Abstract
Context: A newly discovered geranyl prenylated chalcone, semisynthesized from naturally occurring nymphaeol C, has the ability to inhibit the growth of CNS1 (glioblastoma) and 13-06 (malignant glioma) cells. A second-order regression model was established to predict the normalized cell viability of CNS1 and 13-06 cells.
Objective: The goal of this study is to evaluate the influence of prenylated chalcone on the glioblastoma and malignant glioma cell lines. For the first time, response surface methodology (RSM) has been introduced to perform a cell line study.
Materials and methods: A newly discovered prenylated chalcone was used. This compound is a member of the flavonoid family and possesses a common phenylbenzopyrone structure. Two independent factors, including prenylated chalcone concentration and uptake time, were carefully evaluated by a 22 factorial design. RSM was introduced as a new method for CNS1 and 13-06 cell line studies.
Results: For CNS1 cells, the least inhibition uptake time was 20.7 h, and the least inhibition dose was 12.4 μg/ml. For 13-06 cells, the best inhibition uptake time was 26.2 h, and the least inhibition dose was 12.0 μg/ml.
Discussion and conclusion: The RSM model successfully predicted the normalized cell viability of CNS1 and 13-06 cells through the use of prenylated chalcone. The results obtained in this study will be useful for further studies on the use of prenylated chalcone.
Introduction
Brain tumors are the most commonly diagnosed malignant gliomas in adults. These malignant astrocytic tumors exhibit a high proliferation rate and an aggressive growth pattern (CitationOhgaki & Kleihues, 2005). Several studies have suggested that the flavonoids have chemopreventive and therapeutic potential against malignant gliomas (CitationRen et al., 2003). Flavonoids are polyphenols that are found in tea, beer, coffee, and other fruits and vegetables. The use of flavonoids as anticancer agents is being seriously considered (CitationKanadaswami et al., 2005). Prenylated chalcones are intermediate products in the biosynthesis of flavonoids. They have been shown to have several advantageous biological properties including antibacterial, antitumor, and anti-inflammatory properties (CitationMiddleton 1996; CitationDimmock et al., 1999; CitationKondratyuk & Pezzuto, 2004; CitationGo et al., 2005; CitationLiu et al., 2010). It has been shown that WJ9708011, a newly discovered geranyl prenylated chalcone semisynthesized from naturally occurring nymphaeol C (CitationHuang et al., 2007), has potential against the brain gliomas. In this study, a newly discovered prenylated chalcone was used in glioblastoma and malignant glioma cell line studies. The results obtained can be used for the development of new drugs.
New drug development typically involves the following three steps: conducting a cell line study, developing animal models, and carrying out human epidemiological trials. Cell line studies can help identify molecular targets that can be used to elucidate pathways. Two independent variables, dose and uptake time, are the major factors that help determine the biological ability of drugs. The conventional method that involves the evaluation of one variable at a time is a time-consuming process. In addition, the newly developed drug is usually available in limited quantities and is expensive. This makes it impractical to use large amount of a new drug for testing purposes. Hence, a new approach is necessary for improving the development process of new drugs.
Response surface methodology (RSM) is an effective statistical technique for process optimization, and has widely been used in chemical and biological applications for predicting optimal operating conditions (CitationChang et al., 2002; CitationCacace & Mazza, 2006; CitationBas & Boyaci, 2007). However, there have been no studies on the application of RSM for the cell line studies. Unlike traditional methods, RSM allows the evaluation of two or more variables and studies the interactions between these variables. RSM also reduces the number of experiments and thus requires less material. The goal of this study is to evaluate the use of prenylated chalcone in glioblastoma and malignant glioma cell line studies by using RSM.
Methods
CNS1 (glioblastoma) and 13-06 (malignant glioma) cells were obtained from National Cheng Kung University (Taiwan). These cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen™, Gibco, New York, NY) containing 10% heat-inactivated fetal bovine serum. The cells were incubated at 37°C with 5% CO2-enriched air. After the cells reached confluence, subcultures were prepared using 0.02% ethylene diaminetetraacetic acid (EDTA) and 0.05% trypsin. The cells were cultured on 96-well tissue culture plates (approximately 2 × 104 cells) at 37°C with 5% CO2-enriched air. A confluent monolayer culture was achieved after 1 or 2 days.
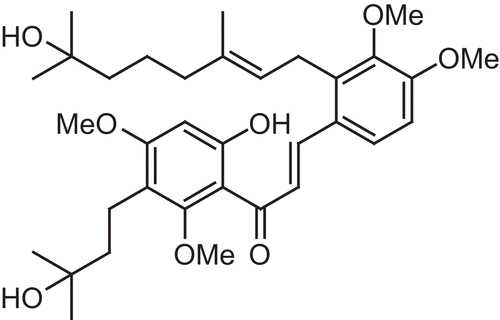
WJ9708011 is a newly discovered prenylated chalcone and a member of the flavonoid family with a common phenylbenzopyrone structure (). The prenylated chalcone concentrations (μg/ml) and uptake times (hours) were determined on the basis of the RSM experimental designs (). Dimethyl sulfoxide (DMSO) was used as the solvent to dissolve prenylated chalcone, and the solubility was 10 mg/1 ml. The cell viability was evaluated using the MTT (3-(4,5-dime thylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide) cell proliferation assay. After incubation, the cells were washed with phosphate buffer, and then 20 μl of MTT reagent was added. The cells were incubated for 2 h at 37°C. The intracellular purple formazan formed and could be solubilized with 110 μl of DMSO. Next, 100 μl of prepared sample was transferred to a 96-well plate and analyzed using an enzyme-linked immunosorbent assay (ELISA) reader (Multiskan RC, Labsystems Inc., Franklin, MA). The absorbance of each sample was measured at a wavelength of 490 nm at 25°C. The cell viability was normalized with the control unit, and expressed as a percentage.
Table 1. Experimental data and the observed response values (normalized cell viability, %) of CNS1 and 13-06 cells with two independent factors, X1 (prenylated chalcone concentration, μg/ml) and X2 (uptake time, hours).
The second-order model, including linear, quadratic, and interactive components, was chosen for the RSM analysis.
where Y is the response; Xi and Xj are input variables; β0 is the intercept; βi are linear coefficients; βii are squared coefficients; βij are interaction coefficients; and ϵ is an error term. The central composite design (CCD) is the most popular design method for second-order models. CCD has 2k factorial experiments, where k represents the number of independent factors. The central point (nc) and the distance [α, α = (2k)1/4] from the axial point to the central point needs to be determined before designing the experiment. For two-factor design, α is 1.414. The statistical evaluation was performed by running an analysis of variance (ANOVA) with SASR (version 9.0, SAS Institute Inc., Cary, NC). The results were compared using Student’s t-test, and the complete analysis had a 0.05 significance level (α = 0.05).
Results and discussion
The influence of the prenylated chalcone concentration and uptake time on the cell viability was evaluated by a 22 factorial design with three replicates (). The data shown in are the mean values of three measurements. A negative value for the normalized cell viability means that the prenylated chalcone compound was able to inhibit the growth of both CNS1 and 13-06 cells. A large variation in the repetition of the central point was because of the difficulty in mixing. A heavy mixing force may damage cells; thus, the mixture was mixed gently and this may have caused the variation. This kind of variation is very common in the conventional cell line studies. Although efforts have been made to minimize variation, a certain degree of variation still exists. A regression analysis was carried out to fit the mathematical model with the experimental data. The coefficients of the second-order model for CNS1 and 13-06 cells are shown in and , respectively. The second-order model expressed by equation 2 was used to predict the cell viability.
Table 2. Regression coefficients of the second-order model for CNS1 cells.
Table 3. Regression coefficients of the second-order model for 13-06 cells.
CNS1 (glioblastoma) cells
The RSM model successfully predicted the cell viability of CNS1 cells. The effect of using prenylated chalcone on CNS1 cells is shown in . The normalized cell viability was significantly affected by the prenylated chalcone concentration and uptake time. When the prenylated chalcone concentration and uptake time were 12.4 μg/ml and 20.7 h, respectively, the normalized cell viability was found to be the maximum (−4.6%). At this stationary point, the prenylated chalcone compound had the lowest ability to inhibit CNS1 cell growth. The RSM model provided the above-mentioned valuable information without running numerous experiments. This is one of the advantages of using the RSM model.
Figure 2. Response surface plot for the use of prenylated chalcone in the cell line study of CNS1 (glioblastoma) cells.
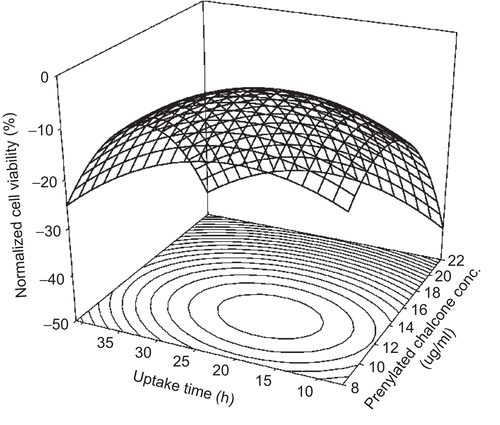
Generally, the use of a high dose and long uptake time will achieve better inhibition. According to , the best inhibition (−44.5%) occurred at 41 h when 22.0 μg/ml prenylated chalcone was used. Although the cell viability of using 22.0 μg/ml prenylated chalcone varied from −34.6 to −44.5% depending on uptake time, the use of such a high dose of prenylated chalcone was not suitable. Such a high dose of prenylated chalcone not only inhibits glioblastoma cells but also damages normal cells. Practical application will help determine the dose of prenylated chalcone that is suitable for use.
The normalized cell viability was calculated using 12.4 μg/ml prenylated chalcone along with different uptake times on the basis of equation 2. The least inhibition performance occurred at 20.7 h. This uptake time of 20.7 h was defined as the least inhibition uptake time (LIUT) for using prenylated chalcone on CNS1 cells. There are two mechanisms underlying this inhibition process. On the one hand, when the uptake time was shorter than the LIUT, the cells were killed mainly because of the original toxicity of prenylated chalcone. When the uptake time was longer than the LIUT, the cells were inhibited by the metabolite of prenylated chalcone. On the other hand, when the uptake time was fixed at 20.7 h, the normalized cell viability was calculated on the basis of equation 2 when prenylated chalcone was used at different doses. As a result, the use of 12.4 μg/ml prenylated chalcone with an uptake time of 20.7 h produced the least inhibition performance of prenylated chalcone in CNS1 cells. This dose (12.4 μg/ml) was defined as the least inhibition dose (LID). The definition of LIUT and LID will be very useful in further cell line studies.
13-06 (malignant glioma) cells
The RSM model successfully predicted the cell viability of 13-06 cells as well. The effect of using prenylated chalcone on the 13-06 cells is shown in . When the prenylated chalcone concentration and uptake time were 12.0 μg/ml and 26.2 h, respectively, the normalized cell viability was calculated to be −24.5%. This condition is noted at the saddle point in the graph. The normalized cell viability was also significantly affected by the prenylated chalcone concentration and uptake time. Overall, prenylated chalcone facilitated better inhibition performance in 13-06 cells than in CNS1 cells.
Figure 3. Response surface plot for the use of prenylated chalcone in the cell line study of 13-06 (malignant glioma) cells.
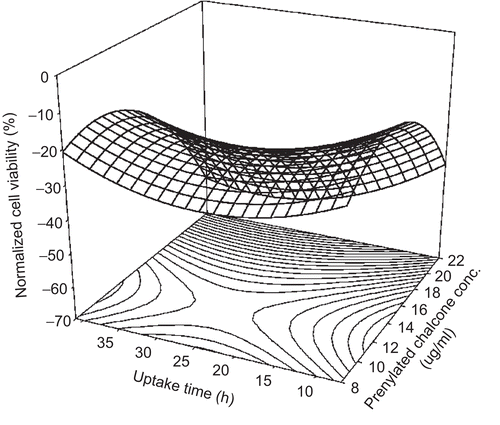
Based on equation 2, the normalized cell viabilities were calculated when different doses of prenylated chalcone were used at an uptake time of 26.2 h. The results showed that the use of 12.0 μg/ml prenylated chalcone at an uptake time of 26.2 h achieved the least inhibition performance. Thus, the LID was 12.0 μg/ml prenylated chalcone. This result was similar to that obtained in the CNS1 study. Meanwhile, the normalized cell viability was calculated when 12.0 μg/ml prenylated chalcone was used at different uptake times. The best inhibition performance was found to be 26.2 h. This uptake time of 26.2 h was defined as the best inhibition uptake time (BIUT). Thus, prenylated chalcone produced the BIUT for 13-06 cells.
Conclusions
The RSM model successfully predicted the normalized cell viability for CNS1 and 13-06 cells when prenylated chalcone was used. The normalized cell viability was used to represent the influence of prenylated chalcone on the inhibition of CNS1 and 13-06 cells. A second-order model created by RSM had two independent factors including prenylated chalcone concentration and uptake time. The LIUT, BIUT, and LID were all determined on the basis of the RSM model. For CNS1 cells, the LIUT was 20.7 h, and the LID was 12.4 μg/ml. For 13-06 cells, the BIUT was 26.2 h, and the LID was 12.0 μg/ml. This study concluded that RSM is a good tool that can be used for developing new cell line studies. The results obtained in this study will be useful in further studies on the use of prenylated chalcone.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bas D, Boyaci IH. (2007). Modeling and optimization I: Usability of response surface methodology. J Food Eng, 78, 836–845.
- Cacace JE, Mazza G. (2006). Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci, 68, 240–248.
- Chang YN, Huang JC, Lee CC, Shih IL, Tzeng YM. (2002). Use of response surface methodology to optimize culture medium for production of lovastatin by Monascus rubber. Enzyme Microb Tech, 30, 889–894.
- Dimmock JR, Elias DW, Beazely MA, Kandepu NM. (1999). Bioactivities of chalcones. Curr Med Chem, 6, 1125–1149.
- Go ML, Wu X, Liu XL. (2005). Chalcones: An update on cytotoxic and chemoprotective properties. Curr Med Chem, 12, 481–499.
- Huang WJ, Huang CH, Wu CL, Lin JK, Chen YW, Lin CL, Chuang SE, Huang CY, Chen CN. (2007). Propolin G, a prenylflavanone, isolated from Taiwanese propolis, induces caspase-dependent apoptosis in brain cancer cells. J Agric Food Chem, 55, 7366–7376.
- Kanadaswami C, Lee LT, Lee PPH, Wang JJH, Ke FC, Huang YT, Lee MT. (2005). The antitumor activities of flavonoids. In Vivo, 19, 895–909.
- Kondratyuk TP, Pezzuto JM. (2004). Natural product polyphenols of relevance to human health. Pharm Biol, 42, 46–63.
- Liu HL, Jiang WB, Xie MX. (2010). Flavonoids: Recent advances as anticancer drugs. Recent Pat Anticancer Drug Discov, 5, 152–164.
- Middleton E. (1996). Biological properties of plant flavonoids: An overview. Pharm Biol, 34, 344–348.
- Ohgaki H, Kleihues P. (2005). Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol, 64, 479–489.
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. (2003). Flavonoids: Promising anticancer agents. Med Res Rev, 23, 519–534.