Abstract
Context: Hesperidin is a flavonoid that has various pharmacological activities including anti-inflammatory, antimicrobial and antiviral activities.
Objective: The aim of the study is the isolation of hesperidin from the peel of Citrus sinensis L. (Rutaceae), and the evaluation of its antioxidant capacity and cytotoxicity against different human carcinoma cell lines.
Materials and methods: In the present work, hesperidin is identified and confirmed using chromatographic and spectral analysis. To correlate between hesperidin concentration and antioxidant capacity of peel extracts, extraction was carried out using 1% HCl-MeOH, MeOH, alkaline solution, the concentration of hesperidin determined qualitatively and quantitatively using high performance thin layer chromatography (HPTLC), high performance liquid chromatography (HPLC) analysis, in vitro antioxidant capacity of hesperidin and the extracts against free radical diphenylpicrylhydrazyl (DPPH•) performed using an electron spin resonance spectrophotometer (ESR). Cytotoxic assay against larynx, cervix, breast and liver carcinoma cell lines was performed.
Results: Hesperidin was found to be moderately active as an antioxidant agent; its capacity reached 36%. In addition, the results revealed that hesperidin exhibited pronounced anticancer activity against the selected cell lines. IC50 were 1.67, 3.33, 4.17, 4.58 µg/mL, respectively.
Discussion and conclusion: Orange peels are considered to be a cheap source for hesperidin which may be used in the pharmaceutical industry as a natural chemopreventive agent. Hesperidin and orange peel extract could possess antioxidant properties with a wide range of therapeutic applications.
Introduction
There is a great tendency to produce pharmaceuticals from natural resources and preferably from agricultural waste. Fruit waste, especially orange peel, was selected as an attractive source of active ingredients of pharmaceuticals because it is available and inexpensive. The accumulation of unused peel may lead to environmental and health problems. The orange peel fungus (Aleuria aurantia) may be confused with Otidea or Caloscypha species that are poisonous or of unknown edibility (CitationNilsson & Persson 1977; CitationYao & Spooner 1995).
Citrus peel is characterized by the presence of phenolics and vitamin C; however, in some cultivars the peel contents are higher than flesh contents (CitationYoo et al., 2004). These compounds were found to have antioxidant activity and can reduce the risk of some cancers and cardiovascular diseases (CitationYoo et al., 2004; CitationPalma et al., 2005).
Hesperidin, a flavanone glycoside, one of the main flavonoids in Citrus displays a wide spectrum of pharmacological effects and is referred to as a bioflavonoid. It, both alone or in combination with other flavonoids, has anticancer activity and can reduce proliferation of colon carcinoma in animal models (CitationTanaka et al., 1997a), also it inhibits skin tumorigenesis in mice (CitationKoyuncu et al., 1999) and had an apoptotic effect on human colon cancer cells (CitationPark et al., 2003). Hesperidin also shows antimutagenic effect against chemical mutants (CitationTanaka et al., 1997b; CitationFranke et al., 1998). Antioxidant properties of hesperidin have been studied but the results varied considerably among different reports (CitationGarg et al., 2001; CitationLee et al., 2002; CitationKim et al., 2004).
Vitamin P (citrin) which is a mixture of two flavonoids: eriodictyol and hesperidin could decrease capillary permeability and fragility in guinea-pigs (CitationGarg et al., 2001). Hesperidin usually in combination with ascorbic acid is employed to reduce capillary fragility. It is indicated in prevention and correction of capillary permeability or fragility in cerebrovascular and cardiovascular diseases, hypertension, hemorrhagic nephritis, and habitual and threatened abortion (CitationBalbaa et al., 1976). Hesperidin is also important for absorption and retention of vitamin C. Hesperidin’s deficiency is linked with abnormal capillary leakiness, pain in the extremities causing aches, weakness and night leg cramps (CitationGarg et al., 2001).
Hesperidin, in combination with other bioflavonoid diosmin (Daflon) is usually used as a therapeutic agent in the treatment of venous insufficiency and to prevent ischemia reperfusion (CitationKorthuis & Gute, 1999). Anti-inflammatory activity (CitationEmim et al., 1994) in addition to antiviral (CitationBae et al., 2000; CitationKim et al., 2000) and anti-hypercholesterolemia (CitationMonforte et al., 1995) activities have been reported as important biological effects of hesperidin.
On the other hand, oxidative compounds could lead to health problems and serious diseases as stroke, heart disease, Alzheimer’s disease, and atherosclerosis because they can oxidize several cellular components including essential protein, non-protein thiol, low density lipoproteins LDL, DNA and membrane phospholipids (CitationKim et al., 2004). Thus, one of the aims of this work is to produce natural antioxidant agents from a cheap source. Hesperidin accumulates in citrus peel in considerably high amounts; its concentration reaches 5.9–7.3% on dry weight bases (CitationManthey & Grohman, 1996).
In brief, the aim of this work is the effective management of large amounts of citrus fruit peel as cheap available source of hesperidin, isolation of hesperidin from the peel, and the study of the cytotoxicity and antioxidant capacity of hesperidin and the peel extracts.
Materials and methods
Plant material
Dried peel of mature orange, Citrus sinensis (L.) Osbeck var. Navel (Rutaceae), was obtained from the Egyptian market, identified by Zakaria Fouad at the Agriculture and Biological Research Division, National Research Centre (NRC), Egypt. A voucher specimen has been deposited at NRC Herbarium with registration number CAIR5800. Identification was confirmed by Salwa Kawashty, Department of Chemotaxonomy, NRC.
Instruments
Spectro UV-VIS: double beam UVD-3500, Labomed, INC; FAB/Mass: Jeol JMs- AX500, +ve mode; 1H-NMR: Jeol 500 MHz, Chemical shift values are in ppm, J values are in Hz; ESR: Bruker, Elexsys, x-band, modulation frequency, 500 MHz, the sample inserted via quartz liquid flat cell, average scans 1, state of aggregation C, field mod. Amplitude 0.0002, Field Mod. Frequency (Hz) 100000, Microwave Frequency (Hz) 9.77568e+09, Microwave Power (w) 0.00202637, and Receiver gain 65, Receiver harmonic 1; HPLC: Shimadzu, UV detector; wavelength 254 nm, Column C18, 5 μm, 0.4 × 25 cm, mobile phase (isocratic); F.R 1.5 mL/min.
Chemicals
Authentic (standard) hesperidin (98%) was purchased from Roth, Germany, DPPH• (2,2-diphenyl-1-picrylhydrazyl 95%) from Sigma, Vitamin C (> 99.5%) from Fluka, Doxorubicin injectable grade (98–102%) from Pharmacia. Solvents used for analysis are all HPLC grade.
Extraction and isolation of hesperidin
Ripe orange peel (100 g) was dried at 50°C, crushed, then treated with 300 mL CaCl2 for 10 min, to coagulate slimy materials, filtered using Buchner, the filtrate was rendered alkaline by NaOH (0.2 N) till pH 11 then the solution was heated at a temperature 40–50°C in a water bath for 1 h. Diatomaceous clay was added while stirring to facilitate filtration, then the solution was acidified by HCl at pH 6, allowed to stand for crystallization for 24 h. Identification of the isolated hesperidin was established by direct comparison and co-chromatography with an authentic sample using HPTLC with solvent system CHCl3-MeOH-H2O (30:60:5, v/v/v) and examination of the spot under UV light before and after exposure to ammonia. The structure of hesperidin was confirmed based on spectral analysis UV, FAB/Ms, 1H-NMR.
Quantitative determination of hesperidin in different extracts
Hesperidin was extracted via three different methods to correlate between hesperidin concentrations and antioxidant capacity of the extracts.
First extract: orange peel (50 g) was dried at 50°C, crushed and extracted by maceration in 200 mL MeOH-1% HCl at room temperature overnight, then filtered. The filtrate was concentrated using a rotary evaporator under reduced pressure.
Second extract: orange peel (50 g) was dried at 50°C, crushed and defatted by maceration in 150 mL petroleum ether 60–80°C, heated at 45°C for 2 h and then filtered; the residue was extracted with 200 mL methanol in a Soxhlet at 65°C for 4 h, then the solution concentrated under reduced pressure.
Third extract: orange peel (50 g) was dried at 50°C, crushed and extracted by 200 mL alkaline NaOH solution (0.2 N) at 10–11 pH, heated at 40–50°C for 1 h and the solution was then acidified using 2 N HCl to reach 4–4.5 pH and kept overnight for precipitation.
Identification of extracted hesperidin in the three different extracts was established by direct comparison and co-chromatography against the authentic sample using HPTLC with solvent system CHCl3-MeOH-H2O (30:60:5, v/v/v) and examination of the spot under UV light before and after exposure to ammonia.
The dry matter of the three extracts was analyzed by HPLC using solvent system acetonitrile-ammonia acetate buffer (80:20, v/v) at pH of 4.4 (isocratic). Authentic hesperidin was dissolved in acetonitrile-ammonia acetate (80:20, v/v) (1 mg/1 mL). The dry matter of each extract (5 mg) was dissolved in 5 mL of the same solvent. Samples were filtered through a 0.45 μ Millipore filter and injected into an HPLC column (25 μL). The retention time for hesperidin, RT = 1.88 min. The concentration of hesperidin in the three extracts was based on the calculation of the percentage peak areas of the samples compared with that of standard hesperidin, the analyses were done in duplicate.
Antioxidant capacity
The antioxidant capacity of hesperidin and different prepared extracts was evaluated by ESR to measure the scavenging power to the free radical DPPH•. Antioxidant capacity was measured by adding 1 mL of 10−3 M DPPH• (in DMSO) to 1 mg of vitamin C as positive control, 1 mg of hesperidin or 1 mg of each dry extract. All tested samples were dissolved in 1 mL DMSO. The integrated areas of DPPH• signals were taken as a measurement of the antioxidant capacity, thus as the area decreased the antioxidant capacity increased. The calculation carried out provided that one mole of the antioxidant agent reacted with one mole of the free radical. The measurements were taken in triplicates after 5 min. Antioxidant capacity was estimated using the following equation:
where A0 = integrated area of DPPH•, A1 = integrated area of DPPH•with tested sample.
Cytotoxic assay
Potential cytotoxic activity of hesperidin was performed at the National Institute of Cancer, Egypt. Cytotoxicity was carried out using a sulforhodamine B (SRB) assay (CitationSkehan et al., 1990) against breast (MCF7) [HTB-22 N+5], larynx (HeP2) [CCL-23 N+11], cervix (HeLa) [CCL2 N+8], and liver (HPg2) [ATCCHB-8065 N+7] carcinoma cell lines. Doxorubicin was used as positive cytotoxic control agent. Cancer cells were plated in (104 cells/well) for 24 h before treatment with tested samples to allow attachment of the cells to the plate. Different concentrations of the tested sample added to the cells mono layer. Triplicate wells were repeated for each dose; the cells were incubated for 48 h at 37°C and 5% CO2 then the cells were fixed, washed and stained with sulforhodamine B stain. Excess stain was washed with acetic acid and the attached stain recovered with Tris EDTA buffer. Color intensity was measured in the ELISA reader and the IC50 of the oil against each carcinoma cell lines was calculated in comparison to standard drugs.
Results and discussion
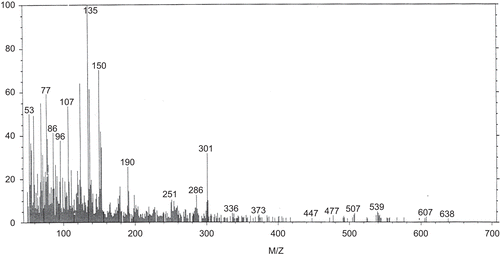
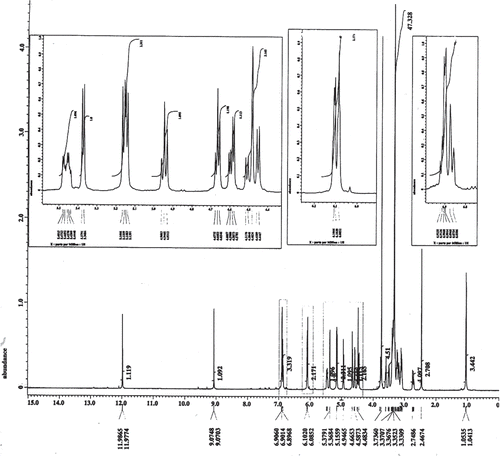
The isolated hesperidin was identified by comparison of its physical and spectroscopic data (UV, FAB/Ms, and 1H-NMR) (, and ) with the corresponding authentic (standard) sample and published data (CitationMabry et al., 1970). The purity of the isolated hesperidin was found to be 97.3% from HPLC analysis. It was found to be yellow amorphous powder, melting point (mp) 250–252°C. Matched with an authentic hesperidin sample using co-chromatography HPTLC (CHCl3-MeOH-H2O 30:60:5, v/v/v), Rf (0.44), the color of the spot under UV light (365 nm) deep purple changed to light blue after exposure to ammonia vapor. UV showed λmax (MeOH) at 284, 328, λmax (NaOMe) at 243, 286, 356 λmax (AlCl3) at 307, 386, λmax (AlCl3/HCl) at 304, 378, λmax (NaOAc) at 284, 328 and λmax (NaoAc/H3BO3) at 284, 328. FAB/Ms; positive mode showed a pseudo-molecular ion peak (M+1) at m/e 610, which constitute to a molecular formula (C28H34O15) with other main fragments m/e (53, 50%; 77, 58%; 107, 55%; 135, 100%; 190, 27%; 301, 35%). 1H-NMR (DMSO); showed the main characteristic signals of hesperidin, δppm 6.9 (m, H-5″); 6.9 (m, H-6′); 6.9 (m, H-2′); 6.08 (d, J = 1.8 Hz, H-6); 6.1 (d, J = 1.8 Hz, H-8); 4.95 (d, J = 7.5 Hz, H-glu); 4.5 (d, J = 1.8 Hz, H-rh); 5.17 (dd, J = 12.6 Hz, 2.5Hz, H-2); 3.25 (dd, J = 17.1 Hz, 12.8 Hz, H-3ax); 2.74 (dd, J = 17.1 Hz, 2.5 Hz, H-3eq); 3.8 (s, OCH3); 1.0 (d, J = 6 Hz, CH3-rh).
Hesperidin concentration and antioxidant capacity
The results showed that total amounts of extracts from the orange peel were 18.5, 18.7 and 16.9 g, for the former three methods of extraction, respectively. HPLC analysis showed that concentration of hesperidin in peel extract using 1% HCl methanol mixture at room temperature was 3.95% ± 0.05%, while defatting and extraction with hot methanol in a Soxhlet yielded 4.65% ± 0.3% total amount of hesperidin, and finally extraction by heating with alkaline solution gave the highest hesperidin concentration reaching 5.97% ± 0.25% on dry weight basis.
The hesperidin yields were 0.73, 0.87 and 1.008 g, respectively. Although the method of extraction with hot alkaline solution gave the lowest crude extract, yet this method gave the highest hesperidin yield. This is in good agreement with studies illustrating that heat and alkalinity accelerate hesperidin extraction from source material due to its basic nature (CitationBalbaa et al., 1976). The data of the present work illustrate that extraction with hot alkaline solution is the optimum extraction method giving the highest hesperidin yield.
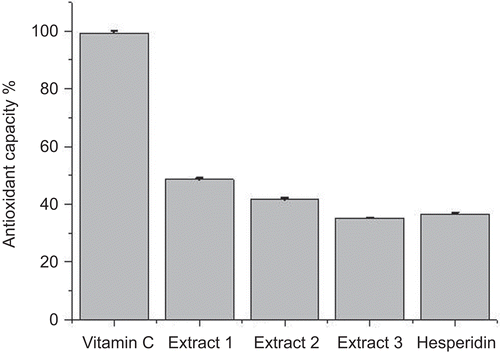
The results obtained show that orange peel extract contains a considerable amount of hesperidin up to 5.97%. Regarding antioxidant capacity of the extracts using ESR, it was found to be inversely correlated to hesperidin concentration (R2 = 0.9623) and reached 48.53% ± 0.5%, 41.63% ± 0.55% and 35.19% ± 0.17% for the three former extracts respectively and 36.65% ± 0.56% for hesperidin comparing with 99.33% ± 0.76% antioxidant capacity of vitamin C (). The increment of antioxidant capacity in the obtained extracts is due to extraction of higher concentration of other antioxidant agents besides hesperidin that exerted synergetic effect (CitationPalma et al., 2005; CitationCirico & Omaye, 2006; CitationMcKay & Blumberg, 2007).
Figure 4. Antioxidant capacity (DPPH scavenging capacity) of vitamin C, Citrus sinensis L. peel extracts and hesperidin.
In the present work, antioxidant capacity of hesperidin and peel extract was measured in vitro for the first time using ESR techniques, since the only report about using ESR for measuring the antioxidant action of hesperidin to nitric oxide radicals was applied in vivo in Wistar rats (CitationTimoshin et al., 2005). ESR measures the exact area of the free radical DPPH• so there is no chance of miss-measurements of antioxidant capacity. On the other hand, the majority of antioxidant assays of hesperidin were carried out by using either the Crocin bleaching inhibition method (CitationFinotti & Majo, 2003), the spectrophotometer method (CitationBrand-Williams et al., 1995) or by measuring oxygen radical absorbance capacity (ORAC) (CitationLin & Chin, 2006).
The antioxidant capacity of hesperidin in the present study is found to be moderately active compared with other reports that found hesperidin to be inactive (CitationGarg et al., 2001), or had slow but long-lasting activity (CitationLee et al., 2002). Other reports consider hesperidin and other flavonoids in addition to vitamin C as the main antioxidant agents in Citrus (CitationPalma et al., 2005). Moreover, hesperidin enhanced antioxidant status in nicotine-induced toxicity (CitationVenugopal & Menona, 2007). The variation of the antioxidant behavior of crude hesperidin extracts in previous reports is due to climate, soil, fruit variety and degree of maturation (CitationWang et al., 1996). These variations lead to change in the phenolics and vitamins in these extracts in addition to the solvent influence. In polyphenol redox systems the solvents play a fundamental role in the chemical behavior of these compounds, and consequently their antioxidant property. This means that the compounds present different chemical forms related to the environment in which they are solved during the measurement of the antioxidant assay (CitationPedrielli et al., 2001; CitationFinotti & Majo, 2003). Also, kinetic factors may be of great importance (CitationFoti & Ruberto, 2001). Furthermore, the antioxidant behavior of the flavanones varies according to the oxidant radical; the antioxidant potential of the flavanones obtained by the radical DPPH• was lower than those obtained by the radical 2,2-azino-bis(3-ethylbenzthiazotin-6-sulfonic acid) (ABTS) (CitationGardner et al., 2000). DPPH•, despite being recommended as an easy, accurate method for measuring antioxidant activity of fruit and vegetable juices, is less sensitive to hydrophilic antioxidants (CitationGil et al., 2000). This may be another reason for variations of antioxidant capacity values obtained in the present work rather than previous reports.
Cytotoxic activity
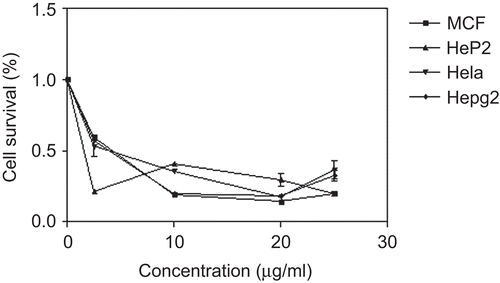
Results show that hesperidin has significant cytotoxic effect against selected human cancer cell lines including larynx, cervix, breast and liver. IC50 values occurred below 10 µg/mL. IC50 values for the formal cell lines were 1.67, 3.33, 4.17, 4.58 µg/mL for hesperidin comparing with 0.4, 0.85, 0.7, 0.6 µg/mL for standard doxorubicin respectively. The larynx cell line was the most affected one and suffered the highest inhibitory action of hesperidin (). The cytotoxic activity of hesperidin is in close agreement with studies that reported hesperidin to have chemoprevention activity, inhibit colon carcinogenesis in rats and have an apoptotic effect in human colon cancer cells (CitationTanaka et al., 1997a; CitationPark et al., 2003) hesperidin also inhibited tumor promotion in skin tumorigenesis in mice (CitationKoyuncu et al., 1999;). Hesperidin vandyl complex showed in vitro antitumoral activity against rat osteosarcoma and human colon adenocarcinoma (CitationEtcheverry et al., 2008). The anticancer potential of hesperidin could be a result of its antioxidant capacity (CitationGarg et al., 2001; CitationKim et al., 2004), increased suppression of cell proliferation (CitationPark et al., 2003) and antimutagenic effect (CitationAmirhosein et al., 2008). Hesperidin and its metabolite, hesperetin were found to be the most potent agents inhibiting neoplastic transformation in murine fibroblasts among dietary flavonoids (CitationFranke et al., 1998).
Figure 5. Cytotoxic activity of hesperidin against breast (MCF7), larynx (HeP2) cervix (HeLa), and liver (HPg2) carcinoma.
The results obtained in the present work illustrate in vitro antioxidant capacity and cytotoxic activity of hesperidin. These results may extrapolate the potential effect of hesperidin in vivo as an essential step for drug production.
In conclusion, orange peels could be considered as a cheap and easy source of the medicinally important hesperidin. It is extracted efficiently with high purity (97.3%). It exhibited highly cytotoxic activity against the selected human carcinoma cell lines and antioxidant activity against free radical DPPH•. It could be used in the pharmaceutical industry as a natural cytotoxic agent as well as a natural coloring agent in food industry. In addition, hesperidin and orange peel extract could possess antioxidant properties with wide range of therapeutic applications. Further work is needed to produce novel drugs from this natural source.
Acknowledgements
Deep thanks are due to Nabaweya Ibrahim, of the National Research Centre, for her valuable advice and critical reading.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amirhosein A, Sayel GH, Fashad N, Ebrahim H, Mehran G. (2008). Chemoprotective effects of hespridin against genotoxicity induced by cyclophosphamide in mice. Arch Pharm Res, 31, 794–797.
- Bae EA, Han MJ, Kim DH. (2000). In vitro inhibitory effect of some flavonoids on rotavirus. Biol Pharm Bull, 23, 1122–1124.
- Balbaa SI, Hilal SH, Zaki AY. (1976). Medicinal plants constituents, second edition. Egypt: Central Agency for University and School Books.
- Brand-Williams W, Cuvelier ME, Berest C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wiss und-Technology, 28, 25–30.
- Cirico TL, Omaye ST. (2006). Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem Toxicol, 44, 510–516.
- Emim JADS, Oliveira AB, Lapa AJ. (1994). Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin and the isoflavonoids duartin and claussequinone, in rats and mice. J Pharm Pharmacol, 46, 118–122.
- Etcheverry SB, Ferrer EG, Naso L, Rivadeneira J, Salinas V, Williams PAM. (2008). Antioxidant effects of the VO (IV) hesperidin complex and its role in cancer chemopreventive. J Biol Inorg Chem, 13, 435–447.
- Finotti E, Majo D. (2003). Influence of solvents on the antioxidant property of flavonoids. Nahran /Food, 47, 168–187.
- Foti M, Ruberto G. (2001). Kinetic solvent effects on phenolic antioxidants determined by spectrophotometric measurements. J Agric Food Chem, 49, 342–348.
- Franke AA, Cooney RV, Custer LJ, Mordan LJ, Tanaka Y. (1998). Inhibition of neoplastic transformation and bioavailability of dietary flavonoid agents. Adv Exp Med Biol, 439, 237–248.
- Gardner PT, White TA, McPhail DB, Duthie GG. (2000). The relative contribution of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem, 68, 471–474.
- Garg A, Garg S, Zaneveld LJD, Singla AK. (2001). Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res, 15, 655–609.
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem, 48, 4581–4589.
- Kim DH, Song MJ, Bae EA. (2000). Inhibitory effect of herbal medicines on rotavirus infectivity. Biol Pharm Bull, 23, 2183–2188.
- Kim J, Jung K, Choi J, Chung H. (2004). Hesperetin: A potent antioxidant against peroxynitrite. Free Radic Res, 38, 761–769.
- Korthuis RJ, Gute DC. (1999). Adhesion molecule expression in post ischemic microvascular dysfunction: activity of a micronised purified flavonoid fraction. J Vasc Res, 36, 15–23.
- Koyuncu H, Berkarda B, Baykut F, Soybir G, Alati C, Gul H, Altun M. (1999). Preventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoter. Anticancer Res, 19, 3237–3241.
- Lee J, Kang Y, Kang J. (2002). Free radical scavenging of flavonoids and their effects on erythrocyte naleak, platelet aggregation and TBARS production. Nutr Sci, 5, 197–202.
- Lin HC, Chin YG. (2006). Induction of cell apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Molecular Nutr Food Res, 50, 1072–1079.
- Mabry TJ, Markham KR, Thomas MB. (1970). The systematic identification of flavonoids. New York, USA: Springer Verlag.
- Manthey JA, Grohman K. (1996). Flavonoids in citrus processing byproducts. J Agric Food Chem, 44, 811–814.
- McKay DL, Blumberg JB. (2007). A review of the bioactivity of South African herbal teas, (Aspalarhus linearis) and honey bush (Cyclopia intermedia). Phytother Res, 21, 1–16.
- Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Lo-Curto RB. (1995). Biological effects of hesperidin, a citrus flavonoid, part 2: Hypolipidemic activity on experimental hypercholesterolemia in the rat. Farmaco, 50, 595–599.
- Nilsson S, Persson O. (1977). Fungi of Northern Europe 1: Larger fungi (excluding gill fungi). Penguin Books.
- Palma S, D’Aquino S, Agabbio M, Schirru S. (2005). Changes in flavonoids, ascorbic acid, polyphenol content and antioxidant activity in cold-stored fortune mandarin. Acta Horti, 682, 617–622.
- Park H, Kim M, Ha E, Chung J. (2003). Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells SUN-C4. Phytomedicine, 15, 147–151.
- Pedrielli P, Peduli GF, Skibsted LH. (2001). Antioxidant mechanism of flavonoids, solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J Agric Food Chem, 49, 3034–3040.
- Skehan P, Storen R, Scudiero D, Monks A, McMahon J, Vistic D, Warren JT, Boekesch H, Kenney S, Boyd MR. (1990). New colorimetric cytotoxic assay for anticancer-drug screening. J Natl Cancer Inst, 82, 1107–1112.
- Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M. (1997b). Modulation of N-methyl-N-amylnitrosamine induced tumorigenesis by dietary feeding of diosomin and hesperidin, alone and in combination. Cacinogenesis, 18, 761–769.
- Tanaka T, Makita H, Kawabata K, Mori H. (1997a). Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperdin. Carcinogenesis, 18, 957–965.
- Timoshin AA, Dorkina EG, Paukova EO, Vanin AF. (2005). Quercetin and hesperidin decrease the formation of nitric oxide radicals in rat liver and heart under the conditions of hepatosis. Biofizika, 50, 1145–1149.
- Venugopal P, Menona AB. (2007). Effect of hespridin on matrix metalloproteinases and antioxidant status during nicotine-induced toxicity. J Toxcol, 238, 90–980
- Wang H, Cao G, Prior RL. (1996). Total antioxidant capacity of fruits. J Agric Food Chem, 4, 701–7405.
- Yao YJ, Spooner BM. (1995). Notes on British taxa referred to Aleuria. Mycological Res, 99, 1515–1518.
- Yoo K, Lee K, Park J, Lee H, Hwang I. (2004). Variation in major antioxidant and total antioxidant activity of yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J Agric & Food Chem, 52, 5907–5913.