. Influence of the decoction of the roots of Nauclea latifolia on hot-plate-induced pain in mice.
Studies of motor coordination (Rota-rod test)
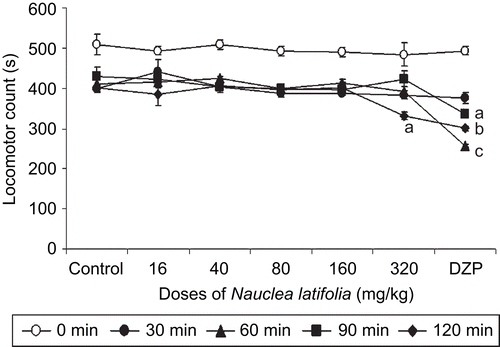
In the Rota-rod test, N. latifolia decoction (16–160 mg/kg) does not exhibit significant effect on mouse motor coordination. Only the 320 mg/kg dose of Nauclea latifolia at 120 min after treatment, significantly reduced (F(7,33) = 188,82; P<0.001) locomotor activity ().
Figure 1. Effects of acute Nauclea latifolia decoction (16, 40, 80, 160 and 320 mg/kg) or diazepam (1 mg/kg) treatment on motor co-ordination of mice on the Rota-rod, Acquisition process of the Rota-rod performance as expressed by means ± SEM of performance time, ap <0.05, bp <0.01, cp <0.001, significantly different compared to the control; data were analyzed by two-way ANOVA, followed by Tukey’s (HSD) multicomparison test; n = 6 animals per group.
Antagonism to bicuculline-induced behavioral excitation
Behavioral aspects such as locomotion, rearing and grooming were increased by bicuculline. Mice treated with bicuculline presented hyperactivity when compared to controls. N. latifolia decoction and diazepam decreased significantly the hyperactivity induced by bicuculline in the open field test (grooming (F(5,28) = 384.5 P <0.0001), rearing (F(5,28) = 40.7, P <0.0001), and immobility (F(5,128) = 93.4 P <0.0001). The number of crossings was also increased for the same dose (except 16 mg/kg) but only 40, 80 and 160 mg/kg were statistically significant (F(5,28) = 103.57, P <0.05), (F(5,28) = 103.57, P <0.01) and (F(5,28) = 103.57, P <0.001), respectively ().
Table 9. Influence of the decoction of the roots of Nauclea latifolia on bicuculline induced-behavioral excitation in 1 h.
Discussion
The hypothermia observed in the present studies after oral administration of N. latifolia decoction suggests an implication of both central and peripheral mechanisms. This is not surprising since it is well known that certain psychoactive central nervous system depressant drugs reduce temperature both in normal and pyretic conditions. Studying the antipyretic properties of N. latifolia decoction by the yeast-induced hyperthermia test illustrated that the extract was active at sedative and anxiolytic doses (CitationNgo Bum et al., 2009). These doses (80 and 160 mg/kg) reduced the rectal temperature in hyperthermic mice, 1 h after treatment, thus restoring the normal temperature (non-hyperthermic mice). It is currently accepted that prostaglandin E2 (PGE2) is the final fever mediator in brain, especially in preoptic areas of the anterior hypothalamus. It would therefore be of interest to determine whether the extract inhibits the syntheses of PGE2 (CitationLi et al., 2008).
Several behavioral nociceptive tests which differ with respect to stimulus quality, intensity and duration, were employed in evaluating the analgesic effect of N. latifolia decoction in order to obtain a holistic picture of the analgesic properties of the plant extract. The models were selected such that both central and peripheral effects were investigated. At the doses tested, the decoction of the roots of N. latifolia was shown to have antinociceptive activity in all the nociceptive models, thus indicating that the extract had both central and peripherally mediated activity. N. latifolia decoction produced an inhibition of acetic acid-induced writhes. This effect was shown to be significant but not specific (CitationVogel & Vogel, 1997). Intraperitoneal injection of acetic acid produces pain through the activation of chemosensitive nociceptors (CitationStai et al., 1995) or irritation of the visceral surface, which leads to the liberation of histamine, bradykinin, prostaglandins and serotonin (CitationGarcia et al., 2000). It has been reported that the writhing assay is sensitive to µ-opioid and non-steroid anti-inflammatory drugs (NSAIDs) that act primarily by a central and peripheral mechanism, respectively. The NSAIDs can inhibit the number of writhes in this model by inhibiting cyclooxygenase in peripheral tissues, thus interfering with the mechanism of transduction in primary afferent nociceptors by blocking the effect and/or release of anti-inflammatory mediators (CitationPanthong et al., 2007). It is therefore possible that the decoction may act via a mechanism similar to NSAIDs. In the present study, inhibition of acetic acid-induced writhing by the decoction indicates significant antinociceptive activity of N. latifolia decoction ().
It is well known that the formalin test involves two phases: a neurogenic one with the release of substance P, and an inflammatory one with the release of serotonin, histamine, bradykinin and prostaglandins (CitationGarcia et al., 2000). The licking response induced by formalin results from a combination of peripheral input and spinal cord sensitization (CitationTjolsen et al., 1992). Taking this into account, the antinociceptive effect of the decoction could be dependent on either peripheral or central sites of action. Central acting drugs such as opioids inhibit both phases of pain by equally inhibiting the effect produced by prostaglandins released at this level in response to inflammation (CitationHunskaar & Hole, 1987) and by endogenous opioids through their action on the central nervous system. The peripheral analgesics such as acetylsalicylic acid only inhibit the second phase, whereas the narcotic analgesics inhibit both phases. The fact that N. latifolia decoction significantly inhibited both phases of the pain induced by the formalin suggests that it may act as narcotic analgesia.
In the present study, the possible mechanism of action of N. latifolia root decoction was investigated in the presence of naloxone, theophylline, l-NAME or glibenclamide. Glibenclamide, an ATP-sensitive K+ channel blocker blocked the analgesic activities of N. latifolia decoction. It is well established that glibenclamide specially blocks ATP-sensitive K+ channels, with no effect on Ca2+- or voltage-dependent K+ channels (CitationAmoros et al., 1990; CitationEdwards & Weston, 1993). Therefore, the present data suggest that the opening of ATP-sensitive K+ channels plays a role in the analgesic action of N. latifolia decoction. The antinociceptive effect of the decoction was also blocked by the NO synthase inhibitor l-NAME, suggesting that its antinociceptive action involves the activation of NO-cyclic (guanosine monophosphate) GMP pathways at peripheral and/or central levels (CitationNozaki-Taguchi & Yamamoto, 1998). It has been clearly established that NO is a downstream signaling molecule released in response to central analgesia such as morphine (CitationCadet et al., 2004). Hence, the release of NO or its production is an important step of the antinociceptive action of N. latifolia decoction. Naloxone, a non-selective opioid antagonist, reversed the antinociceptive of the decoction in both phases of the formalin test. This finding clearly suggests that activation of opioid receptors and/or an increment of endogenous opioids, either centrally or peripherally, might be involved in the antinociceptive effect of N. latifolia decoction (CitationBjörkman et al., 1990).
To corroborate that N. latifolia decoction has analgesic activity, hot-plate and tail immersion tests were conducted. In the tail immersion test, which consists of a thermal stimulus, an increase in the reaction time is generally considered to be an important parameter for evaluating central antinociceptive activity (CitationKnoll, 1967). The analgesic activity of Nauclea latifolia decoction was observed at a dose of 160 mg/kg and the effect was similar to that of morphine. The tail-flick response is believed to be a spinal mediated reflex (CitationChapman et al., 1985). The antinociceptive activity of N. latifolia decoction in the hot-plate test, indicates a central action and likely involves supraspinal as well as spinal components (CitationYaksh & Rudy, 1976; CitationWoolf et al., 1980). Our results show that the antinociceptive effects of the decoction did not vanish but are reduced only slightly after pretreatment with naloxone. The lack of reduction of the analgesic effects after co-treatment of animals with naloxone suggests that N. latifolia decoction acts on the peripheral opioid system and other central receptors involved in pain.
The decoction (16–160 mg/kg) did not attenuate motor coordination (Rota-rod performance) suggesting that actions may not be achieved via neuromuscular blockade. Rather, the effects might involve neurons that control central depressant activities. These results suggest that inhibition of pain is not related to the reduction of spontaneous locomotor activity of animals. At the doses (320 mg/kg) which affected locomotion, the decoction also caused an impairment of rod performance, which is indicative of either CNS depressant or muscle relaxant effects (CitationAmos et al., 2005).
The results also show that N. latifolia decoction inhibits bicuculline-induced hyperactivity. This is shown by the marked reduction in locomotor activity, the duration of rearing and grooming in the bicuculline-induced behavioral excitation test. This model has been used in laboratory animals to evaluate the central depressant properties of the drugs. The model measures the level of excitability of the central nervous system (CitationGoth, 1984). Agents that suppress this behavior were known to do so through central inhibition. The test is a measure of an exploratory behavior that reveals the sedative activity of the agent (CitationFile & Pellow, 1985). Bicuculline, a selective GABAA antagonist (CitationSperber et al., 1989) acts directly on the postsynaptic GABAA receptor complex to induce hyperactivity behavior. The effect of N. latifolia decoction against bicuculline suggests a possible interference with central GABAergic neurotransmission. GABAergic inhibitory interneurons are densely distributed in the superficial dorsal horn and at the basis of the longstanding gate control theory of pain, which postulates that loss of function of these inhibitory interneurons (disinhibition) would result in increased pain (CitationMelzack & Wall, 1965).
Preliminary phytochemical screening of N. latifolia roots decoction indicated the presence of alkaloids, flavonoids, tannins, saponins and others phytochemical constituents (). Flavonoids are well known for their ability to inhibit pain perception (CitationSawadogo et al., 2006). Flavonoids and their related compounds also exhibit inhibition of arachidonic acid peroxidation, which results in reduction of prostaglandin levels thus reducing the fever and pain (CitationBaumann et al., 1980). Saponins have been implicated in opioid receptor mechanism (CitationHuong et al., 1995) through antagonistic activity (CitationWagner et al., 1983) by binding on the sensory nerve terminals. It suggests that flavonoids and saponins were responsible for the antipyretic and antinociceptive effects of N. latifolia roots decoction. But it is noted that active components of N. latifolia remain to be isolated in the further studies.
The present studies, therefore support the claims of traditional medicine that Nauclea latifolia root decoction possesses antipyretic, analgesic and anticonvulsant properties. Overall, these results demonstrate that the central and peripheral effects of Nauclea latifolia root decoction might partially or wholly be due to the stimulation of peripheric opioid receptors through the action of the nitric oxide-cyclic GMP-ATP-sensitive K+ (NO/cGMP/ATP)-channel pathway and/or facilitation of the GABAergic transmission.
Declaration of interest
This research was supported by the University of Ngaoundéré, Cameroon and Grant awarded to Germain Sotoing Taïwe, from the Agence Universitaire de la Francophonie (Bourse Internationale de Formation à la Recherche Doctorale, AUF 2009/2010, Ref.: 1021FR/277/BAC 2009/JGZ/AS) for valuable financial assistance.
References
- Abbah J, Amos S, Ngazal I, Vongtau H, Adzu B, Chindo B, Farida T, Odutola AA, Wammbebe C, Gamaniel KS (2009): Pharmacological evidence favoring the use of Nauclea latifolia in malaria ethnopharmacy: Effect against nociception, inflammation, and pyrexia in rats and mice. J Ethnopharmacol 127: 85–90.
- Akpanabiantu MI, Umoh IB, Udosen EO, Udo AE, Edet EE (2005): Rat serum electrolytes, lipid profile and cardiovascular activity of Nauclea latifolia leaf extract administration. Indian J Clin Biochem 20: 29–34.
- Alexeeff GV, Broadwin R, Liaw J, Dawson SV (2002): Characterization of the LOAEL-to NOAEL uncertainty factor for mild adverse effects from acute inhalation exposures. Regul Toxicol Pharmacol 36: 96–105.
- Amoros S, Schimid AH, Fosset M, Lazdunski M (1990): Glucose, sulfonulureas and neurotransmitter release: Role of ATP-sensitive channels activation. Science 247: 252–854.
- Amos S, Abbah J, Chindo B, Edmond I, Binda L, Adzu B, Buhari S, Odutola AA, Wambebe C, Gamaniel K (2005): Neuropharmacological effects of the aqueous extract of Nauclea latifolia root bark in rats and mice. J Ethnopharmacol 97: 53–57.
- Arbonnier M (2000): Trees, shrubs and lianas of the drylands of West Africa. Mali, Ouagadougou, Centre de Coopération Internationale en Recherche Agronomique pour le Développement/Muséum National d’Histoire Naturelle/Union Mondiale pour la Nature (CIRAD/MNHN/UICN).
- Asongalem EA, Foyet HS, Ngogang J, Folefoc GN, Dimo T, Kamtchouing P (2004): Analgesic and anti-inflammatory activities of Erigeron floribundus. J Ethnopharmacol 91: 301–308.
- Baumann J, Von Brucchau SV, Wurm G (1980): Flavonoids and related compounds as inhibition of arachidonic acid peroxidation. Prostaglandins 20: 627–639.
- Björkman RJ, Hedner J, Hedner T, Henning M (1990): Central, naloxone-reversible antinociceptive by diclofenac in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 342: 171–176.
- Brune K, Alpermann H (1983): Non-acidic inhibition of prostaglandin production, carrageenan oedema and yeast fever. Agents Actions Suppl 13: 360–363.
- Cadet P, Mantione KJ, Bilfinger TV, Stefano GB (2004): Differential expression of the human mu opiate receptor from different primary vascular endothelial cells. Med Sci Monit 10: 351–355.
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE (1985): Pain measurement: An overview. Pain 22: 1–31.
- Edwards G, Weston AH (1993): Induction of a glibenclamide-sensitive K-current by modification of a delayed rectified channel in rat portal vein in insulinoma cells. Br J Pharmacol 110: 1280–1281.
- EEC (1987): EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987.
- File S, Pellow S (1985): The effect of triazolobenzodiazepines in two animal tests of anxiety and on the hole-board. Br J Pharmacol 86: 729–735
- Garcia MD, Fernandez MA, Alvarez A, Saenz MT (2000): Anti-nociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol 91: 69–73.
- Goth A (1984): Medical Pharmacologie, Eleventh edition. St Louis, Toronto, Mosby.
- Gidado A, Ameh DA, Atawodi SE (2005): Effect of Nauclea latifolia leaves aqueous extracts on bloods glucose levels of normal and alloxan-induced diabetic rats. Afr J Biotech 4: 91–93.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30, 103–114.
- Huong NTT, Matsumoko K, Yamasaki K, Duc NM, Nham NT, Watanabe H (1995): Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuated the psychological stress-/and foot shock stress-induced antinociception in mice. Pharmacol Biochem Behav 52: 427–432.
- Knoll J (1967): Screening and grouping of psychopharmacological agent, in: Siegler PE, Mover HJ, eds, Animal and Clinical Pharmacologic Techniques in Drug Evaluation. Chicago, Year Book Medical Publishers, pp. 305–321.
- Lanhers MC, Fleurentin J, Dorfman P, Motrier F, Pelt JM (1991): Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med 57: 225–231.
- Li S, Dou W, Tang Y, Goorha S, Ballou LR, Blatteis CM (2008): Acetaminophen: Antipyretic or hypothermic in mice? In either case, PGHS-1b (COX-3) is irrelevant. Prostaglandins Other Lipid Mediat 85: 89–99.
- Melzack R, Wall PD (1965): Pain mechanisms: A new theory. Science 150: 971–979.
- Ngo Bum EN, Gwa C, Ntchapda F, Nyunai N, Sokeng S, Rakotonirina VS, Rakotonirina A (2002): Effect of the decoction of rhizomes of Cyperus articulatus on bicuculline, N-methyl-d-aspartate and strychnine-induced behavioural excitation and convulsion in mice. J Cam Acad Sci 2: 91–95.
- Ngo Bum EN, Taiwe GS, Moto FCO, Ngoupaye GT, Nkantchoua GCN, Pelanken MM, Rakotonirina SV, Rakotonirina A (2009): Anticonvulsant, anxiolytic and sedative properties of the roots of Nauclea latifolia Smith in mice. Epilepsy Behav 15: 434–440.
- Nozaki-Taguchi N, Yamamoto T (1998): Involvement of nitric oxide in peripheral antinociceptive mediated by kappa-and delta-opioid receptor. Anesth Anal 87: 388–393.
- Pal S, Sen T, Nag Chauduru, AK (1999): Neuropsychopharmacological profile of the methanolic fraction of Bryophyllum pinnatum leaf extract. J Pharm Pharmacol 51: 313–318.
- Panthong A, Norkaew P, Kanjanaphoti D, Taesotikul T, Ananthachoke N, Reutrakul V (2007): Anti-inflammatory, analgesic and antipyretic activities of the extract gamboche from Garcinia hanburnyi Hook f. J Ethnopharmacol 111: 335–340.
- Pieretti S, Piaz VD, Matucci R, Giovannoni MP, Galli A (1999): Antinociceptive activity of a 3(2H)-pyridazinone derivative in mice. Life Sci 65: 1381–1394.
- Sawadogo WR, Meda A, Lamien CE, Kiendrebeogo M, Guissou IP, Nacoulma OG (2006): Phenolic content and antioxidant activity of six acanthaceae from Burkina Faso. J Biol Sci 6: 249–252.
- Shigemori H, Kagata T, Ishiyama H, Morah F, Ohsaki A, Kobayash J (2002): Nucleamides A-E, new monoterpene indole alkaloids from Nauclea latifolia. Chem Pharm Bull 51: 58–61.
- Sperber EF, Wurpel JN, Zhao DY, Moshe SL (1989): Evidence for the involvement of nigral GABAA receptors in seizures of adult rats. Brain Res 480: 378–382.
- Stai HY, Chen YF, Wu TS (1995): Anti-inflammatory and analgesic activities of extract from roots of Angelica pubescens. Planta Med 61: 1–8.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: An evaluation of the method. Pain 51: 5–17.
- Trease GE, Evans WC (1983): Pharmacognosy. London: Ballière Tindall.
- Twaij HAA, Kery A, Al Khazraji NK (1983). Some pharmacological, toxicological and phytochemical investigations on Centaurea phyllocephala. J Ethnopharmacol 9: 299–314.
- USNRC (1996): Guide for the Care and Use of Laboratory Animals, US National Research Council, Washington, D.C., USA.
- Viswanatha SAHM, Thippeswam MDV, Mahendra KCB (2006): Some neuropharmacological effects of methanolic root extract of Cissus quadrangularis in mice. Afr J Biomed Res 9: 64–75.
- Vogel HG, Vogel WH (1997): Analgesic, anti-inflammatory and antipyretic activity, in: Drug Discovery and Evaluation: Pharmacological Assays. Berlin, Heidelderg, Springer, pp. 360–418.
- Wagner H, Ott S, Jurcic K, Morton J, Neszmelyi A (1983): Chemistry, 13C NMR study and pharmacology of two saponins from Colubrina asiatica. Planta Med 48: 136–141.
- Woolf CJ, Mitchell D, Barrett GD (1980): Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain 8: 237–252.
- Yaksh TL, Rudy TA (1976): Analgesia mediated by a direct spinal action of narcotics. Science 192: 1357–1358.
