Abstract
Context: Previously, we showed the essential oils (EO) of the mountain celery [Cryptotaenia japonica Hass (Umbelliferae)] seeds (MCS) to be a prominent hypolipidemic agent.
Objective: We hypothesized the aqueous extract (AE) of its seeds could also exhibit a comparable nutritional effect.
Materials and methods: Experiments were carried out for compositional analysis, antioxidant assay, and hypolipidaemic assay with AE in hamsters.
Results: AE contained soluble arabinogalactan (AGal) with molecular weight (MW) 878 kDa. AE also was enriched in polyphenolics and flavonoids, reaching 30.4 and 2.20 mg/100 g, respectively. AGal consisted of eight monosaccharides (in mols %), galactose (28.75), arabinose (24.84), glucose (17.91), mannose (6.93), ribose (6.03), fucose (5.83), xylose (5.30), and rhamnose (4.41), with average MW 878 kDa. In vitro, AE showed potent ferrous chelating and DPPH scavenging effects but only moderate H2O2 scavenging capability. In hamsters, AE exhibited promising hypolipidemic bioactivity, in particular, the HDL-C and hepatic unsaturated fatty acid (UFA) biosynthesis regarding oleic, linoleic, and arachidonic acids.
Discussion and conclusion: The presence of AGal enhanced the hypolipidemic and antioxidative bioactivity of MCS. MCS is feasibly beneficial to the hepatic de novo UFA synthesis and the hypolipidemics as evidenced by hamster model.
Introduction
Moutain celery [Cryptotaenia japonica Hass (Umbelliferae)] (MC) for hundreds of years served as common daily vegetable dish in many East Asian countries, now has popularly become an artificial cultivar in Middle Taiwan. It has been highly reputed for its folkloric herbal medicines as a hypotensive, a hypocholesterolemic, and an antiobesity agent (CitationCheng et al., 2008).
Previously, our laboratory had identified 109 compounds in the seed essential oils (EO), which included categories (number of compounds) of monoterpenoids (9), sesquiterpenoids (31), and alcohols (22) (CitationCheng et al., 2008). We also demonstrated that all MC-compounded diets except the EO-added prominently exhibited strong hypolipidemic, especially, HDL-C elevating capability (CitationCheng et al., 2008).
Earlier, CitationSaleh et al. (1985) identified a number of active compounds. 3-n-Butylphthalide was demonstrated to be a hypotensive as well as a hyocholesterolemic (CitationLe & Elliott, 1991). Furthermore, five anticarcinoma components in celeries were identified to possess a glutathione S-transferase (GST) activating activity (CitationZheng et al., 1993; CitationTsi et al., 1995). More recently, by preliminary survey we found that the aqueous soluble fraction of MC seeds (AE) contained huge amount of soluble dietary fibers (SDF), polyphenols, and flavonoids. Since the documented data about its soluble dietary fiber are still lacking, considering the easier handling of these seed dishes, we hypothesized that AE could exhibit similar nutritional bioactivity comparable with its essential oils. To confirm this, we performed simultaneously both the in vitro and in vivo animal experiments.
Materials and methods
Chemicals and reagents
Linoleic acid, 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), ferrous chloride (FeCl2), ferrozine, nitroblue tetrazolium (NBT), dihydronicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), ethylene diaminetetraacetate (EDTA) (disodium salt), H2O2 (30%), peroxidase, phenol red, n-butylhydroxyanisole (BHA), n-butylhydroxy toluene (BHT), ascorbic acid, and citric acid were products of Sigma Chemical Co. (St. Louis, MO). All other reagents and chemicals used were of reagent grade.
Source of mountain celery seeds
Fresh MC seeds were purchased from a local farm located in Nan-Tou, a middle Taiwan county. The seeds were stored immediately in the dark at −20°C before treatment (CitationCheng et al., 2008). The plant has been authenticated by the Research Centre for Chinese Herbal Medicines in Taichung City (Taiwan).
Preparation of desiccated powder of mountain celery seeds
Fresh MC seeds were desiccated at 60°C for 4 h as previously cited (CitationCheng et al., 2008). The crushed particles having size ranging within mesh #40-60 were collected (MCSP) ().
Table 1. Ingredients of experimental animal diets.a,b
Preparation of aqueous extract of mountain celery seeds
MCSP (100 g) was transferred into a 1-L reaction vessel, to which 1000 mL of double distilled water was added. The mixture was refluxed at 100°C for 1 h and filtered. The extraction was repeated twice. The filtrates were combined and evaporated with a 5000-mL rotary evaporator to dryness. The total desiccated extract was weighed, and the percentage yield was taken and stored at −20°C for further use.
Analyses
Total phenolics
The total polyphenolic content was assayed according to CitationTaga et al. (1984). The amount of polyphenols was calculated against the calibration curve established and expressed as the mg of gallic acid equivalent (mgGAE) per 100 g of the desiccated.
Total flavonoids
The method of CitationQuettier-Deleu et al. (2000) was followed to determine the total flavonoid content. The content of isoflavonoids was calculated against the calibration curve and expressed in milligrams of quercetin equivalent (mg QE) per 100 g of the desiccated.
Dietary fibers (DF), insoluble dietary fibers (IDF), and SDF
The official procedure of Association of Official Analytical Chemists Citation(AOAC) (1995) for ingredients analysis (method 991.43) was adopted to determine the DF, the IDF, and the SDF.
Molecular weight of SDF
Sample SDF (10 mg) was accurately weighed and placed in a reaction vessel. To the wighed sample, 1 mL of NaOH (1 N) was added. The remaining procedures were conducted according to CitationKer et al. (2005). A total of 50 tubes were collected. The optical density was measured simultaneously at 490 and 280 nm.
Polysaccharides in SDF
The content of polysaccharides in SDF was determined by the method described by CitationKer et al. (2005). The optical density was measured at 490 nm.
GC/MS analysis of polysaccharide composition in SDF—Hydrolysis, reduction, derivatization of monosaccharides
Similar method described by CitationKer et al. (2005) was followed to determine the polysaccharide composition in SDF. Briefly, the final dehydrated product was transferred into a 1-mL reaction vessel, lyophilized, and analyzed with GC/MS as described (CitationKer et al., 2005). After the polysaccharide fraction was separated from the Sephadex G-100 column, each 1 mL of the polysaccharide fraction was added with 1 mL of phenol solution (5%); then it was thoroughly mixed with 5 mL of sulfuric acid and shaken well. After the solution was cooled to room temperature, the absorbance [optical density (OD)] was measured at 490 nm.
Antioxidative capability of the aqueous extract
Chelating capability for ferrous ions
According to CitationLin et al. (2008a), .7 mL of methanol and 0.1 mL of ferric chloride solution (2 mM) were added to 1 mL of aqueous extract (0.5-20 mg/mL). The mixture was left to stand for 30 sec to facilitate the reaction. To the mixture, 0.2 mL of ferrozine (5 mM) was added and then left to stand for 10 min to facilitate the reaction. When reaction was completed, the absorbance of the colored reaction mixture was taken at 562 nm using a Hitachi U-2001 Spectrophotometer. Standard solutions of EDTA and citric acid, each 0.5∼5 mg/mL, were used as the controls.
Scavenging capability for DPPH free radicals
CitationLin et al. (2008a) was followed to determine the DPPH scavenging capability. Briefly, the dried AE was redissolved in 75% ethanol to make a concentration of 20 mg/ mL to serve as a stock. For analysis, the stock solution was diluted with ethanol to 0.5 to 20 mg/mL [aqueous ethanol extract (AEE) solution]. To 4 mL of AEE (0.5-20 mg/ mL), 1 mL of freshly prepared methanol 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals was added. The mixture was left to stand in dark for 30 min. The absorbance was read at 517 nm using a Hitachi U-2001 Spectrophotometer. BHA and L-ascorbic acid were used as the positive controls and citric acid as the negative control.
Scavenging capability for H2O2
The testing procedures were conducted as previously described by CitationCheng et al. (2008). The optical density was measured at 610 nm using a Hitachi U-2001 Spectrophotometer. L -ascorbic acid and BHT were used as reference controls.
Hamster diets, serum, and hepatic lipid extract
Sixty-four male Syrian hamsters, aged 5 to 6 weeks, were purchased from the National Laboratory Animal Centre. All studies performed with this hamster model were approved by the Hungkuang University Ethic Committee in accordance with Helsinki Declaration in 1975. For the first 2 weeks, the hamsters were acclimated by supplying only regular market diet Fu-Sow (Longevity) Brand. Then the hamsters were randomly grouped by body weight into 8 groups (8 hamsters in each group, 2 in each stainless cage) and fed on different diets: the control group C fed on regular diet; the high-lipid diet (Group H). The groups MP1 through MP3 were fed diet H plus 0.5, 1.0, and 3.0% w/w, respectively, and the groups AE1 through AE3 were fed diet H plus 0.05, 0.10, and 0.50% w/w, respectively () (CitationAnonymous, 1977).
The animal room was conditioned at 24 ± 1°C with a relative humidity maintained at 40% to 60%. Light cycle was changed every 12 h. Water and meal takings were ad libitum. Body weight and amount of diets consumed were recorded every 2 days until the end of the experiment. After 10-week feeding, the hamsters were fasted for 12 h before being euthenized with CO2. The hamsters were bled from the abdomen artery. The whole blood was centrifuged at 4°C at 1800g for 10 min. The sera were separated and stored at −70°C for lipid determination. The livers were dissected immediately after euthenizing and washed twice with ice-cold saline (150 mM). After the adhered water was drained off and their weights taken, the livers were transferred into a zippered bag and stored at −70°C for analysis of hepatic lipid content according to CitationFang et al. (2004). The assay methods used for analysis of serum triglycerides, serum total cholesterol, serum LDL-C, and serum HDL-C were in majority adopted from The Manual of Combined Agents provided by the Randox Laboratories Ltd.
Serum total cholesterol, serum low-density lipoprotein cholesterol (LDL-C), serum high-density lipoprotein cholesterol (HDL-C), serum total triglycerides (TG)
For all serum lipid analyses, similar procedures were conducted according to CitationCheng et al. (2008).
Extraction of hepatic lipids
The lipid extraction from hepatic tissues was conducted following the method of CitationLin et al. (2008). The finally obtained hepatic lipid extract (HLE) was stored at −20°C for further analysis.
Determination of hepatic triglycerides, total hepatic cholesterol (CT)
The method described by CitationLin et al. (2008b) was used for determination of hepatic triglycerides and total hepatic cholesterol. Briefly, for analysis of hepatic lipids, hamsters were euthenized on completion of feeding experiment and the livers were excised. After rinsed with Ringer solution, livers were wiped gently with tissue papers to get rid of any moisture adsorbed on the surface before their weights were measured. Preparation of hepatic lipids extracts was performed according to CitationFolch et al. (1957). The final chloroform–methanol (2:1) extracts were combined and made to a volume of 10 mL with the same extraction solvent and stored at −20°C for further analysis. Hepatic lipid extracts (10 μL) was transferred into a 1.5 mL centrifuge tube. Similar procedures were performed as mentioned in the serum lipid analyses.
Determination of total hepatic phospholipids
Similarly, CitationLin et al. (2008b) was followed to determine the total hepatic phospholipids.
Hepatic fatty acid extract
Method of CitationFolch et al. (1957) was followed to extract the hepatic fatty acids. To 3 mL of Folch extractive agent, 2 mL of the HLE was added (CitationFolch et al., 1957). The solution was dried under nitrogen blowing. To the residue, 4 mL of methanol KOH (0.5 N, KOH/CH3OH) was added and refluxed at 100°C for 15 min on the water bath. A solution of BF3/CH3OH (5 mL) was added and the reflux was continued for 2 min. In the final reflux step, n-hexane (3 mL) was added and refluxed for another 1 min. On cooling to room temperatue, a saturated solution of NaCl (2 mL) and the internal standard (methyl n-pentadecanoate/n-hexane, 0.5%, 1 mL) was added. After agitated for 3 min, the mixture was left to stand for separation. The organic layer was transferred into the reaction vessel, added with anhydrous sodium sulfate, shaken well to completely dehydrate the water content in the solution, and filtered. The filtrate was used for assay of hepatic fatty acid content by GC and GC/MS analyses. GC operational conditions and GC/MS qualitative analysis were similarly perfomed according to CitationKer et al. (2005).
Statistical analysis
Data obtained in the same group were analyzed by Student’s t-test with computer statistical software SPSS 10.0 (SPSS, Chicago, IL). Statistical Analysis System (2000) software was used to analyze the variances and Scheffe multiple comparison test was used to test their significances of difference between paired means. Significance of difference was judged by a confidence level of P < 0.05.
Results and discussion
The characteristics of AE and MP
AE occupied a total content of 16.2% in the mountain celery seeds. AE contained much higher contents of total polyphenolics and total flavonoids than MP, reaching 9.21- and 9.16-fold over MP, respectively (). As often cited, polyphenols (1.2 μM of gallic acid or 5 μM of quercetin) are effective LDL antioxidant and strong transition metal–chelating agents (CitationSrivastava et al., 2006).
Table 2. Main bioactive constituents of the pulverized mountain celery seeds (MP) and its aqueous extract (AE).a
Dietary fiber contents
Mountain celery seeds contain quite a few amounts of DF. The SDF content was 41 and 301 mg/100g in MP and AE, respectively. The difference reached 7.34 fold. In contrast, MP had higher content of IDF, which was totally not found in AE ().
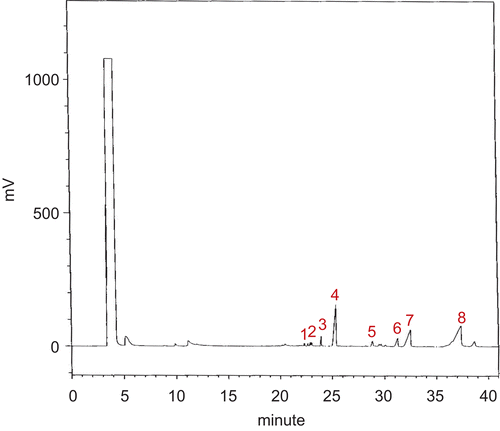
As often cited, SDF at 1% has been considered to be very efficient in suppressing plasma lipid levels, especially hepatic TG and TC (CitationFrias & Sgarbieri, 1998). Literature elsewhere indicated that for human health, a dose of 20 g SDF/day would be sufficient (CitationKnopp et al., 1999). In present study, the GC/MS profile revealed the presence of eight monosacharides (moles %) including galactose (28.75), arabinose (24.84), glucose (17.91), mannose (6.93), ribose (6.03), fucose (5.83), and xylose (5.30), with MW average at 878 kDa (, ), hence named arabinogalactan. CitationXu et al. (2006) demonstrated that the abundant occurrence of galactose, arabinose, and xylose were derived from galactoarabinoxylans, whereas glucose was mostly derived from β-glycan.
Table 3. The monosaccharide profile in arabinogalactan obtained from the mountain celery seeds and its retention time in GC/MS spectrum.
Figure 1. GC/MS profile of monosaccharides in the hydrolysate of the glucoarabinogalactan obtained from the mountain celery seeds. Monosaccharide was assingned by peak number as: 1 = rhamnose. 2 = fucose. 3 = ribose. 4 = arabinose. 5 = xylose. 6 = mannose. 7 = glucose. 8 = galactose.
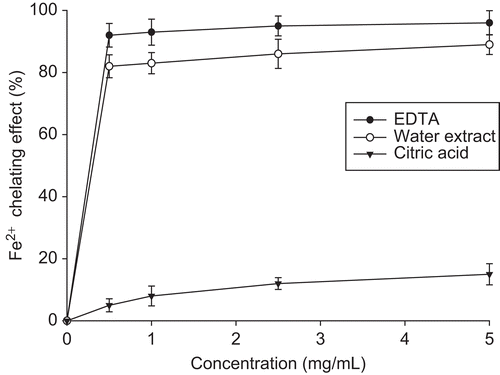
Ferrous ion chelation capability (FICC)
AE showed potent FICC and reached an 82%-89% chelation at concentrations of 0.1-5.0 mg/mL comparing with EDTA and citric acid (). Correspondingly, the FICC may mimic the anticatalytic oxidative power occurring in many biosystems including LDL, implicating the possible role of AE in antiatherosclerogenesis (CitationChait et al., 1993). Prevously, we reported that the MC seed essential oils exhibit moderately low antioxidant bioactivity (CitationCheng et al., 2008), a fact now evidenced by AE.
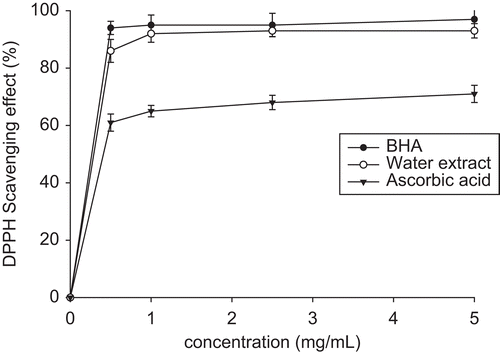
DPPH free radical scavenging capability (FRSC)
AE at concentrations 0.1-5.0 mg/mL exhibited very efficient DPPH FRSC. Comparable effects were seen in AE and BHA (83%-93% vs. 95.35%) but far exceeding that of ascorbic acid ().
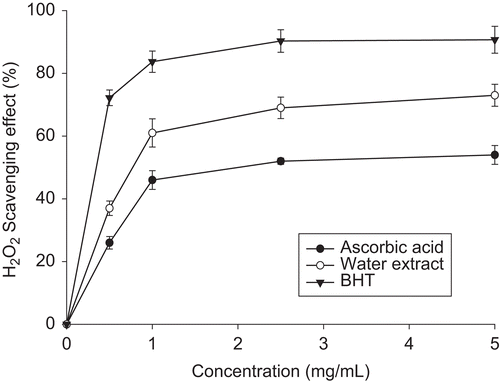
Hydrogen peroxide scavenging capability (HPSC)
AE showed only moderate antioxidant capability against hydrogen peroxide. Even at 5 mg/mL, AE only exhibited 73% of HPSC, being far lower than ascorbic acid yet amazingly higher than BHT (). AE was more effective than the seed essential oil (CitationCheng et al., 2008).
Serum and hepatic lipids
The body weight gain and fed efficiency were seen comparable among all groups (). The weight of liver and kidney appeared almost unchanged among all groups. However, the lipid patterns varied more apparently depending on different diet feeding.
Table 4. Effects of mountain celery seed powder (MP) and its aqueous extract (AE) on serum and hepatic nutritional parameters in male hamsters.a
Serum triglycerides
Both MP and AE effectively reduced the TG levels in a dose-responsive manner. AE showed more effective and prominent suppression than MP (), as seen in AE3 group based on 10-week feeding duration (), implying the SDF of MC exhibiting a significant nutritional bioactivity to affect the in vivo lipid biosynthesis (P < 0.05), consistent with CitationFrias and Sgarbieri (1998) and CitationKnopp et al. (1999).
Serum LDL-C
Both MP and AE significantly reduced the LDL-C, but MP was less effective than AE in this respect. Speculatively, a dose of AE 0.50 g/100 g diet could be sufficiently enough to treat hyperlipidemia ().
Serum HDL-C
Comparing with group H, MP groups failed to raise any HDL-C levels. In contrast, AE effectively raised the HDL-C levels (), resulting in reduced ratio of LDL-C/HDL-C in MP groups to 0.74-0.78 and to 0.52-0.75 in AE groups, implicating that AE could be more efficient to prevent cardiovascular diseases (P < 0.05) (), consistent with our previous report with the essential oils of mountain celery seeds (CitationCheng et al., 2008). Literature often cited that HDL-C level is inversely related with coronary atheroscrotic diseases (CAD) (CitationMoorjani et al., 1988). In this regard, AE can be an effective cardiovascular protective nutraceutic. As often cited, CAD prevention would rely more on the reduction of LDL-C/HDL-C ratio than simply on single parameter either LDL-C or HDL-C (CitationMoorjani et al., 1988).
Hepatic triglycerides
Similar to the above, MP did not show any significant effect on hepatic TG. In this regard, unlike AE that exerted more efficient suppressive activity. All AE groups, especially the AE3, significantly recovered the hyperlipidaemic levels ().
Hepatic total cholesterol
As for hepatic TC reduction, AE was more efficient than MP (), and only higher dose of MP like MP3 was comparable with all AE groups.
Hepatic phospholipids
Both MP and AE were all ineffective to affect the hepatic phospholipid content in all groups. Literature indicated that the higher level of phospholipid would facilitate a greater hypocholesterolemic effect (CitationLiu et al., 1995) (), an implication in phospholipid metabolism of hamsters totally unaffected by MC ().
Hepatic fatty acid profile
The hepatic acid pattern varied greatly depending on diet composition. Both AE and MP effectively suppressed the hepatic palmitic acid (16:0) and stearic acid (18:0) biosynthesis, but instead they elevated hepatic oleic acid (Δ18:1), linoleic acid (Δ18:2) (LA), and arachidonic acid (20:4) (ARA) levels (P < 0.05). Both oleic and linoleic acids are hypolipidemics and vasodilators (CitationHiley & Hoi, 2007; CitationJenko & Vanderhoek, 2008).
α-Linolenic acid (18:3) and LA accelerate the biosynthesis of docosahexaenoic acid (DHA) in many organs (CitationSarkadi-Nagy et al., 2004). DHA, a crucial nervous system n-3 PUFA, may be obtained in the diet or synthesized in vivo from dietary α-linolenic acid (CitationDeMar et al., 2008). Otherwise, the LA is activated by ATP and CoA as cosubstrates to produce linoleic-CoA through the catalytic reaction of acyl CoA lygase (Stryer & Lubert, 2000). Then through a serial steps involving Δ6 desaturase to create site of unsaturation at C-6, the addition of malonyl CoA to increase the chain length by 2 carbons accompanied by the simultaneous release of CO2 by mechanism similar to fatty acid biosynthesis, Δ5 desaturase to create site of unsaturation at C-5, and finally splitting off CoA by action of thiolnase to produce ARA (Stryer & Lubert, 2000). Apparently, these related enzymes were more upregulated by AE than MP (), resulting in higher conversion of arachidonic acid, provided the biosynthesis of linoleic acid is similarly upregulated (). With respect to the biosynthesis of ARA, AE exhibited more prominent effect than MP ().
Table 5. Change of hepatic fatty acid patterns in hamsters after having consumed the mountain celery seed powder and its aqueous extracts.a
The rate limiting reactions are well differentiated in vivo, for the biosynthesis of arachidonic acid may be at the level of the 18:3 to 20:3 elongase (CitationWynn & Ratledge, 2000). Speculatively, the increased fatty acid and triglyceride biosynthesis in rats and hamsters may be due to the activation of squalene synthetase activity (CitationUgawa et al., 2003). As seen, levels of serum TC and TG, and hepatic TC and TG were more effectively suppressed by AE ( and ), implicating AE was more actively affecting these enzymes.
Taken together, AE contains abundant amount of soluble arabinogalactan, polyphenolics, and flavonoids, which virtually contributed to its in vitro powerful ferrous chelating and DPPH scavenging effects. The high-soluble arabinogalactan and polyphenols in AE play a synergistic hypotriglyceridemic and hypocholesterolemic role in hamsters. AE is a very effective hypolidemic for elevation of HDL-C and lowering of LDL-C. Simultaneously, AE is a prominent activator for hepatic unsaturated fatty acid de novo synthesis involving oleic, linoleic, and the arachidonic acids.
Acknowledgment
The work was in part financially supported by Grant NSC 91- 2626-B-241-004, NSC 96-2320-B-241-006-MY3, NSC 97-2313-B-241-007-MY3 and NSC 97-2320-B-039-049-MY3 from the National Science Council, Taiwan.
Declaration of interest
All the authors do not have any conflict of interest in submitting this paper.
References
- Anonymous. (1977). Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr, 107, 1340–1348.
- AOAC. (1995). Official Methods of Analysis (16th edn). Washington, DC: Association of Official Analytical Chemists.
- Berg JM, Tymoczko JL, Stryer L. (2006). Biochemistry (6th edn). New York: W. H. Freeman.
- Chait A, Brazg RL, Tribble DL, Krauss RM. (1993). Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med, 94, 350–356.
- Cheng MC, Lin LY, Yu TH, Peng RY. (2008). Hypolipidemic and antioxidant activity of mountain celery (Cryptotaenia japonica Hassk) seed essential oils. J Agric Food Chem, 56, 3997–4003.
- DeMar JC Jr, DiMartino C, Baca AW, Lefkowitz W, Norman Salem N Jr. (2008). Effect of dietary docosahexaenoic acid on biosynthesis of docosahexaenoic acid from alpha-linolenic acid in young rats. J Lipid Res, 49, 1963–1980.
- Fang YC, Chen BH, Huang RF, Lu YF. (2004). Effect of genistein supplementation on tissue genistein and lipid peroxidation of serum, liver and low-density lipoprotein in hamsters. J Nutr Biochem, 15, 142–148.
- Folch J, Lees M, Sloane Stanley GH. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem, 226, 497–509.
- Frias AC, Sgarbieri VC. (1998). Guar gum effects on food intake, blood serum lipids and glucose levels of Wistar rats. Plant Foods Hum Nutr, 53, 15–28.
- Hiley CR, Hoi PM. (2007). Oleamide: a fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc Drug Rev, 25, 46–60.
- Jenko KJ, Vanderhoek JY. (2008). Conjugated linoleic acids and CLA-containing phospholipids inhibit NO formation in aortic endothelial cells. Lipids, 43, 335–342.
- Ker YB, Chen KC, Chyau CC, Chen CC, Guo JH, Hsieh CL, Wang HE, Peng CC, Chang CH, Peng RY. (2005). Antioxidant capability of polysaccharides fractionated from submerge-cultured Agaricus blazei mycelia. J Agric Food Chem, 53, 7052–7058.
- Knopp RH, Superko HR, Davidson M, Insull W, Dujovne CA, Kwiterovich PO, Zavoral JH, Graham K, O’Connor RR, Edelman DA. (1999). Long-term blood cholesterol-lowering effects of a dietary fiber supplement. Am J Prev Med, 17, 18–23.
- Le QT, Elliott WJ. (1991). Hypotensive and hypocholesterolemic effects of celery oil in the rat can be due to 3-butylphthalide. Clin Res, 392, 173A.
- Lin LY, Peng CC, Liang YJ, Yeh WT, Wang HE, Yu TH, Peng RY. (2008a). Alpinia zerumbet potentially elevates high-density lipoprotein cholesterol level in hamsters. j Agric Food Chem, 56, 4435–4443.
- Lin LY, Peng CC, Yang YL, Peng RY. (2008b). Optimization of bioactive compounds in buckwheat sprouts and their effect on blood cholesterol in hamsters. J Agric Food Chem, 56, 1216–1223.
- Liu CH, Huang MT, Huang PC. (1995). Sources of triacylglycerol accumulation in livers of rats fed a cholesterol-supplemented diet. Lipids, 30, 527–531.
- Moorjani S, Dupont A, Labrie F, Lupien PJ, Gagné C, Brun D, Giguère M, Bélanger A, Cusan L. (1988). Changes in plasma lipoproteins during various androgen suppression therapies in men with prostatic carcinoma: effects of orchiectomy, estrogen, and combination treatment with luteinizing hormone-releasing hormone agonist and flutamide. J Clin Endocrinol Metab, 66, 314–322.
- Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul F, Trotin F. (2000). Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol, 72, 35–42.
- Saleh MM, Zwaving JH, Malingré TM, Bos R. (1985). The essential oil of Apium graveolens var. secalinum and its cercaricidal activity. Pharm Weekbl Sci, 7, 277–279.
- Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Lawrence P, Nathanielsz PW, Brenna JT. (2004). Formula feeding potentiates docosahexaenoic and arachidonic acid biosynthesis in term and preterm baboon neonates. J Lipid Res, 45, 71–80.
- Srivastava A, Harish R, Shivanandappa T. (2006). Novel antioxidant compounds from the aqueous extract of the roots of Decalepis hamiltonii (Wight and Arn.) and their inhibitory effect on low-density lipoprotein oxidation. J Agric Food Chem, 54, 790–795.
- Taga MS, Miller EE, Pratt DE. (1984). China seeds as a source of natural antioxidant. J Am Oil Chem Soc, 61, 928–931.
- Tsi D, Das NP, Tan BK. (1995). Effects of aqueous celery (Apium graveolens) extract on lipid parameters of rats fed a high fat diet. Planta Med, 61, 18–21.
- Ugawa T, Kakuta H, Moritani H, Inagaki O, Shikama H. (2003). YM-53601, a novel squalene synthase inhibitor, suppresses lipogenic biosynthesis and lipid secretion in rodents. Br J Pharmacol, 139, 140–146.
- Wynn JP, Ratledge C. (2000). Evidence that the rate-limiting step for the biosynthesis of arachidonic acid in Mortierella alpina is at the level of the 18:3 to 20:3 elongase. Microbiology (Reading, Engl), 146 (Pt 9), 2325–2331.
- Xu F, Geng ZC, Sun JX, Liu CF, Ren JL, Sun RC, Fowler P, Baird MS. (2006). Fractional and structural characterization of hemicelluloses from perennial ryegrass (Lolium perenne) and cocksfoot grass (Dactylis glomerata). Carbohydr Res, 341, 2073–2082.
- Zheng GQ, Kenney PM, Zhang J, Lam LK. (1993). Chemoprevention of benzo[a]pyrene-induced forestomach cancer in mice by natural phthalides from celery seed oil. Nutr Cancer, 19, 77–86.