Abstract
Context: Terminalia chebula Retz. (Combretaceae) is a medium-sized tree that grows in the wild throughout India. T. chebula has been extensively used in Ayurveda, Unani, and homoeopathic medicine. The fruit has been used as a traditional medicine for a household remedy against various human ailments. Traditionally T. chebula is used to cure chronic ulcer, gastritis, and stomach cancers.
Objective: The present study is to evaluate the antiulcer effect of hydroalcoholic (70%) extract of Terminalia chebula fruit.
Materials and methods: Aspirin, ethanol and cold restraint stress-induced ulcer methods in rats were used for the study. The effects of the extract on gastric secretions, pH, total and free acidity using pylorus ligated methods were also evaluated.
Results: Animals pretreated with doses of 200 and 500 mg/kg hydroalcoholic extract showed significant reduction in lesion index, total affected area and percentage of lesion in comparison with control group (P < 0.05 and P < 0.01) in the aspirin, ethanol and cold restraint stress-induced ulcer models. Similarly extracts increased mucus production in aspirin and ethanol-induced ulcer models. At doses of 200 and 500 mg/kg of T. chebula extract showed antisecretory activity in pylorus ligated model, which lead to a reduction in the gastric juice volume, free acidity, total acidity, and significantly increased gastric pH.
Discussion and conclusion: These findings indicate that hydroalcoholic extract of the fruit T. chebula displays potential antiulcerogenic activity. This activity thus lends pharmacological credence to the suggested use of the plant as a natural remedy in the treatment or management of ulcer.
Introduction
Gastric hyperacidity and gastroduodenal ulcer is a very common global problem today. It is now generally agreed that gastric lesions develop when the delicate balance between some gastroprotective and aggressive factors are lost (CitationGeorge Susan et al., 1999). Major aggressive factors are acid, pepsin, Helicobacter pylori, and bile salts. Defensive factors mainly involve mucus-bicarbonate secretion and prostaglandins. Hypersecretion of gastric acid is a pathological condition which occurs due to uncontrolled secretion of hydrochloric acid from the parietal cells of the gastric mucosa through the proton pumping H+-K+-ATPase (CitationSachs et al., 1995). Even the normal rate of acid secretion may cause ulceration in the breached mucosa when some gastroprotective factors are lost. The modern approach to control gastric ulceration is to inhibit gastric acid secretion, to promote gastroprotection, block apoptosis, and stimulate epithelial cell proliferation for effective healing (CitationWalt, 1992). Most of the antisecretory drugs such as proton pump inhibitors (omeprozole, lansoprazole, etc.) and histamine H2 receptor blocker (ranitidine, famotidine, etc.) are extensively used to control increased acid secretion and acid related disorders caused by stress, NSAIDs, and H. pylori; but there are reports of adverse effects and relapse (CitationYeomans & Tulassy, 1998). On the contrary most of the herbal drugs reduce the offensive factors and are proved to be safe and clinically effective, having better patient tolerance, relatively less expensive and globally competitive. Plant extracts are some of the most attractive sources of new drugs and have been shown to produce promising results in the treatment of gastric ulcers (CitationJainu & Devi, 2006).
Terminalia chebula Retz. (Combretaceae), known as “abhaya” (Sanskrit), “harahra” (Hindi), and “allalekai” (Kannada), is a native of the Indian subcontinent and the adjacent areas such as Pakistan, Nepal, southwestern China, and stretching as far south as Kerala or even Sri Lanka where it is called “aralu”. Fruits of T. chebula are a rich source of gallic acid-based secondary metabolites (20–36%). Major constituents are chebulagic acid, chebulinic acid, and chebulic acid; other constituents are tannic acid, gallic acid, ethyl gallate, ellagic acid, chebulanin, corilagin, and terflavin. Other classes of compounds identified in the fruits are: shikimic acid and related compounds (quinnic acid, dihydroshikimic acid, 5-dehydroshikimic acid), sugar (arabinose, fructose, sucrose), triterpenoids (chebupentol, terminoic acid, arjugenin), and steroids (β-sitosterol, daucosterol) (CitationChattopadhyay & Bhattacharyya, 2007).
T. chebula is used to cure chronic ulcer, carious teeth pain, heart problems (CitationIshtiaq et al., 2007). T. chebula possesses laxative (CitationMiglani et al., 1971), hypolipidemic (CitationShaila & Udup, 1998), antioxidant (CitationLee et al., 2005, Citation2007), hepatoprotectant (CitationTasduq et al., 2006), antiviral (CitationJeong et al., 2002), and antibacterial (CitationSato et al., 1997) activities. The plant is an important ingredient of Triphala™, a popular herbal formulation used as a traditional medicine for chronic disorders such as diabetes (CitationDeb, 2006). One group of researchers found that it is effective in inhibiting the urease activity of Helicobacter pylori (CitationMalckzadeh et al., 2002) and the ubiquitous bacterium implicated in the development of gastritis, ulcers, and stomach cancers.
However, detailed investigations of the antiulcer activity of T. chebula have not been carried out so far. Hence this led us to the study the antiulcer activity of T. chebula in different ulcer-induced models.
Materials and methods
Plant material
The fruit of Terminalia chebula was collected from Nagaon District, Assam, India in the month of May 2007. The plant was authenticated by N. Shiddamallayya, Department of Botany, Regional Research Institute (RRI), Bangalore, India. A voucher specimen of the fruit (RRI/BNG/SMP/Drug Authentication/2007-08, 286) has been deposited in the Department of Pharmacology.
Preparation of extract
The fruits of T. chebula were shade dried and coarsely powdered. The powdered plant material was then subjected to successive extraction with petroleum ether, chloroform and 70% hydroalcohol solvents (500 mL/100 g of dried powder) for 18 h in a Soxhlet extractor. After extraction, the dark green solution obtained was evaporated at 45°C under reduced pressure till a viscous mass material was obtained. The yield of the petroleum, chloroform and hydroalcoholic extracts were found to be 5%, 2.5%, and 32.5% (w/w), respectively. The dried hydroalcoholic extract was stored in an airtight container and placed in a refrigerator. The hydroalcoholic extract was used for the experimental study.
Animals
Wistar albino rats (150-250 g) and albino mice (25–30 g) of either sex were used in this study. The animals were acclimatized for 10 days under standard husbandry conditions as: room temperature 26° ± 2°C; relative humidity 45–55%, and 12 h light and dark cycle. They were allowed free access to standard diet (Pranav Agro Industries, Sangli) and water ad libitum, one week before and during experimental period. The experimental protocol and procedures used in this study were approved by the Institutional Animal Ethics committee (Ref no. IAEC/PP/18/2007-08) and CPCSEA (Reg no. 97/c/06/CPCSEA) and conformed to the guidelines of care and use of animals in research and teaching.
Chemicals and drugs
Anesthetic ether, petroleum ether, chloroform, sodium hydroxide and concentrated hydrochloric acid were procured from Merck, KFC, Bangalore, India. Oxalic acid, Toffer’s reagent and phenol reagent were obtained from Nice Chemicals, Cochin, India. Alcohol (90%) was procured from Gauri Industries, Mandya, India. A gift sample Omeprozole (OMZ) was obtained from Medly Pharmaceuticals, Daman, India and all other chemicals were of high purity and obtained locally.
Phytochemical analysis of the extract
The preliminary phytochemical screening was carried out on the petroleum ether, chloroform, and hydroalcoholic extracts of the fruits of T. chebula for qualitative identification by the standard method described by CitationKhandelwal (2000).
Acute toxicity study
Healthy adult female Swiss albino mice were used for the toxicity study. An acute oral toxicity study was carried out using the fixed-dose method according to the Organization for Economic Cooperation and Development (CitationOECD, 1993) guideline no. 401. The animals were fasted overnight prior to the experimentation. They were continuously observed for 24 h for changes in autonomic or behavioral responses, while mortality was observed in each group for 7 days.
Aspirin induced ulcer
The experiment was performed according to the method of CitationMalairajan et al. (2007). After 12 h of fasting, the rats were randomly divided into four groups of six animals each. Group I represented the control group, and received distilled water orally. Groups II and III received the extract of T. chebula (200 and 500 mg/kg, orally). OMZ (20 mg/kg, orally) was administered to group IV as a reference drug. After 45 min of the treatment, all rats received aspirin (500 mg/kg, orally) to induce gastric ulcer. Four hours later the animals were sacrificed by anesthetic ether (by inhalation, 0.5 mL taken in a pinch of surgical cotton wool). The stomachs were removed, and opened along the greater curvature, and were gently rinsed with distilled water to remove gastric contents and blood before scoring the ulcer. Damage to the mucosa was examined under microscope and ulcer was scored as follows: red coloration (0.5), spot ulcer (1), hemorrhagic streak (1.5), ulcers (2), perforation (3). The mean ulcer score for each animal was expressed as the ulcer index. The percentage of ulcer inhibition was calculated as mean ulcer index of control-mean ulcer index of test/mean ulcer index of control ×100.
The glandular portion of the stomach was carefully separated from the luminal part and the mucus content was estimated. The result is expressed as μg/g wet glandular tissue (CitationMizui & Doteuchi, 1988).
Ethanol induced ulcers
The experiment was performed according to the method of CitationMorimoto et al. (1991). After 12 h of fasting, the rats were randomly divided into four groups of six animals each. Group I represented the control group, which received distilled water orally, and groups II and III were treated with the extract (200 and 500 mg/kg, p.o.). OMZ (20 mg/kg) was administered orally for group IV as a reference drug. One hour after treatment, all rats received 1 mL/200 g of 90% ethanol to induce gastric ulcer. After 1 h, animals were sacrificed by anesthetic ether, the stomachs were removed and opened along with greater curvature and the ulcer index scored. The mucus content was estimated and percentage of ulcer protection was determined.
Cold restraint stress induced ulcers
The procedure was as described for aspirin- and ethanol-induced ulcer models. Animals of different groups were subjected to cold stress 45 min after administration of the extract and OMZ. The rats were deprived of food, but not water, for about 18 h before the experiment. The rats were immobilized by strapping the fore and hind limbs in a restraint cage and kept for 2 h, at a temperature of 4°C (CitationJainu & Devi, 2006). After 2 h, animals were sacrificed by exsanguination after light ether inhalation anesthesia, the stomach was removed and incised along the greater curvature. Ulceration was examined and scored similar to the aspirin- and ethanol-induced ulcer models.
Pylorus ligation induced ulcers
Adult rats were divided into four groups of six animals each and subjected to fasting for 24 h; care was taken to avoid coprophagy. Group I received distilled water orally and served as control. Animals in groups II and III were administered extract of T. chebula orally at dose of 200 and 500 mg/kg respectively. Group IV received OMZ (20 mg/kg, p.o.) as a reference drug. After 1 h of vehicle or extract treatment, the animals were anesthetized using thiopentone sodium (35 mg/kg, i.p.), the abdomen was opened and pylorus ligation was done without causing any damage to blood vessels. The stomach was replaced carefully and the abdominal wall was closed in two layers with interrupted sutures. The animal was deprived of water during the post-operative period. After 4 h the stomach from each animal was dissected and its contents collected into a tube to determine the volume of gastric juice, pH, total and free acidity (CitationShay et al., 1945). The ulcers were scored as described under aspirin (ASP)-induced ulcers.
The gastric juice was collected after 4 h of pylorus ligation and centrifuged for 5 min at 2000 rpm. The supernatant was collected and the volume of gastric juice was expressed as mL/100 g body weight. Total acidity was determined in the supernatant by titrating against 0.1N NaOH, using 2–3 drops of Topfer’s reagent as indicator until canary yellow color was observed. The volume of NaOH required was noted and this corresponded to free acidity. A further 2–3 drops of phenolphthalein was added and titrated with 0.1 N NaOH until pink color was restored and this gave total acidity. Free acidity and total acidity was expressed in terms of 0.1 N HCl per 100 g of gastric contents.
Statistical analysis
The data are represented as mean ± SEM, and statistical significance between treated and control groups was analyzed using ANOVA, followed by Dunnett’s t-test where P < 0.05 was considered statistically significant.
Results
Preliminary phytochemical screening revealed that petroleum ether extract showed positive for phytosterols and fixed oils. The chloroform extract showed positive for the tannins. The hydroalcoholic extract showed positive for the presence of carbohydrates, glycosides, triterpenoids, saponins, tannins, polyphenols, proteins, amino acids and flavonoids. In the acute toxicity study, the animals were treated orally with a high dose of the extract (2000 mg/kg) which did not manifest any significant abnormal signs, changes, or behavioral changes such as body temperature, CNS activity, micturition or defecation when observed for 24 h, except that the animals were lethargic. There was no mortality observed 7 days after administration of the extract at the above-mentioned dose.
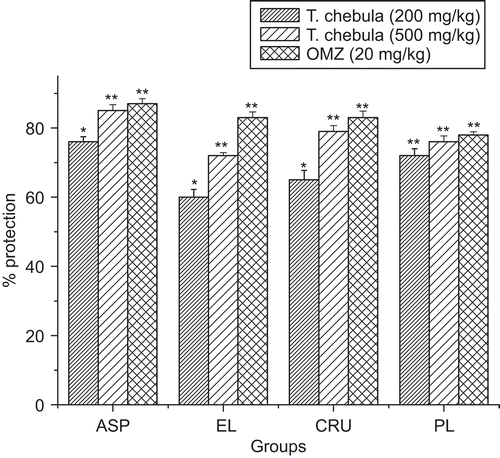
In the aspirin-induced ulcer model, T. chebula extract and OMZ significantly reduced gastric mucosa lesion, compared with the control group (). The percentage inhibition of ulcers was 76% and 85% (200 and 500 mg/kg), the magnitude of which at 500 mg/kg was comparable to 87% caused by OMZ (reference standard drug) at 20 mg/kg (). Animals which received 200 and 500 mg/kg of T. chebula showed significant (P < 0.01 and P < 0.05) increase of mean mucus content when compared to the vehicle control group (). The extract also significantly reduced gastric mucosa lesion compared with the control group in the ethanol-induced ulcer. The percentage of inhibition of mucosa lesion was 60% and 72% for groups treated with (200 and 500 mg/kg of extract) compared to 83% caused by OMZ (20 mg/kg). The mean mucus content was significantly (P < 0.05 and P < 0.01) increased in the extract- and OMZ-treated groups (). In the cold restraint stress-induced ulcer model, T. chebula and OMZ caused a significant (P < 0.01) reduction in gastric mucosa lesion compared with the control group (). The percentage inhibition of gastric mucosa lesion was 65% and 79% (200 and 500 mg/kg of T. chebula) and 83% (20 mg/kg of OMZ) (). T. chebula extract (200 and 500 mg/kg) and OMZ (20 mg/kg) significantly reduced the volume of gastric juice, free acidity, total acidity and also significantly increased gastric pH compared to the control group in pylorus ligated-induced ulcer ().
Table 1. Effect of hydroalcoholic extract of T. chebula on ulcer index score against aspirin-, ethanol- and cold restraint stress-induced gastric ulcer in rats.
Table 2. Effect of hydroalcoholic extract of T. chebula on mucus content against aspirin- and ethanol-induced gastric ulcer in rats.
Table 3. Effect of hydroalcoholic extract of T. chebula on gastric secretion, acidity, pH and ulcer score in pylorus-ligated rats.
Figure 1. Effect of hydroalcoholic extract of T. chebula on percentage protection of ulcer index in different ulcer models. Results are mean ± SEM (n = 6). Statistical comparison was performed by using ANOVA followed by Dunnett’s t-test.*p < 0.05, **p < 0.01were consider statistically significant compared with control group.
Discussion
Ulcers are caused due to imbalance between aggressive and defensive factors of the gastric mucosa (CitationFrancesca & Angelo, 2000). Pepsin and gastric acid make up the offensive factors, whose proteolytic effect is buffered by mucin secretion, mucosal glycoprotein, cell shedding, cell proliferation and prostaglandins (CitationGoyal & Bhattacharya, 1999). Different therapeutic agents including plant extracts are used to inhibit the gastric acid secretion, or to stimulate the mucosal defense mechanism by increasing the mucus production protecting the surface epithelial cells or interfering with the prostaglandin synthesis (CitationGoyal & Sairam, 2002). Gastrointestinal injury is induced by various chemical agents (CitationDesai et al., 1996). Aspirin causes direct irritant effect and mucosal damage by interfering with prostaglandin synthesis (CitationVane, 1971), increasing acid secretion by increasing the H+ ion transport/back diffusion of H+ ions (CitationRao et al., 2000). The plant extract significantly protects the gastric mucosa against aspirin challenges as shown by reduced values of lesion index as compared to the control group suggesting its potent cytoprotective effect. This was further substantiated in gastric mucus content produced by T. chebula. Hydroalcoholic extract of T. chebula showed significant ability to reduce the severity of ulceration of the stomach induced by absolute ethanol. The incidence of ethanol-induced ulcers predominant in the glandular part of stomach was reported to stimulate the formation of leukotriene C4 (LTC4), mast cell secretory products (CitationBaggio et al., 2003) and reactive oxygen species (CitationSalim, 1990) resulting in the damage of rat gastric mucosa starts with microvascular injury, namely a disruption of the vascular endothelium resulting in increased vascular permeability, edema formation and epithelial lifting (CitationSzabo et al., 1985). In the ethanol model, ulcers are caused by perturbations of superficial epithelial cells, notably the mucosal mast cells leading to the release of the vasoactive mediators including histamine, thus causing damage to gastric mucosa. Mucosal blood flow has been attributed to be an important factor in the damage caused by alcohol and is modulated by prostaglandin. The effectiveness of hydroalcoholic extract of T. chebula protection against mucosal damage and increase in gastric mucus content caused by ethanol may be an indication of its effect on prostaglandin production (CitationHollannder et al., 1984). Animals subjected to restraint plus cold for 2 h showed the presence of considerable ulcers in the form of hemorrhagic mucosal lesions in the stomach, which were confined to the glandular segment only. Peripheral sympathetic activation plays an important role in induction of ulcers by restraint (CitationDjahanguiri et al., 1973). Stress-induced ulcer is probably mediated by histamine release with enhanced acid secretion, a reduction in mucous production and generation of free radicals, mast cell activation, alterations in prostaglandin generation, cytokine liberation and breakdown of normal cytoprotective mechanism (CitationMiller, 1987). Ulcers due to cold stress are both due to physiological and psychological factors (CitationStein et al., 1991). The gastroprotective action of hydroalcoholic extract of T. chebula against stress-induced ulceration could be due to its antihistaminic, anticholinergic and/or antisecretory effects.
Pylorus ligation in rats for 4 h, 45 min after oral administration of hydroalcoholic extract of T. chebula, resulted in accumulation of gastric secretions, increase in volume of titrable acid and ulceration. The cause of gastric ulcer after pyloric ligation is believed to be stress-induced increase in gastric hydrochloric acid secretion and/or stasis of acid (CitationGeorge Susan et al., 1999). Pylorus ligation-induced gastric ulcers occur because of an imbalance between aggressive factors and the maintenance of mucosal integrity through the endogenous defiance mechanisms (CitationPiper & Stiel, 1986). Ulcers caused by pyloric ligation are due to increased accumulation of gastric acid and pepsin leading to autodigestion of gastric mucosa and break down of the gastric mucosal barrier (CitationSairam et al., 2002). In the present study, hydroalcoholic extract of T. chebula reduced the volume of free acid by 21% and 24% at the dose levels of 200 and 500 mg/kg, respectively, while; standard drug OMZ (20 mg/kg) reduced the volume of free acid by 31%. The total acidity reduction by extract of T. chebula is 47% and 51% at the dose levels of 200 and 500 mg/kg respectively, whereas the standard reference drug OMZ (20 mg/kg) reduced the total acidity by 60%. CitationNadar and Pillai (1989) also reported that biochemical and histochemical studies revealed decreased β-glucuronidase activity in the Brunner’s gland of duodenal ulcerated rats. Rats treated with a mixture of Ayurvedic medicine (Glycyrrhiza glabra, Terminalia chebula, Piper longum, and Shanka bhasma) recovered faster with concomitant increase in β-blucuronidase activity in the Brunner’s glands. These Ayurvedic medicines improve the secretary status of Brunner’s glands involved in the protection against duodenal ulcer and β-glucuronidase enzyme activity showed gradual increase during recovery. T. chebula fruit exhibited antioxidant activity of different magnitudes of potency (CitationCheng et al., 2003; CitationLee et al., 2005). It has stronger antioxidant activity than α-tocopherols; HPLC analysis with diode array detection indicated the presence of hydroxybenzoic acid derivatives, hydroxycinnamic acid derivatives (chebulagic acid, chebulinic acid, and chebulic acid), flavonol aglycones and their glycosides, as main phenolic compounds (CitationSaleem & Ahotupa, 2001) and chebulic acid compound were isolated from the plant (CitationLee et al., 2007). Although the exact active principle responsible for the antiulcer activity of T. chebula could not be established in this study, it is likely that flavonoid compounds, hydroxybenzoic acid derivatives and flavonol aglycones and their glycosides present in the plant may be involved in this action, as flavonoids have been reported to possess significant antiulcer activity in various ulcer-induced methods (CitationIzzo et al., 1994; CitationParmar & Parmar 1998). Thus our study established a significant antiulcer and cytoprotective effect of T. chebula. However, further studies are required to establish its exact mode of action and active principle involved in its antiulcerogenic effect (CitationZsuzsa et al., 2001).
Acknowledgements
The authors would like to express their gratitude to Premanath Reddy, Chairman and Shalini Reddy, Secretary, Acharya Institute, Bangalore, India, for providing the necessary facilities and support to carry out the research work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baggio CH, Freitas CS, Reick L, Marques MCA. (2003). Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol Res 47:93–98.
- Chattopadhyay RR, Bhattacharyya SK. (2007). Plant review Terminalia chebula: An update. Pharmacog Rev 1:151–156.
- Cheng HY, Lin TC, Yu KH. (2003). Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull 26:1331–1335.
- Deb S. (2006). A Selection of Prime Ayurvedic Plant Drugs. New Delhi, India: Anamaya, 102–103.
- Desai JK, Goyal RK, Parmar NS. (1996). Dopamine receptor subtypes in gastric and duodenal ulceration. Indian J Pharmacol 28:129–142.
- Djahanguiri B, Taubin HL, Landsburg L. (1973). Increased sympathetic activity in the pathogenesis of restraint ulcer in rats. J Pharmaco Exp Therape 184:163–168.
- Francesca Borrelii, Angelo, A Izzo. (2000). The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14:581–591.
- George Susan Anuradha, Sathimoorthy, Sathimoorthy SS. (1999). Effect of alpha tocopherol on gastric ulcer induced by pylorus ligation in rats. Indian J Pharmcol 31:431–433.
- Goyal RK, Bhattacharya SK. (1999). Gastrointestinal mucosal defense and mucosal protective agents. Indian J Exp Biol 29:701–705.
- Goyal RK, Sairam K. (2002). Antiulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasma, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol 34:100–110.
- Hollannder D, Taranawski A, Gergely H, Zipsere KD. (1984). Sucralfate protection of the gastric mucosa against alcohol-induced injury: A prostaglandin-mediated process. Scand J Gastroenterol 101:97–102.
- Ishtiaq M, Hanif W, Khan MA, Ashraf M, Butt AM. (2007). An ethnomedicinal survey and documentation of important medicinal folklore food phytonims of flora of Samahni valley (Azad Kashmir), Pakistan. Pak J Biol Sci 10:2241–2256.
- Izzo AA, Di Carlo G, Mascolo N, Autore G, Capasso F. (1994). Antiulcer effect of flavonoids. Role of endogenous PAF. Phytother Res 6:179–181.
- Jainu M, Devi CSS. (2006). Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. J Ethnopharmacol 104:156–163.
- Jeong AHN, Kim CY, Lee JS, Kim TG, Kim SH, Lee CK, Lee B, Shim CG, Hoon H, Kim J. (2002). Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flovonol glycoside gallates from Euphorbia pekinensis. Planta Med 68:457–459.
- Khandelwal KR. (2000). Practical Pharmacognosy Ttechniques and Experiments Pune, India: Nirali Prakashan, 149–154.
- Lee H, Won NH, Kim KH, Lee H, Jun W, Lee KW. (2005). Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol Pharm Bull 28:1639–1644.
- Lee HS, Jung SH, Yun BN, Lee KW. (2007). Isolation of chebulic acid from Terminalia chebula Retz and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol 81:211–218.
- Malairajan P, Gopalakrishnan G, Narasimhan.S Veni, KJK, Kavimani S. (2007). Antiulcer activity of crude alcoholic extract of Toona ciliata (heart wood). J Ethnopharmacol 110:348–351.
- Malckzadeh F, Ehsanifar H, Shahamat N, Levin M, Colwell RR. (2002). Antibacterial activity of black myrobalan (Terminalia chebula Retz.) against Helicobacter pylori. Int J Antimicrob 18:85–88.
- Miglani BD, Sen P, Sanyal PK. (1971). Purgative action of an oil obtained from Terminalia chebula. Indian J Med Res 52:281–283.
- Miizi Doteuchi M. (1988). Lipid peroxidation a possible role in gastric damage induced by ethanol in rats. Life Sci 42:1757–1760.
- Miller TA. (1987). Mechanism of stress-related mucosal damage. Am J Med 83:8–14.
- Morimoto Y, Shimohara K, Oshima S, Sukamoto T. (1991). Effects of the new antiulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of terprenone and cimetidine. Japanese J Pharmacol 57:595–605.
- Nadar TS, Pillai MM. (1989). Effect of Ayurvedic medicines on beta-glucuronidase activity of Brunner’s glands during recovery from cysteamine induced duodenal ulcers in rats. Indian J Exp Biol 27:959–962.
- OECD. (1993). OECD Guidelines for Testing of Chemicals: Acute Oral Toxicity, No 401. Organization for Economic Cooperation and Development.
- Parmar NS, Parmar S. (1998). Antiulcer potential of flavonoids. India J Physilo Pharmacol 42:343–351.
- Piper DW, Stiel DD. (1986). Pathogenesis of chronic peptic ulcer: Current thinking and clinical applications. Med Prog 2:7–10.
- Rao Ch V, Sairam K, Goel RK. (2000). Experimental evaluation of Bacopa monniera on rat gastric ulceration and secretion. Indian J Physiolo Pharmacol 44:35–41.
- Sachs G, Shin JM et al. (1995). The pharmacology of the gastric acid pump: The H+-K+-ATPase. Ann Rev Pharmacol Toxicol 35:277–305.
- Sairam K, Rao ChV, Babu M.D, Kumar V, Agrawal VK, Goel RK. (2002). Antiulcerogenic activity of methanolic extract of Emblica officinalis. J Ethnopharmacol 82:1–9.
- Saleem M, Ahotupa K. (2001). Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z. Naturforsch [C], 56, 973–978.
- Salim AS. (1990). Removing oxygen derived free radicals stimulates healing of ethanol induced erosive gastritis in the rats. Digestion 47:24–28.
- Sato Y, Oketani H, Singyouchi K, Ohtsubo T, Kihara H, Higuti P. (1997). Extraction and purification of effective antimicrobial constituents of Terminalia chebula Retz. against methicillin-resistant Staphylococcus aureus. Pharm Bull 20:401–404.
- Shaila HP, Udup SL. (1998). Hypolipidemic activity of three indigenous drugs in experimentally induced atherosclerosis. Int J Cardiol 67:119–124.
- Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H. (1945). A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:43–61.
- Stein TA, Keegan LM, Aguste LJ, Bailey B, Wise L. (1991). Stress-induced gastric lesion and the synthesis of prostaglandin and leukotriens. J Surg Res 51:368–371.
- Szabo S, Trier JS, Brown A, Schnoor J. (1985). Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 88:228–236.
- Tasduq SS, Singh AK, Salti NK, Gupta DK, Suri K. (2006). Terminalia chebula fruits prevent liver toxicity caused by sub-chronic adminstration of refampicin, isoniazid and pyrazinamide in combination. Human Exp Toxicol 25:11–18.
- Vane JR. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat New Biol 231:232–235.
- Walt RP. (1992) Misoprostol for the treatment of peptic ulcer and anti-inflammatory-drug-induced gastroduodenal ulceration. N Engl J Med 327:1575–1580.
- Yeomans ND, Tulassy Z. (1998). A comparison of omeprazole with ranitidine for ulcer associated with nonsteroidal anti-inflammatory drugs. N Engl J Med 338:719–726.
- Zsuzsa V, Andrea C, Gyorgy K, Sandor A. (2001). Inhibition of the superoxide anion release and hydrogen peroxide formation in PMNLs by flavonolignans. Phytother Res 15:608–612.
