Abstract
Context: Scientific information on antioxidant properties and phenolic content of less widely used plants can be useful. Therefore, the assessment of such properties remains an interesting and useful task, particularly for finding new sources for natural antioxidants, functional foods, and nutraceuticals.
Objective: As knowledge about antioxidant properties and phenolic content of many plant species used as traditional plant remedies is limited, we determined in vitro the total antioxidant activity and the phenolic content of several plant species traditionally used for ethnoveterinary practices.
Materials and methods: For 24 extracts (70% acetone) from wild and cultivated plant species traditionally used for health care of animals we determined the Trolox equivalent antioxidant capacity (TEAC) by the two assays 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS). The phenolic content was determined by the Folin Ciocalteu method.
Results: Total phenolics, calculated as gallic acid equivalent (GAE), showed variation ranging from 3.18 (Allium sativum L. (Liliaceae)) to 147.68 (Pistacia lentiscus L. (Anacardiaceae)) mgGAE/g dry weight (DW). High TEAC values corresponded to high phenolic content, while plants with low antioxidant activity exhibited low total phenolic content. The TEAC determined through each assay and total phenolic content were positively correlated, R2 = 0.9152 and R2 = 0.8896, respectively, for DPPH and ABTS assay.
Discussion and conclusion: These findings suggest that phenolic content could be used as an indicator of antioxidant properties. The results of this study encourage investigations on Mediterranean plant species as sources of antioxidants.
Introduction
Medicinal plants constitute a major source of new pharmaceuticals and health care products, the attention paid to “natural medicine” should also focus on medicinal plants traditionally used to treat animals. In literature, the information on plant remedies is concerned principally with human therapy (CitationIvanova et al., 2005; CitationDjeridane et al., 2006; CitationKiselova et al., 2006). Information on the status of folk veterinary knowledge was gathered by CitationViegi et al. (2003) and CitationPieroni et al. (2004) in Italy, and by CitationBonet and Vallès (2007) in Catalonia.
Plant secondary metabolites such as polyphenols, play an important role in the defense against free radicals. Medicinal plant parts (roots, leaves, stems, flowers and fruits) are commonly rich in phenolic compounds, such as flavonoids, tannins, stilbenes, coumarins, lignans (CitationCai et al., 2004). The antioxidant properties of polyphenols are due to their redox properties, which allow them to act as reducing agents, hydrogen donators, metal chelators and single oxygen quenchers. Polyphenolics exhibit a wide range of biological effects including antibacterial, anti-inflammatory, antiallergic, hepato-protective, antithrombotic, antiviral, anticarcinogenic and vasodilatory actions; many of these biological functions have been attributed to their free radical scavenging and antioxidant activity (CitationSoobrattee et al., 2005). In view of these potential health benefits, there has been intensive research on natural antioxidants derived from plants.
Scientific information on antioxidant properties of various plants, particularly those that are less widely used in culinary and ethnoveterinarian situations is still rather scarce. Therefore, the assessment of such properties remains an interesting and useful task, particularly for finding new sources for natural antioxidants, functional foods and nutraceuticals. Several different methods are available and have been used to assess the total antioxidant capacity of plant extracts such as the 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) assay (CitationCai et al., 2004), the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (CitationJadhav & Bhutani, 2002), the oxygen radical absorbance capacity (ORAC) assay (CitationCao et al., 1993), the ferric reducing antioxidant power (FRAP) assay (CitationBenzie & Strain, 1996). Previous studies (CitationJadhav & Bhutani, 2002; CitationAuddy et al., 2003; CitationCai et al., 2004) used only one assay method to assess the total antioxidant capacity of plant extracts. However, CitationSchlesier et al. (2002) recommended that at least two methods should be used. In the present study we evaluated the total antioxidant capacities of several species traditionally used for natural health care of animals, using both ABTS and DPPH assays. CitationSurveswaran et al. (2007) indicate such methods as most appropriate to determine antioxidant capacities. Although the Mediterranean flora is remarkable for its diversity and it is a rich source of medicinal plants (CitationDjeridane et al., 2006, Citation2007; CitationBullitta et al., 2007; CitationGonzáles-Tejero et al., 2008), further knowledge is needed about content of polyphenolics, flavonoids, condensed tannins and antioxidant properties of Mediterranean plant species. For this reason we determined the antioxidant capacity, total phenolics (TotP), non-tannic phenolics (NTP), extractable condensed tannins (CT), total flavonoids (TotF) and their relationship with antioxidant capacities in 24 plant species traditionally used for ethnoveterinary practice. The species were selected according to the traditional veterinary practices inventoried by CitationBullitta et al. (2007) through the diffusion of questionnaires in animal breeding farms of rural areas in Sardinia.
Materials and methods
Sample collection
A total of 24 plant species (), recorded in a previous ethnoveterinary study as traditionally used for ethnoveterinary practices (CitationBullitta et al., 2007) were collected in north Sardinian natural pasturelands during spring 2006, only Allium cepa L. (Liliaceae) and Allium sativum L. (Liliaceae) were purchased in a market. The plant growth environment is typically Mediterranean. The rainfall distribution shows a maximum value in winter and a minimum in summer, while temperatures show the opposite trend so that a great water deficit for plants, lasting three to five months, can occur during summer and autumn. About 80% of the total rainfall occurs between October and May. The yearly amount of rainfall is not uniform with lower rainfall in coastal areas (average 350 mm) compared to mountains (average 1000 mm) (CitationBullitta, 1989). Site soil classifications of the different areas of plant collections according to USDA Soil Taxonomy are: Rock outcrop, Lithic Xerorthents; Typic, Vertic and Lithic Xerochrepts, Typic and Lithic Xerorthents. Land capability classes are VIII-VII-VI-V-IV. Land use constraints are high rockiness and stoniness, shallow soils, excess coarse fragments, impermeable substratum, slow drainage, severe erosion hazard (CitationAru et al., 1991). The correct taxonomic identification of specimens was checked according to CitationPignatti (1982) and CitationConti et al. (2005), herbarium specimens [GPE (1 to 24)] were collected and stored at the CNR-ISPAAM in Sassari, Italy. Details on the plant species and their use for the different pathologies of domestic animals are given in . Plants were kept on ice during harvesting, freeze dried and ground to a fine powder for the chemical analysis.
Table 1. Species used as plant remedies on domestic animals (adapted from CitationBullitta et al., 2007).
Extract preparation
Powdered plant samples (50 mg) were treated with 2.5 mL acetone/water (7:3) mixture and shaken for 60 min. The samples were then centrifuged for 10 min at 1683 g in a Biofuge 17 RS Heraeus-Sepatech centrifuge and the supernatant was stored at 4°C until use for the following determinations.
ABTS assay
Antioxidant capacity assay was determined by means of the improved ABTS+ method (CitationSurveswaran et al., 2007; CitationCai et al., 2004). The ABTS radical cation (ABTS+) was prepared by the reaction of 7 mM ABTS and 2.45 mM potassium persulfate, after incubation at 23°C in the dark for 16 h. The ABTS+ solution was then diluted with 80% ethanol to obtain an absorbance of 0.7 ± 0.005 at 734 nm. The ABTS+ solution (3.9 mL; absorbance of 0.7 ± 0.005) was added to 0.1 mL sample and mixed thoroughly. The reaction mixture was allowed to stand at 23°C for 6 min and the absorbance at 734 nm was immediately recorded using an Ultrospec 1100 pro UV/visible spectrophotometer. Trolox, a water-soluble analogue of vitamin E, was used as the reference standard. Solutions (final concentration 0-15 µM) in 80% ethanol were prepared and assayed at the same conditions. The absorbance of the reaction samples was compared to that of the Trolox standard and the results were expressed in terms of Trolox equivalent antioxidant capacity (TEAC), expressed as mmol Trolox equivalents per 100 g dry weight of plant material (mmol TEAC/100 g DW).
DPPH assay
The assay procedure was similar to the ABTS method described above. The DPPH radical (DPPH+) solution (60 µM) was prepared in 80% ethanol according to CitationCai et al. (2003). The same plant extracts diluted with 80% ethanol during the ABTS assay were used in the DPPH assay. The DPPH solution (3.9 mL; absorbance of 0.68 ± 0.005 at 515 nm) was added to 0.1 mL of the tested extracts. The reaction for scavenging DPPH+ radicals was carried out at room temperature in the dark for 120 min and then the reduction in absorbance was recorded at 515 nm using an Ultrospec 1100 pro UV/visible spectrophotometer. A calibrated Trolox standard curve was also made. The results were also expressed as TEAC units (mmol Trolox equivalents per 100 g dry weight of sample).
Total phenolic content
The concentration of total phenolics (TotP) was determined using spectrophotometric analysis with Folin Ciocalteau’s phenol reagent according to CitationSingleton and Rossi (1965). Appropriately diluted test samples, from 5 to 200 µL of extract were added to 1 mL of distilled water, followed by addition of 0.5 mL of Folin Ciocalteau 2 M solution and 2.5 mL of Na2CO3 (20%); the tube was properly shaken and then allowed to stand in darkness. A blue coloration was developed and after 45 min the absorbance was read at 750 nm using an Ultrospec 1100 pro UV/visible spectrophotometer. The amount of total polyphenols was calculated as a gallic acid equivalent from the calibration curve of gallic acid standard solution, and expressed as mg gallic acid equivalent/g dry weight of plant material (mg GAE/g DW).
Non-tannic phenolics
Non-tannic phenolics (NTP) were determined after precipitation of tannin components with polyvinylpolypyrrolidone (PVPP) according to CitationFAO/IAEA (2000). The amount of 0.1 g PVPP was suspended into 1 mL of water and then 1 mL of the extract was added. The solutions were vortexed, refrigerated for 15 min at 4°C and then centrifuged for 10 min at 1683 g. The quantity of 100 µL was added to 900 µL of distilled water and the phenolic content was determined by the Folin Ciocalteu method as described above. The amount of non-tannic phenolics was calculated as a gallic acid equivalent from the calibration curve of gallic acid standard solution, and expressed as mg gallic acid equivalent (GAE)/g dry weight of plant material (mg GAE/g DW).
Condensed tannins
Extractable condensed tannin (CT) determination was performed on an Ultrospec 1100 pro UV/visible spectrophotometer following the butanol-HCl-Fe3+ assay according to CitationPorter et al. (1986). A mixture of butanol-HCl (95:5 v/v) was added to the extracts and iron reagent (2% ferric ammonium sulfate in 2N HCl) was included as catalyzing agent. The mixture was incubated at 100°C in a water bath for 40 min, then cooled before reading the absorbance at 550 nm. The amount of condensed tannins was calculated as a delphinidin equivalent from the calibration curve of delphinidin standard solution and expressed as mg delphinidin equivalent/g dry weight of plant material (mg DE/g DW).
Flavonoids
Total flavonoids (TotF) were determined by colorimetric assay with the AlCl3 method reported by CitationKim et al. (2003). A 1 mL diluted sample was added to a 10 mL volumetric flask containing 4 mL of distilled water. At zero time, 0.3 mL of 5% NaNO2 was added to the flask, after waiting 5 min, 0.3 mL of 10% AlCl3 was added. Then, after 6 min, 2 mL of 1 M NaOH was added to the mixture. Immediately, the solution was made up to 10 mL with 2.4 mL of distilled water and mixed thoroughly. Absorbance of the mixture was determined at 510 nm using an Ultrospec 1100 pro UV/visible spectrophotometer. Catechin was used to make the calibration curve and the flavonoid content was expressed as mg catechin equivalent/g dry weight of plant material (mg CE/g DW).
Statistical analysis
For each sample, the value of antioxidant capacity by ABTS and DPPH methods, content of TotP, NTP, CT and TotF were calculated as the mean of three measurements. Results are presented as mean ± standard deviations (SD). Correlation coefficient (R) and coefficient of determination (R2) were calculated using Microsoft Excel 2000.
Results
The amount of phenolics and the antioxidant activities detected by means of the two in vitro assays (ABTS, DPPH) on the 24 plant species traditionally used as remedies in ethnoveterinary practices are shown in .
Table 2. Total antioxidant capacity (TEAC) by ABTS and DPPH methods, total phenolics (TotP), non-tannic phenolics (NTP), total flavonoids (TotF) and extractable condensed tannins (CT) of the examined plant species (mean ± standard deviation).
ABTS assay exhibited an extremely large variation of antioxidant capacities among species, from 0.74 (Allium cepa) to 131.14 (Pistacia lentiscus L. (Anacardiaceae)) mmol TEAC/100 g DW. The total antioxidant capacity determined through the DPPH assay also showed a wide variation from 0.39 (Allium cepa) to 164.37 (Pistacia lentiscus) mmol TEAC/100 g DW. High antioxidant capacities were found in the same set of species in both assays. The species Cistus creticus L. subsp. eriocephalus (Viv.) Greuter & Bourdet (Cistaceae), P. lentiscus, Euphorbia characias L. (Euphorbiaceae), Prunus spinosa L. subsp. spinosa (Rosaceae), Smilax aspera L. (Liliaceae), Umbilicus rupestris S.D. (Crassulaceae) and Pteridium aquilinum L. Kum (Hypolepidaceae), showing high antioxidant properties and high total phenolic content, were traditionally used to treat animal skin diseases and or gastrointestinal disorders.
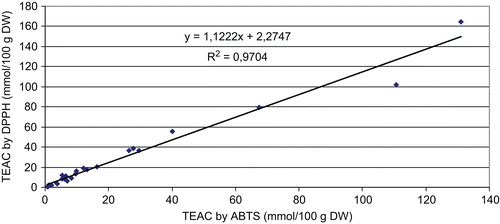
To evaluate the suitability and reliability of the ABTS and DPPH assay methods, linear regression of the values of total antioxidant capacity obtained through the two methods was performed. shows a highly significant linear correlation (R2 = 0.9704 and R = 0.9851) between the total antioxidant capacities evaluated by ABTS and DPPH assays of the samples.
Figure 1. Linear regression between the total antioxidant capacities (TEAC, mmol/100 g DW) determined by ABTS and DPPH assays.
TotP content () showed variation ranging from 3.18 to 147.68 mg GAE/g DW respectively in Allium sativum bulbs and Pistacia lentiscus leaves.
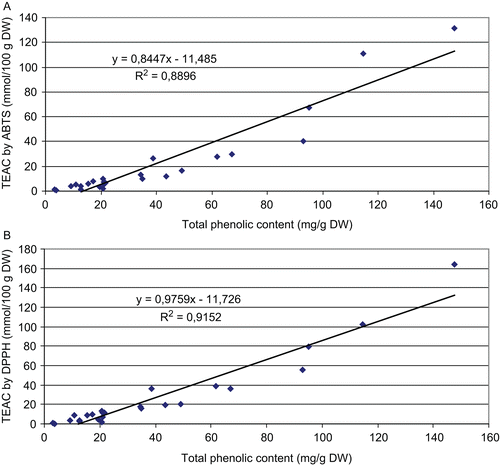
High TEAC values of plants corresponded to a high TotP content and plants showing low TEAC values exhibited relatively low TotP content (). The total antioxidant capacity determined through ABTS assay and TotP was significantly correlated (R2 = 0.8896 and R = 0.9431) (). Significant correlations were also found between the total antioxidant capacity assayed by DPPH method and TotP (R2 = 0.9152 and R = 0.9566) ().
Figure 2. Linear regression between total phenolic content (mg gallic acid/g DW) and antioxidant capacity by ABTS assay (A) and DPPH assay (B).
NTP () showed variation ranging from 3 (Allium sativum) to 117.99 (Pistacia lentiscus) mg GAE/g DW.
TotF () showed variation ranging from 2.62 (Opuntia ficus indica L. Miller (Cactaceae)) up to 50.51 (Prunus spinosa) mg CE/gDW. P. spinosa and other species showing higher values of TotF, Smilax aspera (47.27) and Olea europea L. var. europaea (Oleaceae) (42.93) mg CE/g DW, were used for treatment of skin diseases on all domestic animals (sheep, goats, cattle, horses, pigs, cats and dogs).
CT () were found only in Cistus creticus subsp. eriocephalus, Euphorbia characias, Parietaria officinalis L. (Urticaceae), Pistacia lentiscus, Prunus spinosa, Pteridium aquilinum, Smilax aspera, Umbilicus rupestris S.D. (Crassulaceae), all of them were used to treat skin diseases and/or gastrointestinal disorders in all domestic animals. The species Charybdis maritima L. Speta (Liliaceae) had no therapeutical uses but was indicated as a natural rat repellent.
The correlations (R and R2) between the antioxidant activity revealed by the two assays (ABTS and DPPH) and TotP, NTP, TotF and CT are represented in . While TotP content showed highly significant linear correlation to antioxidant activity (R2 = 0.9704 and R= 0.9851), no significant correlations were found among the antioxidant activity and NTP, TotF, CT. It indicates that besides flavonoids and condensed tannins, there are other phytochemical compounds which contribute to the antioxidant activity of these plants.
Table 3. Correlations (R and R2) between antioxidant capacity of plant extracts by (ABTS and DPPH assays) and total phenolics (TotP), Non-tannic phenolics (NTP), total flavonoids (TotF), extractable condensed tannins (CT)
Discussion
Plants listed in , indicated by farmers as folk remedies for the treatment and prophylaxis of different disorders in domestic animals (CitationBullitta et al., 2007), have been studied for their antioxidant activity and polyphenolic content. Often in literature, the protection against different disorders has been attributed to plant antioxidants such as polyphenols and vitamins and potential sources of antioxidant compounds have been searched in many types of plant materials (CitationIvanova et al., 2005). In our study the most represented families are Liliaceae with four species, Compositae, Oleaceae and Urticaceae with two species each. Species belonging to such families have a common feature of phenolic content lower than 50 mg GAE/g DW and antioxidant capacity lower than 21 mmol TEAC/100 g DW, except for Smilax aspera showing higher antioxidant capacity and phenolic content. In a preliminary inventory of traditional ethnoveterinary practices (CitationBullitta et al. 2007), the genus Allium and the genus Olea with two species each, were reported as the most used, but no remarkable phenolic content and antioxidant capacity were found for such species in the present study.
In agreement with other researchers (CitationCai et al., 2004; CitationShan et al., 2005; CitationSurveswaran et al., 2007) the ABTS assay was used, because it is rapid, robust and accurate for systematically assessing total antioxidant capacity of extracts from plant materials on a large scale. However, the recommendation by CitationSchlesier et al. (2002), to use at least two methods was also taken into account. The ABTS and DPPH assays were also chosen because they are considered by CitationSurveswaran et al. (2007) more accurate and reliable than FRAP assay for assessing total antioxidant capacities of plant extracts.
CitationDjeridane et al. (2007) found in P. lentiscus a TotP value of 23.5 mg GAE/g DW, but their analysis was performed on air dried aerial parts while our analyses were performed on freeze dried leaves. Several plant remedies were traditionally obtained from P. lentiscus, while Malva sylvestris L. (Malvaceae), Matricaria chamomilla L. (Compositae), Parietaria officinalis L. (Urticaceae), also indicated for the preparation of several traditional plant remedies, showed low TEAC and TotP. These results suggest that other phytochemicals might contribute to the beneficial effects of such species reported by farmers and listed by CitationBullitta et al. (2007). Leaves of Prunus spinosa, traditionally indicated to treat wounds infected by worms, showed a good TEAC and the presence of flavonoids and an appropriate level of condensed tannins (2-5% DM) (CitationFrutos et al., 2002), suggesting also beneficial effects as feed to improve animal performances.
The TotP content in leaves of Urtica dioica L. (Urticaceae) was 9.24 mg GAE/g DW, not dissimilar from the value of 6.9 mg GAE/100g DW found by CitationProestos et al. (2006). Opuntia ficus indica cladophylls revealed a very low TEAC 1.08 (ABTS) and 2.35 (DPPH) mmol TEAC/100 g DW, while other researchers found a marked antioxidant activity in the methanolic fruit extracts (CitationButera et al., 2002).
High TEAC values of plants corresponded to a high TotP content and plants showing low TEAC values exhibited relatively low TotP content (). Other studies showed that phenolic compounds are a major antioxidant constituent in selected herbs, vegetables and fruits, and there are direct relationships between their antioxidant activity and total phenolic content (CitationDorman et al., 2004; CitationSurveswaran et al., 2007).
The highly significant correlations obtained in this study support the hypothesis that phenolic compounds contribute significantly to the total antioxidant capacity of the examined plants species, in accordance to the findings of CitationCai et al. (2004) and CitationDjeridane et al. (2006) who found a linear correlation between the content of total phenolic compounds and antioxidant capacity of several plant species.
P. spinosa (39.49 mg DE/g DW) and P. lentiscus (29.98 mg DE/g DW), revealed a CT content within the range considered by CitationFrutos et al. (2002) beneficial for protein metabolism in ruminants, to favor a decreasing in the rumen degradation of dietary proteins and an increasing absorption of amino acids in the small intestine of cattle and sheep.
The different relationships between the antioxidant activity and the total phenolic content can be due to many factors; in fact the total phenolic content does not incorporate all the antioxidants. Also, it must be taken into account the synergism between the antioxidants in the mixture that makes the antioxidant activity not only dependent on the concentration, but also on the structure and the interaction between the antioxidants. This can explain why samples such as Artemisia arborescens L. (Compositae), Hedera helix L. (Araliaceae), Urtica dioica, with similar concentration of total phenolics, vary in their antioxidant activities (TEAC assay). Similarly, Pteridium aquilinum (whole plant) and Umbilicus rupestris which had different total phenolic levels, exhibited a similar antioxidant activity, but it is worth considering that the antioxidant activity of plant extracts may be related to the presence of some individual active phenolic compounds.
Among the seven species traditionally used to treat animal skin diseases and/or gastrointestinal disorders, three have also similar uses for humans. Parietaria officinalis, Pistacia lentiscus and Umbilicus rupestris were in fact reported by CitationBullitta et al. (2007) as traditional remedies against diarrhea and wound disinfectants. Pistacia lentiscus will be further investigated considering its high antioxidant capacity, high total phenolic content, the uses to treat gastrointestinal disorders in all domestic animals and against skin diseases of animals and humans.
Conclusions
This study was aimed at testing whether some plant species used for traditional ethnoveterinary practices could be promising sources of natural antioxidants. The good linear correlations obtained between phenolic concentration and antioxidant capacity determined by the DPPH and ABTS assays suggest that phenolic content could be used as an indicator of antioxidant properties of the examined plant species. The results of this study may support the rediscovery of traditional plant species from the Mediterranean area and their uses as sources of antioxidants. The knowledge of traditional ethnoveterinary practices can be a source of useful information for the isolation of natural extracts to develop new products for natural health care and well-being of domestic animals. Further investigations for potential applications of new, natural antioxidants require anyway, elucidation of the chemical composition of phenolics and in vivo studies in order to better establish the functionality of the examined plant species.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aru A, Baldaccini P, Vacca A (1991). Nota illustrativa alla carta dei suoli della Sardegna. Cagliari, Italy: Stef.
- Auddy B, Ferreira M. Blasina F, Lafon L, Arrendondo F, Dajas F, Tripathy P, Seal T, Murkerjee B. (2003). Screening of antioxidant activity of three Indian medicinal plants traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol, 84, 131–138.
- Benzie IFF, Strain JJ. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem, 239, 70–76.
- Bonet MA, Vallès J. (2007). Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J Ethnopharmacol 110, 130–147.
- Bullitta P. (1989). Sardinia. In: Welcome to the Italian grasslands. XVI International Grassland Congress, Post Congress in Italy. October 12-18, 1989. Publication supported by Agricoltura Ricerca, 32–41.
- Bullitta S, Piluzza G, Viegi L. (2007). Plant resources used for traditional ethnoveterinary phytoterapy in Sardinia (Italy). Genet Resour Crop Evol 54, 1447–1464.
- Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pinto AM, Livrea A. (2002). Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extra properties of its betalains: Betanin and indicaxanthin. J Agric Food Chem 50, 6895–6901.
- Cao GH, Alessio HM, Buettner GR. (1993). Oxigen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med 14, 639–655.
- Cai YZ, Luo Q, Sun M, Corke H. (2003). Antioxidant activity of betalains from plants in the Amaranthaceae. J Agric Food Chem 51, 2288–2294.
- Cai YZ, Luo Q, Sun M, Corke H (2004). Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sci 74, 2157–2184.
- Conti F, Abate G, Alessandrini A, Blasi C. (2005). An Annotated Checklist of the Italian Vascular Flora. Rome: Palombi Editori.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem, 97, 654–660.
- Djeridane A, Yousfi M, Nadjemi B, Vidal N, Lesgards JF, Stocker P. (2007). Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur Food Res Technol, 224, 801–809.
- Dorman H, Bachmayer O, Kosar M, Hiltunen R. (2004). Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem, 52, 762–770.
- FAO/IAEA (2000). Quantification of tannins in tree foliage. Working Document. Vienna: IAEA.
- Frutos P, Hervas G, Ramos FJ, Giràldez FJ, Mantecon AR. (2002). Condensed tannin content of several shrub species from a mountain area in northern Spain, and its relationship to various indicators of nutritive value. Anim Feed Sci Technol, 95, 215–226.
- González-Tejero MR, Casares-Porcel M, Sánchez-Rojas CP, Ramiro-Gutiérrez JM, Molero-Mesa J, Pieroni A, Giusti, ME, Censorii E, de Pasquale C, Della A, Paraskeva-Hadijchambi D, Hadjichambis A, Houmani Z, El-Demerdash M, El-Zayat M, Hmamouchi M, ElJohrig S. (2008). Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J Ethnopharmacol, 116, 341–357.
- Ivanova I, Gerova D, Chervenkov T, Yankova T. (2005). Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol, 96, 145–150.
- Jadhav HR, Bhutani KK. (2002). Antioxidant properties of Indian medicinal plants. Phytother Res, 16, 771–773.
- Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. (2003). Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem, 51, 6509–6515.
- Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. (2006). Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother Res, 20, 961–965.
- Pieroni A, Howard P, Volpato G, Santoro RF. (2004). Natural remedies and nutraceuticals used in ethnoveterinary practices in inland southern Italy. Vet Res Commun, 28, 55–80.
- Pignatti S (1982). Flora d’Italia. Vol. 1-3. Bologna, Edagricole.
- Porter LJ, Hristich LN, Chan BG. (1986). The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry, 25, 223–230.
- Proestos C, Boziaris IS, Nychas GJE, Komaitis M. (2006). Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem, 95, 664–671.
- Schlesier K, Harwat M, Bohm V, Bitsch R. (2002). Assessment of antioxidant activity by using different in vitro methods. Free Rad Res, 36, 177–187.
- Shan B, Cai YZ, Sun M, Corke H. (2005). Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem, 53, 7749–7759.
- Singleton VL, Rossi JA. (1965). Colorimetry of total phenolics with phosphor-molybdic-phosphotungstic acid reagent. Am J Enol Vit, 16, 144–158.
- Soobrattee M., Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorum T. (2005). Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mut Res, 579, 200–213.
- Surveswaran S, Cai Y, Corke H, Sun M. (2007). Systematic evaluation of natural phenolic antioxidant from 133 Indian medicinal plants. Food Chem, 102, 938–953.
- Viegi L, Pieroni A, Guarrera PM, Vangelisti R. (2003). A review of plants used in folk veterinary medicine in Italy as basis for a databank. J Ethnopharmacol, 89, 221–244.
