Abstract
Context: Althaea officinalis Linn. (Malvaideae) flower is commonly used in folk medicine in Lebanon and neighboring countries. Although most of the studies have been conducted on the mucilage-rich roots, little is known about the flower.
Objective: This study investigates the potential role of aqueous extract of Althaea officinalis flower in lipemia, gastric ulcer, inflammation, and platelet aggregation using the rat model.
Material and Methods: Blood lipid profile and liver function were assessed after 1 month of extract intake via drinking water. Anti-inflammatory activity was tested against acute and chronic inflammation induced by carrageenan and formalin, respectively. Antiulcer activity was evaluated using ethanol-induced gastric ulcer. Antiplatelet activity was investigated in vitro using the adenosine 5′-diphosphate (ADP)-induced platelet aggregation bioassay.
Results: The 50 mg/kg body weight dose resulted in significant increase in serum HDL cholesterol level with no effects on stool cholesterol and triacylglycerol. Increasing the dose to 500 mg/kg body weight caused a significant decrease in stool water content. No adverse effect on liver enzymes was observed. Significant anti-inflammatory (acute and chronic inflammation) and antiulcerogenic activities were observed at all used doses (50, 100, and 250 mg/kg body). Time-dependent inhibition of platelet aggregation was demonstrated at 500 µg/ml concentration.
Discussion and conclusion: The aqueous extract of Althaea officinalis flower demonstrated potential benefits in lipemia, inflammation, gastric ulcer, and platelet aggregation with no visible adverse effect.
Introduction
Folk medicine, particularly herbal medicine, is widely practiced worldwide and often without control. Extensive efforts have been invested to investigate the therapeutic and toxic effects of traditionally used medicinal plants. Marshmallow, Althaea officinalis Linn. (Malvaideae), abundant in Middle Eastern and many other countries (CitationMouterde, 1970; CitationQuattrocchi, 2000), is commonly prescribed by herbal medicine practitioners in Lebanon for inflammation, gastritis, and common cold. Reported literature revealed that the majority of studies on the plant were conducted on the mucilage-rich roots, which were shown to have antitussive action (CitationNosal’ova et al., 1992), anti-inflammatory and immunostimulant activity (CitationScheffer & Konig, 1991), hypoglycemic activity (CitationTomoda et al., 1987), and to increase the phagocytic activity of macrophages (CitationWagner & Proksch, 1985). Additional studies reported activity against periodontopathic facultative aerobic and anaerobic bacteria (CitationIauk et al., 2003), and reduction of pigmentation by inhibiting endothelin-1-induced activation of normal human melanocytes (CitationKobayashi et al., 2002).
Although many studies were conducted on the Althaea officinalis root, no work was reported on the biological activity of its flower. The flower of a close species, Althaea rosea (L.), was more investigated and shown to possess analgesic, anti-inflammatory (CitationWang et al., 1989), and antiestrogenic activities (CitationPapiez et al., 2002). In this study, the interest in the Althaea officinalis flower stems from the fact that people of Lebanon and neighboring countries consume infusions prepared from the flowers and not the roots. Decoction of the flower is commonly used in the treatment of gastric ulcer and inflammation. Therefore, this investigation was carried out to explore the medicinal effects of the water extract of Althaea officinalis flower upon inflammation and gastric ulcer in addition to antiplatelet activity. Also, blood lipid profile and potential hepatotoxicity were assessed after 1 month of administration of the extract via drinking water to rats.
Materials and methods
Plant collection and water extract preparation
Althaea officinalis flowers were collected from the Bekaa valley of Lebanon during the summer of 2007. The identification of the plant material was confirmed by plant taxonomy books (CitationMouterde, 1970) and the plant taxonomist, Dr. Ahmad Houry. The plant was dried in the shade. Aqueous extract was prepared by soaking the dried flowers in hot water (85–90°C) for half an hour, followed by filtration and freeze-drying of the filtrate (11.8% yield). All intraperitoneal injections were prepared in 0.9% NaCl solution and subjected to syringe filtration using Millipore filters (0.45 µm pores size).
Animal treatment and hyperlipidemia development
Male Sprague-Dawley rats (Lebanese American University stock) with an average weight of 275 g were used in all experiments. All experimental protocols were approved by the Animal Ethical Committee of the Lebanese American University, which complies with the Guide for the Care and Use of Laboratory Animals (National Research Council of the United States 1985). Animals caged in groups of four were maintained at an ambient temperature of 20–22°C with 12–12 h light-dark cycle. A freshly prepared flower extract was administered to experimental animals via drinking water on a daily basis. Since the consumption of each rat is 10 ml of drinking water per 100 g body weight (b.w.) per day (CitationWaynforth & Flecknell, 1992), solutions of the freeze-dried extract were prepared accordingly. A high fat diet was prepared by mixing 10 g of coconut oil with 90 g of standard rat chow (22% protein, 5.7% fat, and 72.3% carbohydrate) to increase the total fat content to 15% w/w. Animals received an isocaloric diet of 6.5 g of high fat food per 100 g b.w. Rat body weights were routinely measured to ensure homogeneous food consumption.
Rats were divided into four groups of eight animals each. Group I received plain water and served as control, while groups II, III, and IV received the extract solution at 50, 250, and 500 mg/kg b.w., respectively. On day 30, fasted animals were anesthetized using diethyl ether and 8 ml of blood was collected from the inferior vena cava. Serum samples were used for measuring liver enzymes activities (SGOT, SGPT, and ALP) and lipid profile (triacylglycerol, total cholesterol, HDL cholesterol, LDL cholesterol) using appropriate kits (SPINREACT, Spain). The above used doses were safe because doses up to 3000 mg/kg b.w. did not show any lethal effect in tested rats.
Stool analysis
Rat stool samples were collected on day 29 from each rat and weight of each sample was recorded. Stools were left overnight at 60°C in an oven to ensure complete drying. The next day, the dried stools were weighed and the percentage water content was computed and assessed for their triacylglycerol and cholesterol content as described previously by CitationDaher et al. (2003).
Anti-Inflammatory Effect
Carrageenan-induced paw edema in rat
Animals were divided into five groups of six animals each. Acute inflammation was produced in all groups by a single subplantar injection of 0.02 ml of freshly prepared 1% carrageenan in normal saline in the right hind paw of rats (CitationAjith & Janardhanan, 2001). Group I served as a positive control (no treatment), while Groups II, III, and IV received (i.p) the plant extract at a concentration of 100, 250, or 500 mg/kg b.w., respectively. Group V received i.p. diclofenac (10 mg/kg b.w.) and served as a standard reference drug. The extracts and diclofenac were administered 30 min prior to carrageenan injection. The paw thickness was measured using vernier calipers before and 3 h after carrageenan injection (CitationAjith & Janardhanan, 2001).
Formalin-induced paw edema in rats
Animals were divided into five groups of six animals each. Chronic inflammation was produced in all groups by a single subplantar injection of 0.02 ml of 2% formalin in the right hind paw (CitationAjith & Janardhanan, 2001). Thirty minutes prior to formalin injection, Groups I, II, and III received the plant extract (i.p.) at a concentration of 100, 250, or 500 mg/kg b.w., respectively, Group IV received the standard reference drug diclofenac (10 mg/kg b.w., i.p.), and Group V did not receive any treatment and served as a positive control. The administration of the extracts and diclofenac was continued once daily for 6 consecutive days. The paw thickness was measured using vernier calipers before and 6 days after formalin injection (CitationJose et al., 2004). The increase in paw thickness in both models was calculated using the formula:
where Pt is the thickness of paw at time t (i.e., 3 h after carrageenan injection and 6 days after formalin injection) and P0 is the paw thickness at 0 time.
The percent inhibition was calculated using the formula:
where C is the increase in paw thickness of the positive control and T is that of treatments.
Ethanol-induced gastric ulcer in rats
Gastric ulcer was conducted on male Sprague-Dawley rats according to the method described by CitationAlkofahi and Atta (1999). Briefly, animals (250–300 g) were randomly assigned to five groups of six rats each. Animals were deprived of food for 48 h before use to ensure an empty stomach, and kept in cages with raised floors of wide wire mesh to prevent coprophagy. To prevent excessive dehydration during starvation, all groups were supplied with sucrose 8% (w/v) in NaCl 0.2% (w/v), which was removed 1 h before experimentation (CitationAlkofahi & Atta, 1999; CitationGharzouli et al., 1999). Group I served as control, Groups II, III, and IV received oral doses of 50, 100, and 250 mg/kg b.w., and Group V received oral 11.5 mg/kg b.w. of the reference standard drug, cimetidine (CitationXu et al., 1998). All doses were administered intragastrically with water (10 ml/kg b.w.). Two doses were given on the first day at 9:00 h and 17:00 h; a third dose was given on the second day 1.5 h before induction of gastric ulceration. Gastric ulcers in all animals were induced by gastric gavage (10 ml/kg b.w.) using 50% ethanol in water.
One hour after ethanol administration, all animals were killed by an overdose of diethyl ether, stomachs were rapidly removed, opened along their greater curvature, and rinsed under running tap water. Using illuminated stereomicroscope, long lesions were counted and measured along their greater length. Petechial lesions (very small lesions) were also counted and each five were considered as 1 mm of ulcer. The average total length of long ulcers and petechial lesions in each group of rats represented the ulcer index (mm). The curative ratio was determined by the formula:
Platelet aggregation
The platelet aggregation assay was performed according to the method of CitationJose et al. (2004). Briefly, 8 ml of fresh blood from healthy rats was collected in test tubes containing 1.6 ml of anticoagulant solution (5:1 ratio). The anticoagulant solution consisted of sodium citrate 2.4% (w/v), citric acid 1.5% (w/v), and d-glucose 1.8% (w/v). Platelets were isolated and the final platelet pellets were suspended in a buffer solution composed of 113 mM NaCl, 4.3 mM K2HPO4, 16 mM Na2HPO4, 8.3 mM NaH2PO4, and 5.5 mM d-glucose (pH 7.5). The suspension was adjusted to a final optical density of 0.5/ml at 600 nm.
The extract of Althaea officinalis (500 µg) was incubated with a volume of 1 ml washed platelets in three siliconized tubes for 5, 10, and 20 min in a waterbath at 37°C. Approximately 1 mM freshly prepared adenosine 5′-diphosphate (ADP) (20 µl ) was added at the end of the incubation period and mixed thoroughly with the washed platelets, then the optical density at 600 nm was measured at 1 min intervals up to 5 min. For each of the three tubes, a normal and control tube was prepared. A solution of washed platelets incubated in the buffer and to which ADP was added served as a control. A normal solution was prepared by incubating the washed platelets and extract with no ADP added. A graph was plotted for the relative optical density versus time for the control, normal, and extract. The percentage platelet aggregation inhibition was calculated according to the formula:
The percentage aggregation of control ADP was assumed as 100 %.
Statistical analysis
Values are presented as means ± S.E.M. One-way analysis of variance was used to determine the significant difference between the treated groups and the control. All values were considered significant when P < 0.05.
Results
Stool analysis
The intake of the water extract of Althaea officinalis flower, over the study period, revealed a significant decrease of 14.6% in water content with respect to control only when animals received 500 mg of extract per kg body weight. However, the extract appeared to have no effect upon stools triacylglycerol and cholesterol content regardless of the dose used. Data are presented in .
Table 1. Percent fecal water content, fecal triacylglycerol (mg/g), and fecal cholesterol (mg/g) as determined after 29 days of Althaea officinalis flowers water extract intake in drinking water.
Serum lipid profile
After 1 month of extract intake, there were no significant changes in mean serum total cholesterol, LDL cholesterol, and triacylglycerol concentrations with respect to control. However, the mean serum HDL cholesterol levels were relatively higher in all treated groups, incrementing between 9.9 and 26.9%, and significance was only reached with the 50 mg/kg b.w. dose. Data are presented in .
Table 2. Mean serum concentrations of TAG, total cholesterol, LDL-cholesterol, HDL-cholesterol, and glucose in control and treatment groups after 1 month Althaea officinalis flower water extract intake in drinking water.
The effect of Althaea officinalis on the activity of liver enzymes
A general decrease in liver enzymes activities was observed after 1 month of Althaea officinalis flower water extract intake (). A significant decrease (28.5–39%) in the activity of SGOT was noted with all doses used. Similar observation was recorded (8.3–27.2%) with SGPT activity with significance reached at 50 and 500 mg/kg b.w. doses. As for ALP, only the 50 mg/kg b.w. dose resulted in a significant decrease (28.7%) in ALP activity. Calculation of the SGOT:SGPT ratio revealed that all experimental groups exhibited a lower ratio with respect to control.
Table 3. SGOT, SGPT, ALP (U/L), serum activities, and SGOT/SGPT ratio of control and treatment groups after 1 month of supplementation with water extract of Althaea officinalis flower
Anti-inflammatory activity
The water extract of Althaea officinalis flowers exhibited promising anti-inflammatory activities at all doses used (). The inhibition of inflammation ranged between 41–78% and 52–68% in acute and chronic inflammation models, respectively. The 250 mg/kg b.w. dose appeared to be the optimum dose in both models.
Table 4. Effect of water extract of Althaea officinalis flowers upon acute (carrageenan-induced) and chronic (formalin-induced) inflammation.
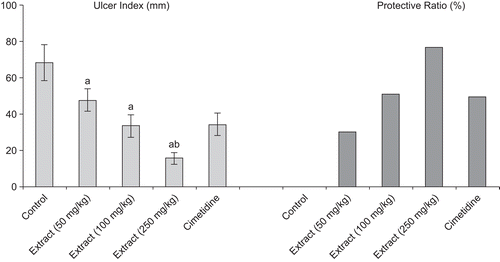
Gastroprotective effect against ethanol-induced gastric ulcer
Ethanol-induced gastric damage was characterized by the presence of elongated and petechial lesions found in the glandular region. Animals receiving the extract showed a significant dose-dependent protection against ethanol-induced ulcer. With respect to control group, animals receiving the 250 mg/kg b.w. dose showed a better protection than cimetidine, the reference drug ().
Platelet aggregation activity
Addition of ADP, an inducer of aggregation, to washed rat platelets is manifested by a marked decrease in OD at 600 nm. Preincubation of the platelets with the water extract of the flowers of Althaea officinalis (500 µg/ml) caused an increase in OD indicating an inhibition of platelet aggregation induced by ADP. The inhibitory effect (17.9–72%) was time-dependent and significance was achieved after 10 min preincubation with the extract ().
Table 5. Effect of water extract of Althaea officinalis flowers (500 µg) on platelet aggregation induced by ADP (1 mM) when incubated with washed human platelets for 5, 10, and 20 min.
Discussion
This study reveals some medicinal potentials of the aqueous extract of Althaea officinalis flower. After 4 weeks of extract intake, only the highest dose caused a mild but significant decrease in the water content of stools. Normally, such a high dose is not commonly used in the community and this explains why the flower was never claimed to cause constipation. Furthermore, stool analyses showed that the extract has a neutral effect on the intestinal absorptive efficiency of cholesterol and triacylglycerol.
These data revealed a significant increase in HDL-cholesterol with the lowest dose of the extract with no change in LDL cholesterol. The increase in HDL-cholesterol may be attributed to pectin in the flower because HDL-cholesterol levels were found to be significantly higher in rats fed pectin (CitationBobek & Chorvathova, 1984). Althaea officinalis flower is known to contain flavonoids (CitationGudej, 1989); however, the efficacy of the flavonoids contribution to hypolipidemia is controversial (CitationBladé et al., 2010). Higher doses of the extract failed to significantly improve serum HDL cholesterol for unknown reasons. It could be that some other components in the extract have reached concentrations high enough to antagonize the beneficial effect of pectin. It is worth mentioning that the incremental trend (≥10%) in HDL cholesterol was still observed at higher doses.
The liver is an essential organ in the body and the major one for metabolism of drugs and exogenous toxins. For this reason, reports of hepatotoxicity must be included in any study conducted on medicinal herbs (CitationGrunhage et al., 2003). After 1 month of extract intake, the activities of SGOT and SGPT in serum were decreased indicating a hepatoprotective rather than hepatotoxic effects of the extract on the liver cells. In addition, the ratios of SGOT/SGPT were all less than 2 meaning that there is no fear of liver toxicity (CitationCohen & Kaplan, 1979), and hence the extract may be safe to consume. The ALP activities in the treatment groups were also less or similar to that of the control group indicating no risk of cholestasis (CitationPratt & Kaplan, 2001).
The potential anti-inflammatory activity of the water extract of Althaea officinalis flowers was investigated using in vivo models of acute and chronic inflammation induced by carrageenan and formalin, respectively. The extract exhibited high levels of anti-inflammatory activities in both models of inflammation. The 250 mg/kg b.w. dose appeared to be the optimum dose in both models producing an effect comparable to that of diclofenac, the reference anti-inflammatory drug. Studies conducted on roots of Althaea officinalis along with these data reveal a promising role of the plant in inflammation. Flavonoids, in general, have been recognized to possess anti-inflammatory activity by decreasing the release of inflammatory mediators, stabilizing cell membranes (CitationMartini et al., 2004) and inhibiting cyclooxygenase enzyme activity (CitationJose et al., 2004). In addition, the mucilage was shown to possess anti-inflammatory activity (CitationGalati et al., 2005). Whether the mucilage, flavonoids, both, or other extract components are responsible for the anti-inflammatory effects need to be investigated.
Many medicinal plants have been reported to possess gastroprotective properties against chemically induced ulcer (CitationAlkofahi & Atta, 1999; CitationBorrelli & Izzo, 2000; CitationZayachkivska et al., 2005). In this study, Althaea officinalis flower extract demonstrated a dose-dependent protection against ethanol-induced gastric ulcer. It has been shown that mucilage and flavonoids have the property of covering and protecting gastric mucosa, thereby reducing the incidence of gastric ulcer (CitationIzzo et al., 1994). The gastroprotective effect observed in this study could be attributed to active compounds found in the extract such as flavonoids and mucilage polysaccharides.
The effect of water extract of Althaea officinalis flowers on platelets aggregation was investigated. The in vitro 500 µg/ml dose was selected to mimic an oral dose of 50 mg/kg b.w., taking into consideration the rat blood volume and medium extract bioavailability. The inhibition of platelet aggregation was time dependent in the presence of ADP, a potent activator of platelets aggregation (CitationJose et al., 2004). Previous studies showed that flavonoids inhibit platelet function through binding to the thromboxane A2 receptor (CitationGuerrero et al., 2005). Therefore, flavonoids present in the flower of Althaea officinalis may be responsible for the inhibition of platelet aggregation observed and support the use of flavonoids in folk medicine in the protection from thrombosis and heart shock.
Conclusion
This investigation demonstrates many potential benefits of the Althaea officinalis flower aqueous extract, thus confirming the traditional use of the flower in treating gastric ulcer and inflammation. The lack of hepatotoxicity, the improvement of HDL cholesterol level, and the antiplatelet activity are additional benefits to be noticed. The different activities observed represent a starting point and ‘essential database’ for future studies where further purification and assessment of the activity of each component may be done.
Acknowledgements
We thank Miss Jessica Saliba and Miss Simona Ghanem for the histological processing; Mr. Jean Karam for taking care of the animals; and Mrs. Helena Bou Farah for lab assistance.
Declaration of interest
The authors report no conflicts of interest.
References
- Ajith TA, Janardhanan KK. (2001). Antioxidant and anti-inflammatory activities of methanol extract of Phellinus rimosus (Berk) Pilat. Indian J Exp Biol, 39, 1166–1169.
- Alkofahi A, Atta AH. (1999). Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol, 67, 341–345.
- Bladé C, Arola L, Salvadó MJ. (2010). Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res, 54, 37–59.
- Bobek P, Chorváthová V. (1984). Effect of pectin on cholesterol distribution in the lipoproteins of streptozotocin-diabetic rats fed cholesterol diet. Physiol Bohemoslov, 33, 230–236.
- Borrelli F, Izzo AA. (2000). The plant kingdom as a source of anti-ulcer remedies. Phytother Res, 14, 581–591.
- Cohen JA, Kaplan MM. (1979). The SGOT/SGPT ratio–an indicator of alcoholic liver disease. Dig Dis Sci, 24, 835–838.
- Daher CF, Baroody GM, Howland RJ. (2003). Effect of a surfactant, Tween 80, on the formation and secretion of chylomicrons in the rat. Food Chem Toxicol, 41, 575–582.
- Galati EM, Cavallaro A, Ainis T, Tripodo MM, Bonaccorsi I, Contartese G, Taviano MF, Fimiani V. (2005). Anti-inflammatory effect of lemon mucilage: in vivo and in vitro studies. Immunopharmacol Immunotoxicol, 27, 661–670.
- Gharzouli K, Khennouf S, Amira S, Gharzouli A. (1999). Effects of aqueous extracts from Quercus ilex L. root bark, Punica granatum L. fruit peel and Artemisia herba-alba Asso leaves on ethanol-induced gastric damage in rats. Phytother Res, 13, 42–45.
- Grünhage F, Fischer HP, Sauerbruch T, Reichel C. (2003). [Drug- and toxin-induced hepatotoxicity]. Z Gastroenterol, 41, 565–578.
- Gudej J. (1989). Determination of flavonoids in leaves, flowers and roots of Althaea officinalis L. Farm Pol 46, 153–155.
- Guerrero JA, Lozano ML, Castillo J, Benavente-García O, Vicente V, Rivera J. (2005). Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost, 3, 369–376.
- Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G. (2003). Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res, 17, 599–604.
- Izzo AA, Di Carlo G, Mascolo N, Autore G, Capasso F (1994). Antiulcer effect of flavonoids. Role of endogenous PAF. Phytother Res 29, 87–93.
- Jose N, Ajith TA, Janardhanan KK. (2004). Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation. Phytother Res, 18, 43–46.
- Kobayashi A, Hachiya A, Ohuchi A, Kitahara T, Takema Y. (2002). Inhibitory mechanism of an extract of Althaea officinalis L. on endothelin-1-induced melanocyte activation. Biol Pharm Bull, 25, 229–234.
- Martini ND, Katerere DR, Eloff JN. (2004). Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol, 93, 207–212.
- Mouterde P (1970). New Flora of Lebanon and Syria. Dar El-Mashreq (Ed.). Beirut, Lebanon, pp. 44–55.
- Nosál’ova G, Strapková A, Kardosová A, Capek P, Zathurecký L, Bukovská E. (1992). [Antitussive action of extracts and polysaccharides of marsh mallow (Althea officinalis L., var. robusta)]. Pharmazie, 47, 224–226.
- Papiez M, Gancarczyk M, Bilinska B. (2002). The compounds from the hollyhock extract (Althaea rosea Cav. var. nigra) affect the aromatization in rat testicular cells in vivo and in vitro. Folia Histochem Cytobiol, 40, 353–359.
- Pratt DS, Kaplan MM (2001). Evaluation of liver function. In: Harrison TR and Fauci AS, Harrison’s Principles of Internal Medicine (15th ed.). New York: McGraw-Hill, pp. 1711–1714.
- Quattrocchi U (2000). World Dictionary of Plant Names. Boca Raton, Florida: CRC Press, Vol 1, pp.105–106.
- Scheffer J, Konig W (1991). Radix althaeae und flores chamomillae extrakte auf entzündungsreaktionen humaner neutrophiler granulozyten, monozyten und rattenmastzellen. The Third Phytotherapy Congress, Abstracts. p.9.
- Tomoda M, Shimizu N, Oshima Y, Takahashi M, Murakami M, Hikino H (1987). Hypoglycemic activity of twenty plant mucilages and three modified products. Plant Med, 53, 8–12.
- Wagner H, Proksch A (1985). Immunostimulatory drugs of fungi and higher plants. In: Wagner H, Hikino H, and Farnsworth NR, Economic and Medical Plant Research, Orlando, FL: Academic Press, vol 1, pp 111–153.
- Wang DF, Shang JY, Yu QH. (1989). [Analgesic and anti-inflammatory effects of the flower of Althaea rosea (L.) Cav.]. Zhongguo Zhong Yao Za Zhi, 14, 46–8, 64.
- Waynforth HB, Flecknell PA (1992). Experimental and Surgical Technique in the Rat. Academic Press, London, pp. 341–358.
- Xu CD, Gan RB, Chen SN, Jiang SH, Xu JY. (1998). Protection of gastric mucosa from ethanol induced injury by recombinant epidermal growth factor in rats. World J Gastroenterol, 4, 437–438.
- Zayachkivska OS, Konturek SJ, Drozdowicz D, Konturek PC, Brzozowski T, Ghegotsky MR. (2005). Gastroprotective effects of flavonoids in plant extracts. J Physiol Pharmacol, 56 Suppl 1, 219–231.