Abstract
Context: Copaiba oil is an oleoresin made up of resin acids and volatile compounds, and it is obtained by tapping the trunks of trees that are members of the Copaifera L. (Leguminoseae) genus and are found in tropical parts of Latin America.
Objective: This study analyzed the chemical composition of Copaifera multijuga Hayne oil and conducted preclinical trials to investigate anti-inflammatory effects and any action it may have on the central nervous system (CNS) of rats.
Materials and methods: The chemical analysis was carried out using gas chromatography with mass spectroscopy. Anti-inflammatory activity was measured by leucocytes mobilization, by chemotaxis assay in Boyden’s chamber, and by pleurisy model in rats. CNS effect was determined by plus maze and open-field assays. The statistical test applied was analysis of variance (ANOVA) followed by Tukey’s test or ANOVA followed by Duncan’s test.
Results: The oil was composed of sesquiterpenes with the predominance of β-caryophyllene (36.0%), followed by α-copaene (18.8%), β-bisabolene (8.5%), and α-trans-bergamotene (7.0%). Data demonstrated that at 100 and 200 mg/kg doses and at a concentration of 200 μl/ml copaiba essential oil presented anti-inflammatory effects both in vivo and in vitro based on reduced leukocyte migration to the rats’ pleural cavity and to the chemotactic agent lipopolysaccharide solution, respectively. During the experiments investigating CNS effects, locomotive and exploratory activities were reduced and the animals’ anxiety increased at 100 and 200 mg/kg.
Conclusion: The results obtained suggest that copaiba oil has an interesting anti-inflammatory effect and important effect on the CNS.
Introduction
The application of plants for medicine purposes is one of the oldest medical practices of humanity (CitationSchulz et al., 2002). Research regarding the evaluation of safe use of herbs in Brazil is incipient, as well as the commercialization control by official organs in fairs, public markets, or natural products stores (CitationVeiga & Pinto, 2005).
Copaiba oil is extracted from trees of the Copaifera L. (Leguminoseae), a genus that grows in the Amazon forest region. It has been used by the local people since ancient times and is popularly used to promote tissue healing, as an anti-inflammatory and antimicrobial agent, and for the treatment of respiratory and dermatological diseases (CitationCascon & Gilbert, 2000).
This oil is basically made up of sesquiterpenes and diterpenes, and to date more than 40 sesquiterpenes and 28 diterpenes have been described (CitationTappin et al., 2004; CitationVeiga & Pinto, 2002). The principal sesquiterpene found in the oil of different species of Copaifera sp. is β-caryophyllene, followed by α-humulene, α-bergamotene, and δ-cadinene. The composition of the diterpenes varies depending on species (CitationVeiga et al., 2007), and copalic acid was the only diterpene found in all oils analyzed (CitationVeiga & Pinto, 2002). CitationCascon and Gilbert (2000) chemically evaluated three species of Copaifera, Copaifera multijuga Hayne, Copaifera guianesis Desf., and Copaifera duckey Dwyer. Analysis of C. multijuga oil detected predominantly β-caryophyllene, followed by α-copaene, α-bergamotene, α-humulene, and δ-cadinene. The main diterpene acids found in C. multijuga were copalic, enantioagathic, and 3-acetoxy-copalic acids. Nevertheless, C. guianesis showed a smaller proportion of sesquiterpenes and diterpene acids. β-Caryophyllene, α-copaene, α-bergamotene, and α-humulene have been found together with β-selinene and β-bisabolene. Bicyclic diterpene acids of the labdane group (cativic, copalic, eperu-8(17)-en-15,18-dioic and polyalthic acids, tetracyclic acids of the kaurane series (kaur-16-en-19-oic and kauran-19-oic acids) and bicyclic clerodane (hardwickiic acid) were detected. C. duckei analysis detected β-caryophyllene, α-bergamotene, β-bisabolene, β-selinene, α-selinene, α-bisabolene, copalic and polyalthic acids, eperuic, chrolechinic, eperu-8(17)-en-15,18-dioic, clerodan-15,18-dioic acids, and no detectable contents of kaur-16-en-19-oic acid.
Previous research into copaiba oil observed analgesic, gastroprotective, and antitumor effects and also protection against Schistosoma mansoni cercariae (CitationVeiga & Pinto, 2002; CitationLima et al., 2003). Some authors studied the anti-inflammatory activity of the copaiba oil with different models, such as carrageenan-induced edema inhibition, cotton-pellet granuloma formation inhibition, vascular permeability, and prevention of acetic acid colitis, inhibition of bradycinin-induced paw edema in rats (CitationVeiga & Pinto, 2002; CitationPaiva et al., 2002; Veiga et al., 2006, Citation2007). Few studies have been described validating the pharmacological use of C. multijuga oil, and there are no published studies about effects of this material on the central nervous system (CNS). Therefore, the objective of this study was to conduct preclinical trials to investigate its anti-inflammatory effects and any action it may possibly have on the CNS.
Materials and methods
Reagents
Reagents used for this experiment are as follows: ethyl ether, carrageenan, sodium chloride, saline phosphate buffer (SPB), Turk solution, Hanks solution, heparin, and Tween. All the reagents were obtained from Merck and Sigma.
Copaiba oil
The copaiba oil was obtained by tapping the trunk of trees from a cultivation of C. multijuga trees in the Amazon state.
Chromatographic analysis
The copaiba oil was analyzed using gas chromatography (GC) and gas chromatography with mass spectroscopy (GC-MS), using a Shimadzu GC-17A chromatograph, equipped with a silica capillary column (30 mm, 0.25 mm, 0.25 µm, coated with DB-5). Temperature was programmed from 60 to 300°C at 3°C/min. Injector and detector temperatures were set at 220°C and 250°C, respectively. Helium was used as carrier gas at a flow rate of 1.0 ml/min. The chromatograph was equipped with flame ionization detectors, whereas the GC-MS had a quadruple MS system (QP 5000), operating at 70 eV and mass range 40-400 amu. The relative composition of the oil was obtained from electronic integration. The components of the oils were identified by comparison of retention indices (determined relatively to the retention times of n-alkanes homologous series) and mass spectra with those of authentic samples, data from Nist GS-MS library, and with the literature (CitationApel et al., 2004).
Animals
Adult male Wistar rats were obtained from the Nova Petrópolis central animal house weighing 180 to 220 g. These animals were kept in normal animal house conditions with free access to water and chow, at a temperature of 25 ± 2°C, in a constant 12 h light−dark cycle and with humidity monitored (40−60%). Animals were managed using the principles and guidelines for the care of laboratory animals according to CitationZimmermann (1983). This research was evaluated by the Research Ethics Committee at Feevale, and all animals were killed using methods that conform to Federal Veterinary Medical Council (Conselho Federal de Medicina Veterinária) Resolution 714 of 20 June, 2002.
Treatment
Pleurisy trial
Animals were orally treated with copaiba oil at doses of 100 and 200 mg/kg, whereas controls were given water (negative control) or indomethacin (positive control) at a dosage of 10 mg/kg, 1 h before induction of pleurisy. Each group contained eight animals.
Plus maze and open-field tests
To assess CNS effects, animals were treated with copaiba oil at doses of 100 and 200 mg/kg, whereas controls were given water. Treatment was given orally 1 h before the pharmacological tests. Each group contained 12 animals.
Induction of pleurisy
After treatment, pleurisy was induced using the method described by CitationSpector (1956). Animals were anesthetized with ethyl ether and peripheral venous blood samples were taken from their tails for use in total and differential leukocyte counts with Neubauer counting chambers, as described by CitationSannomiya et al. (1990), and blood smears, respectively. Soon after, the thoracic muscles of the intercostal border of the animals were dissected, until the intercostal space could be viewed and into which a hollow needle was introduced and 0.1 ml of a 1-mg/ml suspension of carrageenan in saline solution (sodium chloride at 0.9%) was injected directly into the pleural cavity of each animal. The animals were returned to their cages for 4 h for inflammation to develop. After this period, the rats were reanesthetized and a second peripheral blood sample was taken from the tail of each animal. They were then subjected to exsanguination by sectioning the internal jugular vein and carotid artery to obtain the pleural exudate. After respiratory arrest, bilateral parasternal thoracotomy was performed and the thoracic cavity was exposed. The pleural space was then washed with 2 ml of heparinized SPB. The pleural exudate was collected with a Pasteur pipette and the cells were washed three times with SPB. The total number of cells was determined by reading via an optical microscope in a Neubauer counting chamber. Pleural exudate differential leukocyte counts were carried out on stained smears (May-Grüenwald Giemsa), determining the percentage of mononuclear and polymorphonuclear cells.
Determination of the number of leukocytes in circulation
Total and differential counts of leukocytes in peripheral blood were carried out on the blood samples taken from the animals’ tails immediately before administration of the inflammatory stimulus and again before killing the animals. For the total count, samples were diluted 1:20 (V/V) with Turk solution in Thoma pipettes and homogenized manually for 1 min before counting the cells with Neubauer counting chambers. Differential counts were carried out on blood smears that had been fixed and May-Grüenwald Giemsa stained. One hundred cells were counted for each slide and the percentages of each cell type were determined.
Chemotaxis
This evaluation was performed using the CitationBoyden’s (1962) method as modified by CitationZigmond and Hirsch (1973). Experimental details have been described previously by CitationMello et al. (1992) and CitationFarsky (1994). Cylindrical chambers were cut into transparent acrylic tiles. These consisted of two compartments (each with a 0.5 ml capacity), separated by a nitrocellulose filter with a diameter of 13 mm and 8 μm pores.
The lower compartment was filled with a solution containing the chemotactic factor and the upper compartment was filled with a suspension of peritoneal neutrophils, making it possible to evaluate cell migration through the filter, at the concentration gradient of the chemotactic factor, which was established between the two compartments.
Harvesting the chemotactic factor
Interaction between plasma and bacterial antigens activates both the classical and alternative complement system pathways and results in the formation of factors that are chemotactic for leukocytes. The chemotactic material used in these experiments was obtained by incubating homologous plasma for 30 min at 37°C with an Escherichia coli lipopolysaccharide solution (LPS), at a concentration of 65 μg/ml. The plasma was then diluted (V/V) with Hanks solution to obtain a concentration of 20%.
Collection and preparation of the leukocyte suspension
Neutrophils were obtained from the peritoneal cavity of the rats 4 h after injection of 20 ml of a glycogen solution at 1%. To harvest the cells, the animals were killed by exposure to ethyl ether and then 60 ml of Hanks solution, containing 1 IU/ml of heparin, was injected into the peritoneal cavity. The cells obtained were washed with Hanks solution. They were then washed twice with Hanks solution. The number of cells put into the upper compartment of the chemotaxis chamber was 1.5 × 106 suspended in 0.4 ml of Hanks solution. These cells were treated with the copaiba oil being studied, at concentrations of 50, 100, and 200 μl/ml, suspended in Hanks solution (Tween 80:Hanks, 9:1 V/V) in a vibrating water bath (37°C) for 30 min.
Determination of leukocyte migration activity
Chambers were prepared in duplicate and incubated at 37°C for 1 h in a humid atmosphere. The filters were then removed, fixed, stained, diaphanized, and mounted between slide and coverslip. The filters were read under an optical microscope at 40× magnification. Focused on the upper plane of the filter, the observation plane of the microscope was reduced until just two cells were visible in focus. The distance in micrometers between the upper plane of the filter and that of these two cells in focus allows the neutrophil migration capacity to be evaluated (CitationZigmond & Hirsch, 1973). Readings were taken for five fields for each filter, and the result was expressed as a mean.
Exposure to open field
This technique was performed using a varnished wooden box measuring 50 × 60 × 40 cm and with a glass front. The floor was covered with linoleum and divided into 12 rectangles of 15.0 cm × 13.3 cm with dark lines. Each test session lasted 5 min. Animals were placed in the lower left hand corner of the box and left free to explore their surroundings. The time spent in the first rectangle before leaving it, orientation maneuvers (rearings), and the number of crossings from one rectangle to another were all recorded (CitationIzquierdo, 1979; CitationIzquierdo et al., 1984; CitationNetto et al., 1986).
Plus maze
This is an animal model of anxiety that has been used to study new anxiolytic agents and to understand the neurobiological processes associated with anxiety.
This apparatus consists of a plus-sign-shaped apparatus elevated approximately 50 cm from the floor. Two of the arms are open (50 × 10cm) and the two arms perpendicular to these are walled (50 × 10 × 40 cm), whereas none of the four arms has a roof. Each animal was placed in the center of the maze and the number of times it entered open and closed arms was recorded. The mean time that the animals remained in the arms was also measured. This model was standardized by CitationPellow et al. (1985).
Statistical analysis
The results of the pleurisy experiment were analyzed using Student’s t-test and/or analysis of variance (ANOVA), followed by Tukey test. The open-field and plus maze test results were first subjected to ANOVA and then this was followed by the Duncan test. The chemotaxis experiment was analyzed using Student’s t-test. P values less than 0.05 were considered statistically significant (shown as * or 1 or a), and P values less than 0.01 were considered highly significant (shown as ** or 2).
Results
Chromatographic analysis
The results of the chemical composition of the copaiba oil by GC-MS are presented in . Overall, 30 compounds representing 98.4% of the oil were identified. The oil is characterized only by sesquiterpenes, in which sesquiterpene hydrocarbons represent 97.5% of the constituents and oxygenated sesquiterpenes respond by only 0.9% of the content. The predominant compound identified was β-caryophyllene, accounting for 36.0% of the oil, followed by α-copaene (18.8%), β-bisabolene (8.5%), α-trans-bergamotene (7.0%), and δ-cadinene (6.1%). The components of the oils were identified by comparison of retention indices (determined relatively to the retention times of n-alkanes homologous series) and mass spectra with those of authentic samples, data from Nist GS-MS library, and with the literature (CitationApel et al., 2004).
Table 1. Copaiba essential oil composition obtained by GC-MS.
Pleurisy experiment
The results of the pleurisy experiment can be obtained from . It was observed that all groups exhibited significant increases in peripheral blood total leukocyte counts after induction of the inflammatory process. No significant differences were detected between the groups.
Table 2. Effects of Copaifera sp. oil on peripheral blood total leukocyte count during induction of a carrageenan-induced inflammatory process.
The differential white blood count demonstrated a significant reduction (P < 0.05) in the number of lymphocytes after induction of inflammation, with relation to the control group. Animals treated with copaiba oil at doses of 100 and 200 mg/kg exhibited reductions (P < 0.01) in lymphocyte counts before administration of carrageenan, in comparison with the control group. An increase in these leukocytes was also observed after the inflammatory process in the group treated with 100 mg/kg of the oil.
A significant increase (P < 0.01) in neutrophils was observed in all groups after induction of pleurisy, although no significant differences were identified between groups before and after inflammation, or with relation to the control group.
It was observed that the numbers of monocytes underwent a significant reduction (P < 0.01) in the group treated with indomethacin, both before and after the inflammatory process, and in the group treated with copaiba at the 100 mg/kg dosage before administration of the phlogistic agent, when compared with the control. Furthermore, the numbers of this type of cell increased in the 100 mg/kg group after induction of inflammation. The group treated with 200 mg/kg did not exhibit a significant increase in monocytes.
Analysis of the pleural exudates demonstrated a significant reduction (P < 0.01) in migrated leukocytes in all treatment groups with relation to the control group (). The same was observed in polymorphonuclear cells, which were reduced in all groups except the control group. No monocyte abnormalities were observed in any of the groups. Therefore, it can be postulated that copaiba oil has anti-inflammatory properties at the doses tested.
Table 3. Leukocyte migration to pleural exudate of rats after administration of carrageenan.
Chemotaxis
In the chemotaxis experiment, a reduction (P < 0.01) was observed in the leukocyte migration rate of cells treated with indomethacin, when compared with control cells. Cells treated with copaiba oil at concentrations of 50 and 100 μl/ml did not exhibit suppression of leukocyte migration. However, those treated with copaiba at a concentration of 200 μl/ml exhibited a significant reduction (P < 0.01) in leukocyte migration, when compared with the control group ().
Table 4. Effects of Copaifera sp. oil on leukocyte migration in vitro.
Open field
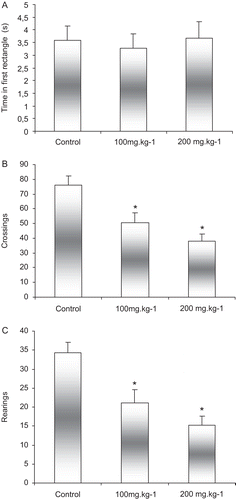
The results of the open-field test can be observed from . In this experiment, no significant differences were observed between groups in terms of the time spent in the first rectangle. However, there was a reduction in locomotor activity demonstrated by a reduced number of crossings in the groups treated with copaiba in relation to the control group (P < 0.01). With relation to the exploratory activities, the groups treated with copaiba demonstrated a reduced number rearing responses in relation to the control group (P < 0.01). The significant differences among groups regarding the number of rearings performed suggest that the treatment with copaíba (100 and 200 mg/kg) affect exploration of the animals in this task.
Figure 1. Effects of administration of copaiba oil on: (A) time in first rectangle before moving on, (B) number of crossings, and (C) number of rearing responses. Animals were given oral copaiba oil or vehicle 1 h before the test. Results are given as mean ± standard deviation. N = 12 animals per group. *P < 0.05 compared with controls; ANOVA/Duncan test.
Plus maze
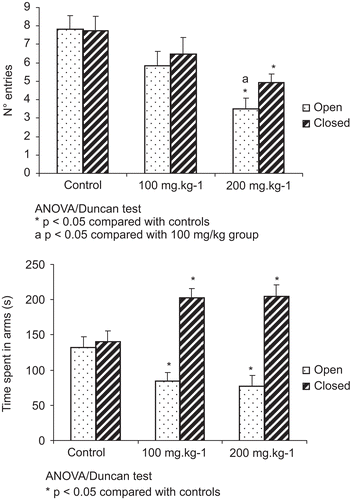
In this experiment, it was observed that the group treated with 200 mg/kg of copaiba oil was significantly different from the other groups, with significantly fewer entries into open arms than the control group and 100 mg/kg (P < 0.01). Furthermore, the number of entries into closed arms was less than that in the control group (P < 0.05), but did not differ significantly from the 100 mg/kg group (). With relation to time spent in open and closed arms, the groups treated with copaiba exhibited a significant difference in relation to the control group (P < 0.05), with less time spent in open arms and more time in closed arms. The treatment groups did not exhibit differences from each other ().
Figure 2. Effects of administration of copaiba oil on: (A) number of entries and (B) time spent in open and closed arms. Animals were given oral copaiba oil or vehicle 1 h before the test. Results are given as mean ± standard deviation. N = 12 animals per group. *P < 0.05 compared with controls. aP < 0.05 compared with 100 mg/kg group; ANOVA/Duncan test.
Discussion
The most abundant sesquiterpene in the chemical composition of Copaifera sp. oil is β-caryophyllene, which has anti-inflammatory, local anesthetic, antimicrobial, and antioxidant properties (CitationGhelardini et al., 2001; CitationVeiga et al., 2007). In this study, it proved possible to confirm this information by means of chromatographic analysis of the oil, showing that this substance was present in the highest proportion (36.0%). The other compounds found in the oil, all of them were also sesquiterpenes, agree with what has been found in earlier studies (CitationVeiga & Pinto, 2002). Sesquiterpenes are the most commonly found compounds in copaiba oils, and generally prove to be the same in oils from different Copaifera species, varying quantitatively but not qualitatively (CitationLima et al., 2003).
The results obtained in this study suggest that C. multijuga oil exhibited an anti-inflammatory effect in the induced pleurisy model in vivo and chemotaxis in vitro, demonstrated by reduced leukocyte migration to the rats’ pleural cavity and to the chemotactic agent LPS, respectively. These results corroborate with previous anti-inflammatory studies, and they also suggest that the compounds responsible for this pharmacological effect are independent of pharmacokinetic processes, because both in vivo and in vitro tests demonstrated suppression of leukocyte migration.
This effect can be explained by the chemical composition of oil, because it contains a mixture of sesquiterpenes and diterpenes with proven anti-inflammatory properties. Studies undertaken by CitationVeiga et al. (2007) suggested that the oil of several Copaifera species had pronounced anti-inflammatory effects because they significantly suppressed nitric oxide (NO) production in vitro and reduced leukocyte migration in a zymosan-induced pleurisy model. Nitric oxide is a powerful vasodilator, capable of increasing vascular permeability and, consequently, edema, in addition to increasing production of proinflammatory prostaglandins (CitationCerqueira & Yoshida, 2002). CitationVeiga et al. (2007) suggested that this anti-inflammatory effect was due to the presence of sesquiterpenes such as β-caryophyllene and diterpenes, such as kaurenoic acid. Volatile oils whose most abundant component is β-caryophyllene have exhibited marked anti-inflammatory effects in edema induced by carrageenan and prostaglandin E1 in rats, and also anti-arthritic effects (CitationCho et al., 2007). In a study carried out by CitationCho et al. (2007), this sesquiterpene demonstrated attenuation of colitis induced by dextran sodium sulphate in mice, attenuating the inflammatory process by means of reducing infiltration of neutrophils into inflamed tissue, and reducing serum levels and gene expression of IL-6. β-Caryophyllene is a compound that has been approved by the Food and Drug Administration for use in food, because it has low toxicity. Furthermore, in contrast to non-steroidal anti-inflammatory, this sesquiterpene does not have undesirable gastric effects and even seems to have gastrocytoprotective effects in rats (CitationGhelardini et al., 2001). A study carried out by CitationPaiva et al. (2002) found that kaurenoic acid prevented colitis induced with acetic acid in rats due to its antiproliferative and antioxidant effects. Because copaiba oil is a mixture of a variety of components, it may possess synergic effects between different compounds, increasing the biological activity in comparison with the substances in isolation. This synergy could be related to protection against enzymes by an active ingredient, facilitating transport through membranes, and regulating messengers and gene expression, among other effects. Many different substances that are present in these plants may complement the biological action by neutralizing factors involved in resistance to it (CitationGilbert & Alves, 2003).
The elevated plus maze is an animal model of anxiety that is used for the study of new anxiolytic agents and to aid in the understanding of the neurobiological processes associated with anxiety. Animals exposed to the plus maze exhibit fear of the open arms, characterized by behavioral and physiological manifestations. This study found that the anxiety of the treated animals increased when submitted to the plus maze, shown by the reduced number of entries into the open and closed arms and by the significant increase in time spent in the closed arms, in addition to reduced locomotor and exploratory activity in the open field test. Animals that were given the 200 mg/ kg dose exhibited a greater reduction in locomotor and exploratory activity than those treated with the 100 mg/kg dose. The mechanisms through which copaiba oil provokes these effects are not clear.
Conclusion
The results obtained suggest that C. multijuga oil has an anti-inflammatory effect both in vivo and in vitro, because it inhibits leukocyte migration to the pleural cavity of rats and has an antichemotactic effect in the presence of the LPS agent, probably due to the sesquiterpenes found in its chemical composition. They also suggest that the oil possibly has an effect on the CNS, because the results demonstrated an anxiogenic effect and reduced locomotion and exploratory capacity. More studies are needed to find out whether any of the compounds isolated have CNS effects. Neurochemical studies are needed to elucidate the mechanisms associated with the behavioral effects that were observed.
Acknowledgements
The authors are grateful to the Office of the Dean for Research and Innovation (PROPI - Pró-Reitoria de Pesquisa, e Inovação) at the Universidade Feevale for providing financial support and making facilities available.
Declaration of interest
The authors report no declaration of interest.
References
- Apel MA, Sobral M, Schapoval EES, Henriques AT, Menut C, Bessiere JM. (2004). Chemical composition of the essential oils of Eugenia hymalis and Eugenia stigmatosa. Part VI: Section Biflorae. J Essent Oil Res, 16, 437–439.
- Boyden S. (1962). The chemotatic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med, 15, 433–466.
- Cascon V, Gilbert B. (2000). Characterization of the chemical composition of oleoresins of Copaifera guianensis Desf., Copaifera duckei Dwyer and Copaifera multijuga Hayne. Phytochemistry, 55, 773–778.
- Cechinel Filho V, Yunes RA. (1998). Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais: Conceitos sobre modificação estrutural para otimização da atividade. Quim Nova, 21, 99–105.
- Cerqueira NF, Yoshida WB. (2002). Óxido nítrico: Revisão. Acta Cir Bras, 17, 417–423.
- Cho JY, Chang HJ, Lee SK, Kim HJ, Hwang JK, Chun HS. (2007). Amelioration of dextran sulfate sodium-induced colitis in mice by oral administration of beta-caryophyllene, a sesquiterpene. Life Sci, 80, 932–939.
- Elisabetsky E. (2002). Fitoterapia com base científica. Rev Ciência Hoje, 31, 78–79.
- Farsky SHP. (1994). Influência dos glicocorticóides endógenos sobre a interação leucócito-endotélio e sobre a capacidade de migração celular na inflamação. São Paulo, USP, Thesis, p. 99.
- Ghelardini C, Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A. (2001). Local anaesthetic activity of beta-caryophyllene. Farmaco, 56, 387–389.
- Gilbert B, Alves LF. (2003). Synergy in plant medicines. Curr Med Chem, 10, 13–20.
- Izquierdo I. (1979). Effect of naloxone and morphine on various forms of memory in the rat: possible role of engogenous opiate mechanisms in memory consolidation. Psychopharmacology (Berl), 66, 199–203.
- Izquierdo I, Souza DO, Dias RD, Perry ML, Carrasco MA, Volkmer N, Netto CA. (1984). Effect of various behavioral training and testing procedures on brain beta-endorphin-like immunoreactivity and the possible role of beta-endorphin in behavioral regulation. Psychoneuroendocrinology, 9, 381–389.
- Lima SRM, Veiga Junior VF, Christo HB, Pinto AC, Fernandes PD. (2003). In vivo and in vitro studies on the anticancer activity of Copaifera multijuga Hayne and its fractions. Phytother Res, 17, 1048–1053.
- Mello SB, Farsky SH, Sannomiya P, Garcia-Leme J. (1992). Inhibition of neutrophil chemotaxis and chemokinesis associated with a plasma protein in aging rats: selective depression of cell responses mediated by complement-derived chemoattractants. J Leukoc Biol, 51, 46–52.
- Netto CA, Dias RD, Izquierdo I. (1986). Differential effect of posttraining naloxone, beta-endorphin, leu-enkephalin and electroconvulsive shock administration upon memory of an open-field habituation and of a water-finding task. Psychoneuroendocrinology, 11, 437–446.
- Paiva LA, Gurgel LA, Silva RM, Tomé AR, Gramosa NV, Silveira ER, Santos FA, Rao VS. (2002). Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffi on acetic acid-induced colitis in rats. Vascul Pharmacol, 39, 303–307.
- Pellow S, Chopin P, File SE, Briley M. (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149–167.
- Sannomiya P, Pereira MA, Garcia-Leme J. (1990). Inhibition of leukocyte chemotaxis by serum factor in diabetes mellitus: selective depression of cell responses mediated by complement-derived chemoattractants. Agents Actions, 30, 369–376.
- Schulz V, Hänsel R, Tyler VE. (2002). Fitoterapia Racional: Um Guia de Fitoterapia para Ciências da Saúde. São Paulo, Manole, p. 386.
- Spector WG. (1956). The mediation of altered capillary permeability in acute inflammation. J Pathol Bacteriol, 72, 367–380.
- Tappin MRR, Pereira JFG, Lima LA, Siani AC, Mazzei JL, Ramos MFS. (2004). Análise química quantitativa para a padronização do óleo de copaíba por cromatografia em fase gasosa de alta resolução. Quim Nova, 27, 236–240.
- Veiga Junior VF, Pinto AC. (2002). O gênero Copaifera L. Quim Nova, 25, 273–286.
- Veiga Junior VF, Rosas EC, Carvalho MV, Henriques MG, Pinto AC. (2007). Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne–a comparative study. J Ethnopharmacol, 112, 248–254.
- Veiga Junior VF, Pinto A. (2005). Plantas medicinais: Cura segura? Quim Nova. 28 519–528.
- Zigmond SH, Hirsch JG. (1973). Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med, 137, 387–410.
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110.
