Abstract
Context: Earthworm Eisenia foetida (Lumbricus rubellus), a traditional Chinese medicine, is used for treating many diseases, and its coelomic fluid has extensive biological functions.
Objective: The hemolytic, antibacterial and antitumor activities of an earthworm protein purified from coelomic fluid were investigated in vitro.
Materials and methods: We used ultrafiltration, gel chromatography, and ion exchange chromatography in sequence to isolate and purify an earthworm protein from coelomic fluid (ECFP), and ECFP was characterized by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Hemolytic assay and antibacterial tests were applied to determine the cytolytic activity of ECFP. The MTT method was carried out to evaluate the antitumor effect of ECFP on HeLa cells and LTEP-A2 cells.
Results: ECFP, with molecular weight determined to be approximately 38.6 kilodaltons (KDa), was shown to possess significant hemolytic activity to chicken red blood cells (CRBC) (minimal hemolytic concentration 0.39 µg/mL). Antibacterial effect of ECFP obviously tested against Escherichia coli (minimal bactericidal concentration, MBC 180 µg/ mL) and Staphylococcus aureus (MBC 90 µg/mL) were observed. Moreover, ECFP notably inhibited the proliferation of HeLa cells (IC50 77 µg/mL) and LTEP-A2 cells (IC50 126 µg/mL) both in a time- and dose-dependent manner.
Discussion and conclusion: ECFP could serve as a component of the innate defense system of earthworms against foreign organisms, and thus it has potential pharmaceutical application in the future.
Introduction
As a traditional Chinese medicine, earthworms (Lumbricus rubellus) have been used to treat many diseases for thousands of years in China (CitationLiu et al., 2004). It is reported that earthworms can be used to treat chronic bronchitis, bronchial asthma, psychosis, digestive tract ulcer, peptic ulcer, epidemic parotitis, herpes zoster, urticaria, burn, scald, bladder calculi, urinating obstacle, cancer, etc. in clinics (CitationLiu, 1983; CitationLiang, 1984; CitationMu, 1988). Modern medical research has indicated that the coelomic fluid (CF) of earthworms contains an abundance of bioactive substances including lectin (CitationSuzuki et al., 2009), polysaccharide (CitationWang et al., 2007), protease (CitationSugimoto et al., 2003), antibacterial peptide (CitationWang et al., 2003), metalloenzyme (CitationSturzenbaum et al., 2001), fibrinolytic enzyme (CitationWang et al., 2005), and so on. Earthworm proteins and peptides have exhibited various biological activities (CitationLiu et al., 2004; CitationWang et al., 2007). Fetidin, with apparent molecular weight of 40 KDa, was purified from earthworm coelomic fluid and its bioactivities of antibacterial action, hemolysis and hemocoagulation were estimated (CitationMilochau et al., 1997). A 42 KDa protein, named coelomic cytolytic factor 1 (CCF-1), was reported to have cytolytic, opsonizing and hemolytic properties (CitationBeschin et al., 1998). Lumbricin I which was isolated and characterized from the earthworm showed antimicrobial activity in vitro against a broad spectrum of microorganisms without hemolytic activity (CitationCho et al., 1998).
Recently, active progresses in studies of earthworm proteins have been reported, and a large number of new earthworm proteins have been found from coelomic fluid. More and more researchers have focused on the investigation of the bioactive proteins in earthworm coelomic fluid. However, the components of earthworm coelomic fluid are too complex to obtain the high pure protein. On the basis of the isolation and purification of a protein (ECFP) from coelomic fluid of the earthworm Eisenia foetida, we further investigated its biological activities and analyzed the mechanisms of the biological activity of coelomic fluid using different methods in vitro. These results might help us to determine the pharmaceutical application of earthworm proteins in the future.
Materials and methods
Earthworms, bacterial strains and cell lines
The earthworms (Lumbricidae) at nearly the same developmental stage (determined by feeder) were purchased from Tianjin Liming-Jia Earthworm Farm, Tianjin, China, and then were reared in our laboratory. The animal experiments were approved by the Committee of HeiLongJiang BaYi Agricultural University. Bacterial strains (Escherichia coli and Staphylococcus aureus) were provided by the College of Animal Science and Veterinary Medicine, HeiLongJiang BaYi Agricultural University. Human Cervical Cancer HeLa cell line and Human Lung Carcinoma LTEP-a-2 cell line were purchased from Nanjing Keygen Biotech, Nanjing, China.
Reagents
RPMI-1640 media and protein ladder were purchased from Gibco (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Shanghai Sunway Biotech Company (Shanghai, China). Tris hydroxymethyl aminomethane (Tris), ethylene diamine tetra-acetic acid (EDTA), bovine serum albumin (BSA), sodium dodecyl sulfate (SDS), acrylamide, dimethylsulfoxide (DMSO), 2-mercaptoethanol, and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). Other analytical reagents were purchased from Shenyang Chemical Reagent Factory (Shenyang, China).
Preparation of the crude earthworm protein from coelomic fluid
The worms were reared on artificial media in plastic containers at 20°C for 90 days. The earthworms were washed and immersed in tap water for 12 h to extrude clay. These earthworms were then dried on filter paper and excited with a 6 voltage (V) electronic stimulation to induce strong contraction and extrusion of yellow coelomic fluid through the epidermal dorsal pore (CitationMilochau et al., 1997). The coelomic fluid was dissolved into the same volume of phosphate buffer solution (PBS, 5 mM, pH 6.8, containing 1% EDTA and 1% 2-mercaptoethanol), and then was centrifuged at 4°C, 15,000 g for 30 min. The supernatant fluid was quickly collected and ultrafiltrated by 1000 KDa and 5 KDa molecular weight cut-off ultrafiltration membranes on Labscale™ TFF System (Millipore, USA) in turn. Finally, the main components with molecular weight of 5 KDa to 1000 KDa were obtained.
Gel chromatography
The gel chromatography was designed to remove pigment from the coelomic fluid. AKTA purifier 100 purification system (Amersham, Sweden) was used for purification of the crude protein. Sephadex G25 S column (1.6 × 25 mm, Amersham) was installed on the purification system and equilibrated by elution buffer (50 mmol/L Tris + 50 mmol/L NaCl, pH 8.0). Crude protein (5 KDa1000 KDa) (0.5 mL) was gently loaded onto the column. It was eluted at a flow rate of 1 mL/min to the ultraviolet absorbance (UV) and returning to the baseline at room temperature, and the UV absorbance was monitored at 280 nm. The protein fractions were collected and stored at 4°C.
Ion exchange chromatography
A negative ion exchange column Q Sepharose® FF (7 × 25 mm, Amersham) was equilibrated with low salt buffer (buffer A, 50 mmol/L Tris + 50 mmol/L NaCl, pH 8.0). The fraction (peak 1 pooled in gel chromatography) was gently loaded onto the negative ion exchange column. After washing with buffer A to the UV absorbance and returning to the baseline, the adsorbed proteins were eluted with a linear gradient of high salt buffer (buffer B, 50 mmol/L Tris + 1 mol/L NaCl, pH 8.0) from 0% to 100% at a flow rate of 1 mL/min at room temperature. The elution profile was monitored at 280 nm, and the purified coelomic fluid protein (peak 1, named ECFP) was pooled and stored at 4°C.
Determination of protein concentration
Protein concentration was determined by the previous normal method (CitationBradford, 1976), and the reagent with bovine serum albumin (BSA) was defined as the standard. The standard curve was established as follows: y = 0.0071 × - 0.0023 (R2 = 0.9992) [x-axis represented the protein concentration and y-axis represented optical density (OD)].
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Prior to SDS-PAGE assay, the standard curve related to the relative mobility and logarithm of standard molecular weight (protein ladder) was determined. Relative mobility = migration distance of protein/migration distance of indictor (CitationCui et al., 2001). SDS-PAGE was performed on the gel electrophoresis chamber (0.1 × 10 × 10 cm) using 10% polyacrylamide gel topped by a 5% stacking gel in the presence of 1% SDS. The migration buffer consisted of 25 mM Tis and 192 mM glycine (pH 8.5). After migration, gel of 1 mm thickness was stained with Coomassie brilliant blue R-250, and decolored with destaining solution (45% methanol:10% iced acetic acid:45% water).
Hemolytic assay
The hemolytic activity test was carried out in 96-well microtiter plates upon chicken red blood cells (CRBC) (CitationMilochau et al., 1997) by mixing 50 μL buffer (0.075 mol/L PBS + 0.075 mol/L NaCl, pH 7.2) with 50 μL serial double dilution of ECFP respectively, followed by adding 50 μL CRBC solution (2% V/V) (stored in Alsever’s solution at 4°C). These samples were incubated for 2 h at room temperature to determine the highest dilution of ECFP inducing hemolytic erythrocytes. The same experiment was realized with 50 μL of distilled water and incubated with 50 μL CRBC in order to determine whether hemolytic assay was affected with osmolarity of media.
Antibacterial test
Inhibitory activity on microorganism was monitored by a liquid growth inhibition assay as described previously (CitationBulet et al., 1991). Escherichia coli and Staphylococcus aureus were grown to logarithmic phase in a Luria-Bertani liquid media, followed by centrifugation at 1500 rpm for 5 min. Two strains were inoculated into iced PBS (5 mM, pH 6.8), and the optical density (570 nm) were determined to be the initial reference using ultraviolet spectrophotometer (TU1801, Beijing Persee, China). Bacterial suspensions (3 mL) containing ECFP with the final concentration of 22.5, 45, 90, 180, 360 μg/mL (serial double dilution) were seeded into a 15 mL tube and cultured at 180 rpm for 2 h at 37°C. Every concentration was repeated three times and a control test was performed with physiological saline. After immersing in an iced-bath for 20 min, the optical density of 570 nm was determined. The ECFP concentration which displayed inhibition of bacterial growth was determined as minimal inhibitory concentration (MIC).
In addition, the Oxford cup-plate assay was performed for evaluation of ECFP antibacterial activity (CitationWang et al., 2007). In brief, bacteria at logarithmic phase (200 μL cell suspension) with a final cell density of 1 × 106 colony-forming units/mL were seeded onto a 90-mm diameter Petri dish containing solidified Luria-Bertani agar media. ECFP (100 μL) with final concentrations of 22.5, 45, 90, 180, and 360 μg/mL respectively, was overlaid into the Oxford cup, and a control test was performed with PBS. These dishes were incubated overnight at 37°C, and antimicrobial activity was determined by measuring the diameter of the inhibitory zone for bacterial growth around the Oxford cup. The ECFP concentration which exhibited antibacterial activity on bacterial growth was defined to be the minimum bactericidal concentration (MBC).
Antitumor effect
MTT assay (CitationHua et al., 2009; CitationQin et al., 2010) was used for determining the antitumor activity of ECFP. HeLa cells and LTEP-A2 cells were stored in liquid nitrogen in our laboratory. Briefly, the cells were recovered quickly, followed by adjusting of 4 × 104/mL cell suspension. Cells were grown in 100 μL of RPMI-1640 media (supplemented with 10% FBS) into 96-well plates at 37°C in the CO2 incubator (HEPA Class 100, Thermo, USA). The ECFP was added into each well with the final protein concentration of 10, 20, 40, 80, and 160 μg/mL (serial double dilution), respectively. The total medium volume of each well was 200 μL, and every dosage was repeated three times in the same plate. After further incubation for 24, 48, 72 and 96 h, 20 μL of MTT (5 mg/mL in PBS (pH 7.4, 10 mM)) were added to each well, followed by incubation for an additional 4 h. The medium was discarded and 150 μL DMSO was added into each well to dissolve the purple-blue formazan precipitate, and incubated for 20 min. The optical density of 570 nm was measured with a microplate reader (550 Bio-Rad, USA) and the proliferation inhibition rate (%) was calculated according to the following formula: (1 − experimental OD value/control OD value)×100. Inhibitory concentration of 50% (IC50) was calculated according to Modified Karber’s method (CitationQin et al., 2010).
Statistical analysis
The results were presented as arithmetic mean of at three replicates ± SE. The analysis of variance (one-way ANOVA) was performed for evaluating statistical significance, and a P value of less than 0.05 (P <0.05) was used for statistical significance.
Results
Preparation of the crude protein
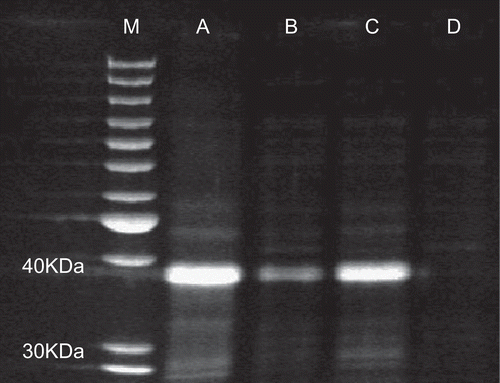
Earthworms were collected and submitted to electronic stimulation to extrude proteins responsible for biological activities. The extracted raw coelomic fluid was ultrafiltrated by 1000 KDa and 5 KDa molecular weight cut-off ultrafiltration membranes in sequence. The major components of raw coelomic fluid were characterized with the SDS-PAGE assay. Four components ranging from different molecular weights were obtained including component A (not ultrafiltration), B (<1000 KDa), C (5 KDa–1000 KDa), and D (<5 KDa). The results indicated that the main proteins or the abundant proteins in crude coelomic fluid had a molecular weight of 30 KDa - 40 KDa, and could be concentrated with 5 KDa cut-off membrane as shown in (lane C).
Figure 1. The crude coelomic fluid of earthworm was characterized with SDS-PAGE. Lane M: protein ladder; lane A: the filtrated earthworm coelomic fluid (not ultrafiltration); lane B: the ultrafiltrated earthworm coelomic fluid (< 1000 KDa); lane C: the ultrafiltrated earthworm coelomic fluid (5 KDa1–000 KDa); lane D: the ultrafiltrated earthworm coelomic fluid (< 5 KDa).
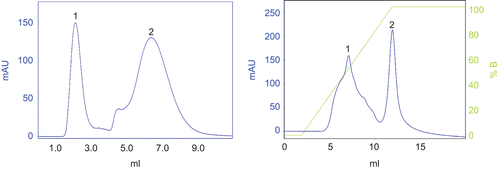
Purification of the crude protein
Purification of the crude protein (5 KDa1000 KDa) was attempted to analyze biological activities of the isolated protein. Two main proteins (peak 1, 2) were obtained from raw coelomic fluid purified by gel chromatography (). SDS-PAGE assay indicated the molecular weight of peak 1 was between 3040 Ka (, line B), and peak 1 was used for the next purification. The protein (peak 1 pooled in gel chromatography) was adsorbed on the ion exchange column and eluted with a linear gradient of high salt buffer from 0% to 100% in 10 min (, right). Two main proteins were isolated, and peak 1 with higher absorbance at 280 nm was the bioactive protein (ECFP) and found to present hemolytic, antibacterial and antitumor activities.
SDS-PAGE assays
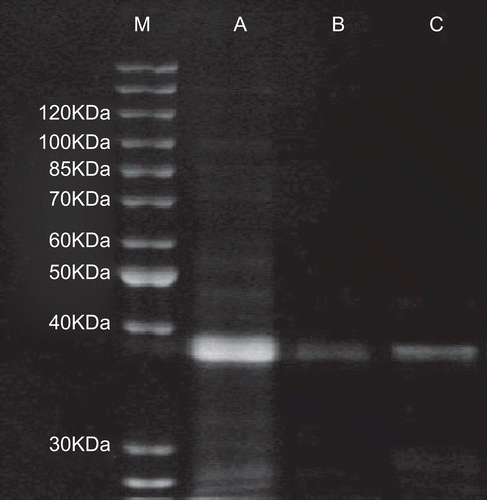
The established standard curve is presented in . The x-axis represents the relative mobility, and the y-axis represents the logarithm of the standard molecular weight of protein (protein ladder). SDS-PAGE analysis showed a main band of molecular weight approximately 38.6 KDa calculated according to the standard curve (, lane C). Only one main protein band was visualized by SDS-PAGE, which demonstrated that the crude proteins had been purified with gel chromatography and ion exchange chromatography.
Figure 3. Standard curve related to the relative mobility and logarithm of standard molecular weight was established. The x-axis represents the relative mobility, and the y-axis represents the logarithm of the standard molecular weight of protein (protein ladder).
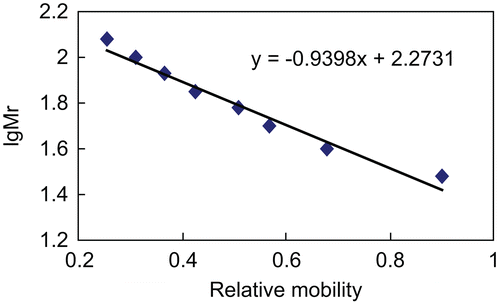
Figure 4. The purified earthworm proteins were characterized with SDS-PAGE. Lane M: protein ladder; lane A: the ultrafiltrated earthworm coelomic fluid (5 KDa–1000 KDa); lane B: peak 1 purified after the gel chromatography; C: peak 1 (ECFP with molecular weight of 38.6 KDa) purified after the ion exchange chromatography.
Hemolytic activity
The results of ECFP hemolytic activity is represented in . The ECFP was found to present significant hemolysis on CRBC when the concentration was above 1.56 μg/mL, and the weaker hemolytic activity (25% hemolysis) was observed at the concentration of 0.78 μg/mL and 0.39 μg/mL. The increasing of the ECFP concentration had stronger hemolytic activity in a dose-dependent manner. However, no hemolysis was detected at 0.19 μg/mL, which indicated that 0.39 μg/mL was the minimal hemolytic concentration.
Table 1. Hemolytic activity of the ECFP on CRBC.
Antibacterial activity
According to the methodology described by the National Committee for Clinical Laboratory Standards (CitationNCCLS, 2008), through testing the antimicrobial activity of the ECFP using bacterial turbidity of colorimetric and Oxford cup plate assay, we found that ECFP showed a significant antibacterial activity against the tested Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus as shown in . After incubation with bacteria, the ECFP exhibited obvious growth inhibition on Escherichia coli with a MIC of 90 μg/mL. Similar inhibitory activity of ECFP was detected on Staphylococcus aureus with a MIC of 45 μg/mL. Furthermore, minimal bactericidal concentrations (MBC) were determined to be 180 μg/ mL on Escherichia coli and 90 μg/mL on Staphylococcus aureus respectively, which implied that there was a different dosage effect between the Gram-negative and Gram-positive bacteria.
Table 2. Antibacterial activity of the ECFP on bacteria.
Antitumor activity
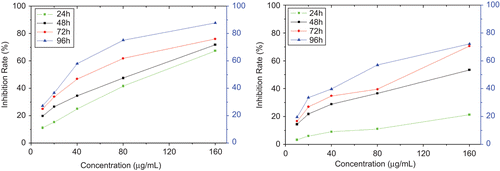
To investigate the antitumor activity of the ECFP on the tumor cells, HeLa cells and LTEP-A2 cells were cultured in the presence of ECFP at different concentrations for 96 h, and the inhibition rates on tumor cells were determined with MTT method. With the prolongation of the treated time, the inhibition effects on cell proliferation significantly increased. Similarly, the higher the dosage of ECFP the cells were exposed to, the higher inhibition rates were observed (). The exposure of HeLa cells and LTEP-A2 cells to ECFP had caused the significant inhibition of cell proliferation both in a time- and dose-dependent manner, and their IC50 was determined to be 77 μg/mL, and 126 μg/mL, respectively. Moreover, the inhibition rate on HeLa cells was notably higher than that of LTEP-A2 cells in the same treatment, which suggested that there might be existing different inhibitory effects on two tumor cell lines.
Figure 5. HeLa cells and LTEP-A2 cells were exposed to ECFP with final concentration of 10, 20, 40, 80, and 160 μg/mL for 96 h, and MTT method was performed for determining the cell proliferation. Every dosage was repeated three times. The ECFP (38.6 KDa) inhibited the proliferation of HeLa cells (left, IC50 77 μg/mL) and LTEP-A2 cells (right, IC50 126 μg/mL) in vitro compared with the control both in a time- and dose-dependent manner.
Discussion
Earthworm coelomic fluid (Annelid, Oligochaeta) contains numerous important defense components including hemolytic, cytolytic, agglutinating, bacteriostatic/bactericidal, and proteolytic substances (CitationCho et al., 1998; CitationWang et al., 2007). As one of the most vital defense components, antimicrobial peptides are now considered as one of the universal host defense tools of living organisms against microbial infection. Up to now, the molecular weight of antibacterial peptides are generally found to be below 60 KDa (CitationTasiemski et al., 2007). In this study we described the isolation and purification of an earthworm protein from coelomic fluid (ECFP) with the approximate molecular weight of 38.6 KDa. This is another crucial report on the bioactive protein from earthworm coelomic fluid, and the bioactive results demonstrated that the ECFP exerted naturally potent hemolytic activity on erythrocytes, antibacterial activity and antitumor activity on proliferation of tumor cells, which could probably be involved as a basic component of innate immunity in earthworms. Furthermore, many earthworm peptides and proteins from coelomic fluid with antibacterial and antitumor activities have been discovered (CitationWang et al., 2003; CitationEllen et al., 2007).
In the experiment, the raw coelomic fluid was ultrafiltrated with 1000 KDa and 5 KDa molecular weight cut-off ultrafiltration membranes to obtain the crude proteins. The ultrafiltration allowed peptides and proteins with the molecular weight above 1000 KDa and below 5 KDa to be completely removed, and arrived at good condensation of bioactive protein (, lane C). By gel chromatography the two primary components of protein in the ultrafiltrated liquid were eluted in turn, as well as removing most of the yellow pigments present in the ultrafiltrated fluid. The following negative ion exchange chromatography has yielded the bioactive protein characterized with the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method, and the bioactive protein showed a single protein band with approximate molecular weight of 38.6 KDa. The elution profile of the ion exchange chromatography showed that the bioactive component was located in only one restricted zone which suggested a good purification. The ECFP had the ability to mediate the reaction of hemolysis, antibacteria, and antitumor on proliferation of tumor cells, which displayed another particular interest of proteins from coelomic fluid since the biological earthworm proteins were investigated (CitationCho et al., 1998).
ECFP was revealed to induce hemolysis of chicken red blood cells (CRBC) without adding any other assistant chemical substances. Hemolytic effect increased with the amount of added protein to total hemolysis in a dose-dependent manner. This similar phenomena was also observed in the experiment on the hemolytic activity of fetidin which could act on red blood membrane and not even require any divalent cations (CitationMilochau et al., 1997). The earthworm cytolytic peptide’s interaction with membranes, relevant mechanism and structure have already been studied. It was reported that a cytolytic protein (Eiseniapore, 38 KDa) was isolated from coelomic fluid of the earthworm Eisenia fetida, and the Eiseniapore was capable of inducing hemolysis of mammalian erythrocytes by pore formation on cell membrane with diameter above 3 nm (CitationSven et al., 1999). Many studies about cytolytic proteins from various species concluded with the negative phospholipid nature of membrane receptors (CitationMilochau et al., 1997). It was suggested from our results that 38.6 KDa ECFP might irreversibly act on red cell membrane to induce hemolysis. However, whether the hemolytic process was caused by membrane receptors was uncertain and should be further investigated.
Many earthworm hemolytic peptides and proteins also exerted the bacteriostatic/bactericidal activity and cytotoxic activity (CitationHarris et al., 2009). A novel antimicrobial peptide (lumbricin I) was found from earthworm, and lumbricin I exhibited antimicrobial activity in vitro against a broad spectrum of microorganisms (CitationCho et al., 1998). Six antimicrobial peptides (antibacterial vermipeptide family, AVPF) were isolated and purified from coelomic fluid of earthworm, and AVPF showed extensive antimicrobial activities in vitro (CitationWang et al., 2007). In our experiment the purified ECFP exhibited weaker antibacterial activity on Escherichia coli (MBC 180 μg/mL) than that on Staphylococcus aureus (MBC 90 μg/mL), which was probably due to the tendency of interaction with the bacterial cell wall. Moreover, the earthworm protein from coelomic fluid (100 μg/mL) exerted toxic effects on HeLa cells with an inhibition rate of 84.22%, leading to cell lysis, and morphological changes of typical apoptosis were observed (CitationLiu et al., 2007). In our study, ECFP also showed significant inhibition of cell proliferation on HeLa cells and LTEP-A2 cells both in a time- and dose-dependent manner. Tumor cells treated with ECFP decreased the malignant phenotype. The high concentration of ECFP (above 40 μg/mL) had induced tumor cell necrosis, similar to the previous reports which stated that earthworm protein from coelomic fluid notably decreased the ratios of live tumor cells (CitationEngelmann et al., 2005). In addition, ECFP had a stronger inhibition effect on HeLa cells (IC50 77 μg/mL) than that on LTEP-A2 cells (IC50 126 μg/mL), which suggested a discrepancy of inhibition rate between two tumor cells. Along with the increasing of dosage and prolongation of exposure time, round and transparent granules, lipid granules and destroyed cell membrane were viewed, which demonstrated that the ECFP’s biological activity was achieved through the interaction with the foreign cell membrane in good agreement with previous studies (CitationSven et al., 1999).
In conclusion, the protein (ECFP) with molecular weight of 38.6 KDa which was isolated and purified with gel chromatography and ion exchange chromatography has the hemolytic, antibacterial and antitumor activities in a dose-dependent manner, and the mechanism of the biological activity was probably subject to interaction with foreign cell membrane. We presume that the protein serves as a component of the earthworm defense system, and future research will focus on the protein structure and its biological role in vivo. It may be an important protein not only because of the fundamental research, but also because of the potential pharmaceutical application as an antibacterial or antitumor drug.
Acknowledgments
We are sincerely grateful to Liu Quan, Yan-Ping He, and Run-Ting Li who have contributed to the purification of the protein.
Declaration of interest
The authors report no conflict of interests and the study was supported by the Master’s Research Foundation, HeiLongJiang BaYi Agricultural University (S2005-38).
References
- Beschin A, Bilej M, Hanssens F, Raymakers J, Van Dyck E, Revets H, Brys L, Gomez J, De Baetselier P, Timmermans M. (1998). Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J Biol Chem, 273, 24948–24954.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Bulet P, Cociancich S, Dimarcq JL, Lambert J, Reichhart JM, Hoffmann D, Hetru C, Hoffmann JA. (1991). Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem, 266, 24520–24525.
- Cho JH, Park CB, Yoon YG, Kim SC. (1998). Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: Purification, cDNA cloning and molecular characterization. Biochim Biophys Acta, 1408, 67–76.
- Cui DB, Zheng YJ, Wang YJ, Zhang LH. (2001). Purification of antibacterial peptides from earthworm. J Dalian Instit L Indust, 23, 265–269.
- Ellen K, Werner M, Edwin LC. (2007). Coelomic fluid proteins as basic components of innate immunity in earthworms. Eur J Soil Biol, 43, S110–115.
- Engelmann P, Cooper EL, Nemeth P. (2005). Anticipating innate immunity without a toll. Mol Immunol, 42, 931–942.
- Harris F, Dennison SR, Phoenix DA. (2009). Anionic antimicrobial peptides from eukaryotic organisms. Curr Prot Pept Sci, 10, 585–606.
- Hua Z, Fang LQ, Hong W, Yan H, Wang YH, Cui YD. (2009). Effects of goat placental immunoregulatory factor on non-specific immunity of mice. Isr J Vet Med, 64, 64–71.
- Liang YL. (1984). Application of earthworm in treating asthma. J Tradit Chin Med, 4, 15–16.
- Liu XF. (1983). Twenty-six cases of Allium tuberous root and earthworm on herpes zoster. Henan J Tradit Chin Med, 6, 14–16.
- Liu YQ, Sun Y, Sun ZJ, Li SJ, Wang C. (2007). Coelomic fluid of the earthworm Eisenia fetida induces apoptosis of HeLa cells in vitro. Eur J Soil Biol, 43, S143–148.
- Liu YQ, Sun ZJ, Wang C, Li SJ, Liu YZ. (2004). Purification of a novel antibacterial short peptide in earthworm Eisenia foetida. Acta Biochim Biophys Sin, 36, 297–302.
- Milochau A, Lassègues M, Valembois P. (1997). Purification, characterization and activities of two hemolytic and antibacterial proteins from coelomic fluid of the annelid Eisenia fetida andrei. Biochim Biophys Acta 1337, 123–132.
- Mu DJ. (1988). Report of 40 cases digestive ulcer treated with earthworm powder. J Tradit Chin Med, 29, 21–23.
- NCCLS (2008). Performance standards for antimicrobial susceptibility testing; ninth informational supplement. NCCLS document M100-S9. Wayne, PA: National Committee for Clinical Laboratory Standards.
- Qin XG, Zhang H, Wang S, Wang YH, Cui YD. (2010). Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharm Biol, 48, 275–281.
- Sturzenbaum SR, Cater S, Morgan AJ, Kille P. (2001). Earthworm pre-procarboxypeptidase: A copper responsive enzyme. Biometals, 14, 85–94.
- Sugimoto M, Ishihara K, Nakajima N. (2003). Structure and function of an isozyme of earthworm proteases as a new biocatalyst. J Mol Cat B-Enzy, 23, 405–409.
- Suzuki R, Kuno A, Hasegawa T, Hirabayashi J, Kasai KI, Momma M, Fujimoto Z. (2009). Sugar-complex structures of the C-half domain of the galactose-binding lectin EW29 from the earthworm Lumbricus terrestris. Acta Crystallogr D, 65, 49–57.
- Sven L, Ellen K, Werner M, Edwin LC. (1999). Biochemical characteristics of Eiseniapore, a pore-forming protein in the coelomic fluid of earthworms. Eur J Biochem, 262, 547–556.
- Tasiemski A, Schikorski D, Le Marrec-Croq F, Camp CPV, Boidin-Wichlacz U, Sautiere PE. (2007). Hedistin: A novel antimicrobial peptide containing bromotryptophan constitutively the marine annelid, expressed in the NK cells-like of Nereis diversicolor. Dev Comp Immunol, 31, 749–762.
- Wang C, Sun ZJ, Liu YQ, Zhang XC, Xu GZ. (2007b). A novel antimicrobial vermipeptide family from earthworm Eisenia fetida. Eur J Soil Biol, 43, S127–134.
- Wang C, Sun ZJ, Liu YQ, Zheng DM, Liu XL, Li SZ. (2007b). Earthworm polysaccharide and its antibacterial function on plant-pathogen microbes in vitro. Eur J Soil Biol, 43, S135–142.
- Wang F, Wang C, Li M, Zhang JP, Gui LL, An XM, Chang WR. (2005). Crystal structure of earthworm fibrinolytic enzyme component B: A novel, glycosylated two-chained trypsin. J Mol Biol, 348, 671–685.
- Wang X, Wang XX, Zhang Y, Qu XM, Yang SL. (2003). An antimicrobial peptide of the earthworm Pheretima tschiliensis: cDNA cloning, expression and immunolocalization. Biotechnol Lett, 25, 1317–1323.