Abstract
Context: Solanum sisymbriifolium Lam. (Solanaceae), commonly known as sticky nightshade, is traditionally used for central nervous system (CNS) disorders. Although solasodine has been isolated from this plant, little is known about its anticonvulsant and CNS depressant actions.
Objective: We investigated anticonvulsant and CNS depressant effects of solasodine isolated from S. sisymbriifolium using several experimental models.
Materials and methods: Swiss albino mice (n = 6) were employed for pentylenetetrazole (PTZ) and picrotoxin (PCT)-induced convulsions and thiopental-induced sleep time. Different groups of Wistar albino rats (n = 6) were subjected to maximal electroshock (MES) test. Solasodine, a steroidal glycoalkaloid, was isolated from dried fruits of S. sisymbriifolium and identified by GC-MS.
Results: The results showed that intraperitoneal (i.p.) injection of solasodine (25 mg/kg) significantly delayed (p < 0.01) latency of hind limb tonic extensor (HLTE) phase in the PCT-induced convulsions. In the MES model, solasodine significantly reduced (p < 0.001) duration of HLTE at 25, 50, and 100 mg/kg, i.p. in a dose-dependent manner. Interestingly, solasodine did not produce any significant reduction in PTZ-induced convulsions. Prior treatment of solasodine (25, 50, and 100 mg/kg, i.p.) significantly potentiated thiopental-provoked sleep in a dose-dependent manner (p < 0.001).
Discussion and conclusion: Our study, for the first time, shows potent anticonvulsant and CNS depressant activities of solasodine. It is likely that solasodine, in part, is responsible for the anticonvulsant and sedative properties of S. sisymbriifolium. The future study should focus on the exact mechanism of action of solasodine.
Introduction
Epilepsy is a common and frequently devastating disorder affecting an estimated 4050 million people worldwide (CitationRudiger, 2002). The incidence and prevalence of epilepsy varies with age (CitationBanerjee et al., 2009) and is highest among children below 7 years of age and in individuals of above 55 years (CitationJalalpure et al., 2009). In India, the prevalence of epilepsy is about 5.5 to 7.9 per 1000 people, which is about 1/18th of the world population (CitationNag, 2000).
A number of synthetic antiepileptic drugs (AEDs) such as diazepam (DZP), phenytoin (PHE), carbamazepine, phenobarbital, felbamate, valproate, and lamotrigine are used for the treatment of epilepsy (CitationVyawahare et al., 2007). Effectiveness of these drugs does not hold true with the entire range of population suffering from this disorder and they are unable to control epileptic seizures in as many as 25% of patients (CitationJalalpure et al., 2009). In developing countries the treatment of epilepsy is inaccessible and unaffordable (CitationOjewole, 2008). Moreover, the modern approach to treat epilepsy can result in serious side effects including high risk of toxicity and drugdrug interactions (CitationJalalpure et al., 2009). These limitations of conventional AEDs demand the development of newer antiepileptic agents (CitationGupta et al., 1999; CitationGupta & Malhotra, 2000). Therefore, there is a dire need to develop cheap, effective, and safe agents from plants and other natural sources (CitationOjewole, 2008). Scientists are keen to obtain drugs from plant origin due to their specific curative properties and relatively low adverse effects (CitationVaidhya, 1997).
The family Solanaceae is well known to create steroidal alkaloids through common biogenetic precursors and includes many important agricultural crops such as potato, tomato, eggplant, and capsicum (CitationDinan et al., 2001). CitationNohara et al. (2007) demonstrated considerable amount of spirosolane, solanidane, spirostane, and furostane glycosides in different species of Solanum including Solanum sisymbriifolium Lam.
S. sisymbriifolium Lam. (Solanaceae), commonly known as sticky nightshade (English), is a viscid, densely spiny herb. It is native to South America but also distributed throughout hotter parts of India (CitationChakravarty et al., 1996). The plant is traditionally used for treatment of hypertension, diarrhea, respiratory tract infections, and various central nervous system (CNS) disorders such as epilepsy and depression (CitationIbarrola et al., 2000; CitationFerro et al., 2005). Many spirosolane-type and solanidane-type alkaloids are found in this plant (CitationChakravarty et al., 1996). Solasodine (molecular formula: C27H43NO2), a steroidal alkaloid, has been isolated from this plant (CitationMazumdar, 1984; CitationChand et al., 1995; CitationWeissenberg, 2001). It is an aglycone part of many solanaceous steroidal glycoalkaloids such as solamargine and solasonine (CitationWeissenberg, 2001; CitationSmith et al., 2008).
A body of evidence demonstrates antifungal (CitationWolters, 1976; CitationNes et al., 1982; CitationRowan et al., 1983; CitationKusano et al., 1987), cytotoxic (CitationLin et al., 1990; CitationNakamura et al., 1996), hepatoprotective (CitationLin et al., 1988), hypotensive (CitationBasu & Lahiri, 1977), and antiatherosclerotic (CitationDixit et al., 1992) activities of solasodine. Solasodine glycosides are also clinically and histologically effective in the treatment of skin tumors (CitationCham et al., 1987, Citation1991). Recently, CitationEmmanuel et al. (2006) reported anti-inflammatory activity of solasodine isolated from Solanum trilobatum. The scientific studies on antiepileptic and CNS depressant activities of solasodine have not been reported till now. In view of the above, we hypothesized that solasodine, in part, could be involved in the CNS related properties of S. sisymbriifolium. To test this hypothesis, we evaluated anticonvulsant and sedative effects of solasodine using experimental models.
Methods
Collection of plant materials
The fruits of S. sisymbriifolium were collected from the medicinal garden of Saurashtra University, Rajkot, India and authenticated by Pran Jeevan Parmar, Botanical Survey of India, Jodhpur, Rajasthan. A voucher specimen (SU/DPS/Herb/12) was deposited in the Department of Pharmaceutical Sciences, Saurashtra University, Rajkot, India for future reference.
Isolation of solasodine
A suspension of dried, finely ground powder of S. sisymbriifolium fruits (100 g) in toluene (300 mL), water (200 mL), and concentrated HCl (32%, 100 mL), was refluxed under stirring for 5 h. The reaction mixture was subsequently alkalinized with 40% aqueous NaOH (200 mL) and refluxed again under stirring for 2 h. Following phase separation, the upper pale-yellow toluene layer was siphoned out and the remaining dark brown aqueous mixture was extracted three times with 100 mL portions of toluene. The combined toluene extracts were clarified with charcoal, and then concentrated to a small volume. Concentrate is alternatively extracted with an equal volume of 25% aqueous acetic acid by stirring twice for 1 h at room temperature. The aqueous acid layer was separated off from the toluene layer and then alkalinized with 25% aqueous NH3. The mixture was briefly heated, then cooled at room temperature to obtain precipitates. The precipitated solasodine was filtered off, washed with cold water, and dried in air (CitationWeissenberg, 2001). The gas chromatography-mass spectrometry (GC-MS) analysis was performed by model QP2010 (Shimadzu, Kyoto, Japan) according to these experimental conditions: silica capillary column: SGE-BPX 5 (30 mm × 0.25 mm × 0.25 µm), injector temperature: 230°C, detector temperature: 250°C, temperature program: initially 80°C, and then raised to 260°C at a rate of 4°C/min and finally held at 260°C for 10 min, electron impact: 70 eV, carrier gas: helium at 1 mL/min, scanning speed: 0.84 scan/sec from 25 m/z to 1000 Da, split ratio: 1:30. The identification of the components was made through comparison of substance mass spectrum with the database of the GC-MS (NIST107). The melting point and GC-MS analysis of isolated solasodine was compared with the standard available data.
Experimental animals
Wistar albino rats of either sex (250–300 g; age 4 months) were subjected to maximal electroshock (MES)-induced seizures test (n = 6). Pentylenetetrazole (PTZ) and picrotoxin (PCT)-induced convulsions, and thiopental-induced sleep time tests were conducted on Swiss albino mice (18–25 g) (n = 6). All the animals were housed in groups in polypropylene cages and placed in a climate-controlled central animal house having a temperature of 22° ± 2°C, relative humidity 60% ± 5%, and 12 h light/dark cycle (lights on at 08:00 h and off at 20:00 h). Animals had free access to standard pellet diet (Amrut, Pranav Agro Industries Ltd, Vadodara, India) and water ad libitum. All the protocols were approved (SU/DPS/IAEC/1010) by the Institutional Animal Ethics Committee (IAEC) of the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India, New Delhi. The animals were habituated prior to the experiments to reduce non-specific stress. For this they were kept individually in the cages for 30 min to acclimatize to the new environment.
Drugs
DZP, PTZ, and PCT were purchased from Sigma (St. Louis, MO). PHE was received as a gift sample from Piramal Healthcare, Himachal Pradesh, India. All the drug solutions were freshly prepared and the solvents used were of analytical grade.
Administration of drugs
PHE and thiopental were dissolved in saline, while DZP and solasodine were prepared as suspension in saline using 1% sodium carboxymethyl cellulose (SCMC) as the suspending agent. Animals were assigned to different treatment groups (n = 6). Solasodine, DZP, and PHE were administered by intraperitoneal (i.p.) route. The control group received the vehicle (1% SCMC, 1 mL/kg, i.p.).
Determination of LD50
The toxicity study was performed as per the method described by CitationLitchfield and Wilcoxon (1949) and the median lethal dose was determined. Briefly, different groups of mice (n = 10) were administered with different doses of solasodine (125, 250, 500, 1000, 1500, and 2000 mg/kg, i.p.) suspended in 1% SCMC in saline (0.9% sodium chloride). The animals were examined every 30 min up to a period of 3 h and then, occasionally for a further 4 h; and finally, at 24 h. The LD50 was calculated.
Pentylenetetrazole and picrotoxin-induced convulsions
A modified method of CitationVellucci and Webster (1984) was followed. Briefly, seizures were induced with PTZ (75 mg/kg, i.p.) or PCT (12 mg/kg, i.p.). During a 45-min test period the animals were observed for various symptoms such as ear and facial twitching, convulsive waves axially through the body, myoclonic body jerks, straub tail, generalized clonic convulsions, twitching and turning to one side, hind limb tonic extensor (HLTE) phase, stupor, and recovery or death. An animal was considered as convulsed if it showed HLTE phase (CitationYemitan & Adeyemi, 2005; CitationQuintans-Júnior et al., 2008). Different groups of animals were treated with solasodine (25, 50, and 100 mg/kg, i.p.), DZP (4 mg/kg, i.p.) or 1% SCMC in saline (control group), 30 min prior to the administration of PTZ or PCT. The ability of solasodine or DZP to prevent or delay the onset of the HLTE phase was considered as anticonvulsant activity.
Maximal electroshock-induced convulsions
The method of CitationSayyah et al. (2005) was followed. Briefly, different groups of rats (n = 6) were treated with solasodine (25, 50, and 100 mg/kg, i.p.), PHE (25 mg/kg, i.p., standard group) or 1% SCMC in saline (control group). Thirty minutes later, all the animals received electrical stimulus (150 mA, 50 Hz, 0.2 sec duration) through ear-clip electrodes using a convulsiometer (Instrument Company, Ambala, India). A drop of electrolyte solution (0.9% sodium chloride) was applied on the ears of the animals for better electrode contact. The appearance and total duration of the HLTE phase were observed over a period of 20 min. Animals showing absence or delayed appearance (more than 10 s) or reduction in HLTE duration were considered protected.
Thiopental-induced sleeping time
The procedure of CitationSukma et al. (2002) was followed. Briefly, 30 min after the administration of solasodine (25, 50, and 100 mg/kg, i.p.) or SCMC (1%, i.p.), mice received thiopental (50 mg/kg, i.p.). The time elapsed from thiopental injection to loss of the righting reflex was taken as sleeping latency. The time elapsed between the loss and voluntary recovery from the righting reflex was considered as the total sleeping time.
Statistical analysis
The data are presented as mean ± SEM. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by the Tukey test. Differences were considered significant at p < 0.05.
Results
Isolation of solasodine
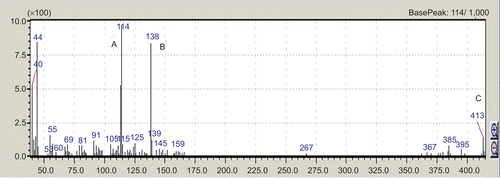
The solasodine was isolated from dried berries of S. sisymbriifolium (yield 0.9% w/w). The melting point of solasodine was 200°C. GC-MS study showed m/z value at 413 which is the molecular weight of solasodine; base peak at 114 m/z and intense fragment at 138 m/z ().
Determination of LD50
This study showed that the LD50 of solasodine in mice was 1500 mg/kg i.p. Three doses corresponding to 1/60th, 1/30th, and 1/15th of LD50 value, i.e., 25, 50, and 100 mg/kg, i.p., respectively, were selected for various experimental models.
Picrotoxin- and pentylenetetrazole-induced convulsions
As shown in , PCT (12 mg/kg, i.p.) produced severe convulsions (hind limb and tonic-clonic seizures) in the control group. Solasodine (25 mg/kg, i.p.) significantly delayed (p < 0.01) latency of HLTE by 29.9 ± 2.7 s. Animals treated with the higher doses of solasodine (50 and 100 mg/kg, i.p.) did not produce significant antiseizure effects in this model. DZP (4 mg/kg, i.p.) significantly delayed (p < 0.001) latency of HLTE phase and produced tonic antiseizure effects by protecting 80% of the animals. In the PTZ-induced convulsions test, prior treatment of solasodine (25, 50, and 100 mg/kg, i.p.) failed to produce any significant effects on the latency of the HLTE phase. DZP (4 mg/kg, i.p.), a standard antiseizure drug, produced marked delay in the latency of the HLTE phase ().
Table 1. Effect of solasodine on picrotoxin-induced convulsions in mice.
Table 2. Effect of solasodine on pentylenetetrazole-induced convulsion test in mice.
Maximal electroshock-induced convulsions
depicts antiseizure effects of solasodine in the MES model. In this test, application of electro stimulus (150 mA, for 0.2 s) produced severe convulsions, marked by a long HLTE phase, in the control group animals. Solasodine, at the doses of 25, 50, and 100 mg/kg, i.p. decreased the duration of HLTE phase by 7.4 ± 0.4, 6.2 ± 0.3, and 2.4 ± 0.2 s, respectively. The effect was dose-dependent and highly significant (p < 0.001) as compared to the control group (11 ± 1 s). PHE (25 mg/kg, i.p.), a standard antiseizure drug, completely abolished the HLTE phase and protected all animals.
Table 3. Effect of solasodine on maximal electroshock-induced convulsions in rats.
Thiopental-induced sleep time
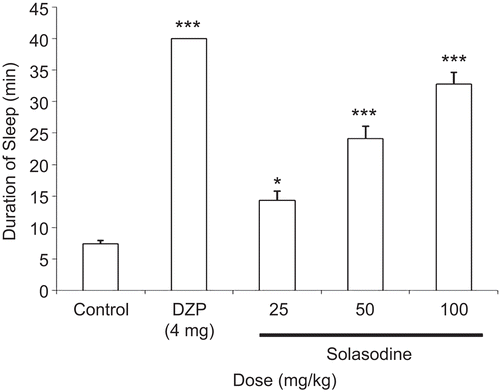
As shown in , solasodine at a dose of 25 mg/kg, i.p., significantly (p < 0.05) prolonged thiopental-provoked sleep time by 14.3 ± 1.5 s, whereas solasodine (50 and 100 mg/kg, i.p.) significantly (p < 0.001) prolonged thiopental-induced sleeping time by 24.1 ± 1.9 and 32.8 ± 1.9 s, respectively, when compared to control group (7.4 ± 0.5 s). The effect was dose-dependent.
Discussion
In the present study, solasodine, isolated from S. sisymbriifolium, showed anticonvulsant and CNS depressant effects in the experimental animals. S. sisymbriifolium is traditionally prescribed for various CNS-related conditions (CitationIbarrola et al., 2000; CitationFerro et al., 2005). CitationChand et al. (1995) reported solasodine as one of the chief constituents from the fruits of this plant. In view of this we hypothesized that solasodine, in part, could account for the neurobehavioral effects of S. sisymbriifolium. To test this hypothesis we evaluated antiepileptic and sedative effects of solasodine using MES-, PCT-, and PTZ-induced seizures and thiopental-provoked sleep models.
In this study the melting point of solasodine was 200°C. It has been reported that solasodine has a melting point of about 199-200°C (CitationWeissenberg, 2001). Our results showed that the yield value of isolated solasodine was 0.9% w/w. This is in agreement with the previous study in which reported percentage of solasodine from berries of S. sisymbriifolium was 0.93% w/w (CitationSubramani et al., 1989). The results of the GC-MS study (base peak at 114 m/z and intense fragment peak at 138 m/z) were in accordance with the earlier study (CitationKeeler et al., 1990). These results confirmed the presence and purity of isolated solasodine.
PTZ and PCT (epileptogenic chemicals) produced marked seizures in the control animals. In these tests, the animals behaved in a characteristic manner and showed progressive signs of epilepsy such as ear and facial twitching, convulsive waves axially through the body, myoclonic body jerks, straub tail, generalized clonic convulsions, twitching and turning of animals to one side, HLTE phase (the severe generalized tonic convulsion phase), stupor, and recovery or death. In the PCT model, prior treatment of solasodine (25 mg/kg, i.p.) significantly (p < 0.01) attenuated the latency of HLTE phase. Recently it has been reported that PCT blocks the GABAA receptor-linked chloride ion channels (CitationMeldrum & Rogawski, 2007) which normally open to allow increased chloride ion conductance into brain cells (CitationAmabeoku et al., 2007) and thus elicits seizures by inhibition and/or attenuation of gamma aminobutyric acid (GABAergic) neurotransmission (CitationKatzung, 1992; CitationLeonard, 2000; CitationRang et al., 2003; CitationOjewole, 2008). The seizures induced by PCT are analogous to the petit mal type of epilepsy in humans (CitationMurali et al., 2008). Inhibition of PCT-provoked convulsions by solasodine may be attributed to the augmentation of GABAergic transmissions. Interestingly, higher doses of solasodine (50 and 100 mg/kg, i.p.) failed to produce significant antiseizure effects in this model. The effect is similar to certain CNS depressant drugs like DZP, which exerts antiepileptic effects at lower doses only. The possible explanation could be the saturation of the GABAA receptors by the higher doses of these drugs.
In the PTZ model, solasodine (25, 50, and 100 mg/kg, i.p.) failed to prevent PTZ-induced seizures. The PTZ test represents a valid model for human generalized myoclonic seizures and also generalized seizures of the petit-mal (absence)-type (CitationMalawska & Scatturin, 2003). Drugs protecting against seizures induced by PTZ, reduce T-type calcium currents (CitationMurali et al., 2008) and are considered to be useful for control of myoclonic and absence seizures (CitationNisar et al., 2009). Since PTZ is a potent GABAA receptors antagonist (CitationJalalpure et al., 2009) and solasodine is ineffective in this model, it is likely that solasodine might not act through this mechanism.
Solasodine (25, 50, and 100 mg/kg, i.p.) showed dose-dependent significant (p < 0.001) anticonvulsant activity in the MES-induced convulsions paradigm. It is well-established that antiepileptic drugs prevent MES-induced HLTE phase (tonic seizures) by inhibition of voltage-dependent Na+ channels (CitationRogawski & Porter, 1990; CitationMandhane et al., 2007). Protection offered by solasodine against HLTE phase in this model predicts inhibition of voltage-dependent Na+ channels to prevent the seizure discharge in the brain.
In the thiopental-provoked sleep model, solasodine dose-dependently potentiated the effects of thiopental. The prolongation of thiopental-induced sleeping time may be attributed to an inhibition of thiopental metabolism or to an action on the central mechanism involved in the regulation of sleep (CitationN’Gouemo et al., 1994), thus suggesting solasodine as a neurosedative drug (CitationCapasso et al., 1996). The results of acute toxicity studies indicated a broad margin of safety of solasodine in mice.
In the light of the above discussion, it is likely that antiepileptic effects of solasodine may be attributed to the opening of chloride ion channels and enhancing GABAergic neurotransmission which plays an important role in epilepsy (CitationIto et al., 2005; CitationPerucca, 2005).
Conclusion
The present study for the first time reports potent antiepileptic and CNS depressant effects of solasodine. The possible mechanisms for these effects could be the facilitation of chloride ions through GABAA receptors and inhibition of voltage-dependent Na+ channels. Our results support the traditional claims of S. sisymbriifolium for the treatment of epilepsy and related disorders. Future study should focus on the identification of exact mechanisms of action of solasodine for the potential CNS modulating properties using different animal models.
Acknowledgements
Special thanks to Pran Jeevan Parmar of the Botanical Survey of India for identification and authentication of the plant. We are grateful to Dr. Navin Sheth, Head of the Department of Pharmaceutical Sciences, Saurashtra University, Rajkot, Gujarat (India) for providing the facilities during the course of this study. The gift sample of PHE by Piramal Healthcare, Himachal Pradesh (India), is gratefully acknowledged.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amabeoku GJ, Green I, Kabatende J. (2007). Anticonvulsant activity of Cotyledon orbiculata L. (Crassulaceae) leaf extract in mice. J Ethnopharmacol, 112, 101–107.
- Banerjee PN, Filippi D, Hauser WA. (2009). The descriptive epidemiology of epilepsy- A review. Epilepsy Res, 85, 31–45.
- Basu A, Lahiri SC. (1977). Some pharmacological actions of solasonine. Indian J Exp Biol, 15, 285–289.
- Capasso A, Aquino R, De Simone F, Sorrentino L. (1996). Neuropharmacological effects of extracts from Sickingia williamsii. J Pharm Pharmacol, 48, 592–595.
- Chakravarty AK, Mukhopadhyay S, Saha S, Pakrashi SC. (1996). A neolignan and sterols in fruits of S. sisymbriifolium. Phytochemistry, 41, 935–939.
- Cham BE, Daunter B, Evans RA. (1991). Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett, 59, 183–192.
- Cham BE, Gilliver M, Wilson L. (1987). Antitumor effects of glycoalkaloids isolated from Solanum sodomaeum. Planta Med, 53, 34–36.
- Chand R, Kumar S, Sharma AK, Srivastava L. (1995). Variation of solasodine in Solanum sisymbrifolium and Solanum xanthocarpum with plant growth and development. Indian Drugs, 32, 362–365.
- Dinan L, Harmatha J, Lafont R. (2001). Chromatographic procedures for the isolation of plant steroid. J Chromatogr, 935, 105–123.
- Dixit VP, Verma M, Mathur NT, Mathur R, Sharma S. (1992). Hypocholesterolaemic and antiatherosclerotic effects of solasodine (C27H43O2N) in cholesterol fed rabbits. Phytother Res, 6, 270–273.
- Emmanuel S, Ignacimuthu S, Perumalsamy R, Amalraj T. (2006). Anti-inflammatory activity of Solanum trilobatum. Fitoterapia, 77, 611–612.
- Ferro EA, Alvarenga NL, Ibarrola DA, Hellión-Ibarrola MC, Ravelo AG. (2005). A new steroidal saponin from Solanum sisymbriifolium roots. Fitoterapia, 76, 577–579.
- Gupta YK, Malhotra J, George B, Kulkarni SK. (1999). Methods and consideration for experimental evalution of antiepileptic drugs. Indian J Physiol Pharmacol, 43, 25–43.
- Gupta YK, Malhotra J. (2000). Antiepileptic drug therapy in the twenty first century. Indian J Physiol Pharmacol, 44, 8–23.
- lbarrola DA, Hellión-lbarrola MC, Montalbetti Y, Heinichen O, Alvarenga N, Figueredo A, Ferro EA. (2000). Isolation of hypotensive compounds from Solanum sisymbriifolium Lam. J Ethnopharmacol, 70, 301–307.
- Ito M, Ohmori I, Nakahori T, Ouchida M, Ohtsuka Y. (2005). Mutation screen of GABRA1, GABRB2 and GABRG2 genes in Japanese patients with absence seizures. Neurosci Lett, 383, 220–224.
- Jalalpure SS, Salahuddin M, Shaikh IM, Manvi FV. (2009). Anticonvulsant effects of Calotropis procera root in rats. Pharma Biol, 47, 162–167.
- Katzung W. (1992). Legal and illegal drugs- Status, selected problems, trends. Z Arztl Fortbild, 86, 689–699
- Keeler RF, Dale I, Baker C, Gaffield W. (1990). Spirosolane-containing Solanum species and induction of congenital craniofacial malformations. Toxicon, 28, 873–884.
- Kusano G, Takahashi A, Sugiyama K, Nozoe S. (1987). Antifungal properties of Solanum alkaloids. Chem Pharm Bull, 35, 4862–4867.
- Leonard B. (2000). Hormones, brain and neuropsychopharmacology- Second International Congress. Indian Drugs, 1176–1181.
- Lin CN, Chung MI, Gan KH. (1988). Novel antihepatotoxic principles of Solanum incanum. Planta Med, 54, 222.
- Lin CN, Lu CM, Cheng MK, Gan KH, Won SJ. (1990). The cytotoxic principles of Solanum incanum. J Nat Prod, 53, 513–516.
- Litchfield JT, Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther, 96, 99–113.
- Malawska B, Scatturin A. (2003). Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs. Mini Rev Med Chem, 3, 341–348.
- Mandhane SN, Aavula K, Rajamannar T. (2007). Timed pentylenetetrazol infusion test: A comparative analysis with s.c. PTZ and MES models of anticonvulsant screening in mice. Seizure, 16, 636–644.
- Mazumdar BC. (1984). Steroidal sapogenins in two wild species of Solanum. Sci Cult, 50, 122–123.
- Meldrum BS, Rogawski MA. (2007). Molecular targets for antiepileptic drug development. Neurotherapeutics, 4, 18–61.
- Murali G, Panneerselvam KS, Panneerselvam C. (2008). Age-associated alterations of lipofuscin, membrane-bound ATPases and intracellular calcium in cortex, striatum and hippocampus of rat brain: Protective role of glutathione monoester. Int J Dev Neurosci, 26, 211–215.
- N’Gouemo P, Baldy-Moulinier M, N’Guemby-Bina C. (1994). Effects of an ethanolic extract of Desmodium adscendens on central nervous system in rodents. J Ethnopharmacol, 52, 77–83.
- Nag D. (2000). Gender and epilepsy: A clinician’s experience. Neurol Ind, 48, 99–104.
- Nakamura T, Komori C, Lee Y, Hashimoto F, Yahara S, Nohara T, Ejima A. (1996). Cytotoxic activities of Solanum steroidal glycosides. Biol Pharm Bull, 19, 564–566.
- Nes WD, Hanners PK, Bean GA, Patterson GW. (1982). Inhibition of growth and sitosterol-induced sexual reproduction in Phytophtora cactorum by steroidal alkaloids. Phytopathology, 72, 447–450.
- Nisar M, Ahmad M, Wadood N, Lodhi MA, Shaheen F, Choudhary MI. (2009). New diterpenoid alkaloids from Aconitum heterophyllum Wall: Selective butyrylcholinestrase inhibitors. J Enzyme Inhib Med Chem, 24, 47–51.
- Nohara T, Ikeda T, Fujiwara Y, Matsushita S, Noguchi E, Yoshimitsu H, Ono M. (2007). Physiological functions of solanaceous and tomato steroidal glycosides. J Nat Med, 61, 1–13.
- Ojewole JA. (2008). Anticonvulsant property of Sutherlandia frutescens R. BR. (variety Incana E. MEY.) [Fabaceae] shoot aqueous extract. Brain Res Bull, 75, 126–132.
- Perucca E. (2005). An introduction to antiepileptic drugs. Epilepsia, 46, 31–37.
- Quintans-Júnior LJ, Souza TT, Leite BS, Lessa MN, Bonjardim LR, Santos MR, Alves, PB, Blank AF, Antoniolli AR. (2008). Phytochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine, 15, 619–624.
- Rang HP, Dale MM, Ritter JM, Moore PK. (2003). Pharmacology, 5th ed. Churchill Livingstone, Edinburgh, Elsevier, pp. 550–561.
- Rogawski MA, Porter RJ. (1990). Antiepileptic drugs: Pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev, 42, 223–286.
- Rowan DD, Macdonald PE, Skipp RA. (1983). Antifungal stress metabolites from Solanum aviculare. Phytochemistry, 22, 2102–2104.
- Rudiger K. (2002). Voltage-gated sodium channels in epilepsy. Epilepsia, 43, 1278–1295.
- Sayyah M, Moaied S, Kamalinejad M. (2005). Anticonvulsant activity of Heracleum persicum seed. J Ethnopharmacol, 98, 209–211.
- Smith SW, Giesbrecht E, Thompson M, Nelson LS, Hoffman RS. (2008). Solanaceous steroidal glycoalkaloids and poisoning by Solanum torvum, the normally edible susumber berry. Toxicon, 52, 667–676.
- Subramani J, Josekutty PC, Mehta AR, Bhatt PN. (1989). Solasodine levels in Solanum sisymbriifolium. Indian J Exp Biol, 27, 189.
- Sukma M, Chaiyo C, Murakami Y, Tohda M, Matsumoto K, Watanabe H. (2002). CNS inhibitory effects of barakol, a constituent of Cassia siamia Lamk. J Ethnopharmacol, 83, 87–94.
- Vaidhya AB. (1997). The status and scope of Indian medicinal plants acting on central nervous system. Indian J Pharmacol, 29, 340–343.
- Vellucci SV, Webster RA. (1984). Antagonism of caffeine-induced seizures in mice by Ro15-1788. Eur J Pharmacol, 97, 289–293.
- Vyawahare NS, Khandelwal AR, Bathra VR, Nikam AP. (2007). Herbal anticonvulsants. J Herb Med Toxicol, 1, 9–14.
- Weissenberg M. (2001). Isolation of solasodine and other steroidal alkaloids and sapogenins by direct hydrolysis-extraction of Solanum plants or glycosides there from. Phytochemistry, 58, 501–508.
- Wolters B. (1976). Screening studies on the transformation and degradation of secondary plant substances by microorganisms by using TLC-ready-sheets as carriers of the substance. Planta Med, 29, 41–53.
- Yemitan OK, Adeyemi OO. (2005). CNS depressant activity of Lecaniodiscus cupanioides. Fitoterapia, 76, 412–418.