Abstract
Context: Garlic, Allium sativum L. (Liliaceae), possesses high therapeutic and pharmacological properties. Hypoglycemic activity is attributed to alliin (S-allyl cysteine sulfoxide), the main active principle localized in garlic cloves.
Objective: To compare the production and therapeutic efficiency of alliin extracted from garlic leaves of plants grown under ex situ and in situ conditions.
Materials and methods: Alliin content of leaves was quantified and aqueous leaf extracts (from ex situ and in situ grown plants) were given to normal and alloxan-induced diabetic rats for five weeks.
Results: Alliin production noted ~50% enhancement in leaves from plants grown under in situ conditions. Serum glucose, triglycerides, total lipids, total cholesterol, low-density lipoprotein (LDL)-, and very low-density lipoprotein (VLDL)-cholesterol in diabetic rats treated with alliin produced from in situ grown plants noted significant reduction of ~54%, 15%, 14%, 20%, 24%, and 15%, while 35%, 14%, 10%, 12%, 17% and 11% reduction was noted in diabetic rats treated with alliin produced from ex situ grown plants in comparison with those administered with distilled water. High-density lipoprotein (HDL)-cholesterol did not show any significant change. Leaf extract of plants lowered serum enzyme levels (alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase) toward the norm better than glibenclamide. The histopathological alteration in pancreas caused by alloxan was also reduced by leaf extract.
Discussion and conclusion: These findings demonstrate leaf extract obtained from plants grown under in situ condition possess higher therapeutic efficiency in comparison with leaf extract obtained from plants grown under ex situ condition. Studies suggest that environmental factors influence production of alliin and its therapeutic potential.
Keywords::
Introduction
Garlic possesses high therapeutic and pharmacological properties including antibiotic, antitumor, antithrombotic, hypocholesterolemic, fibrinolytic, hypertensive effects (CitationEidi et al., 2005; CitationEl-Demerdash et al., 2005; CitationThomson et al., 2007; CitationNasim et al., 2009a). The hypoglycemic activity of plant contributing to treatment of diabetes has been attributed to S-allyl cysteine sulfoxide, the main active principle localized in cloves (CitationJones et al., 2007; CitationNasim et al., 2009b). Literature suggests that garlic administration decreased serum glucose and enhanced contractile response to phenylephrine, significantly improved impaired endothelium-dependent relaxations, and increased serum antioxidant levels in diabetic rats (CitationBaluchnejadmojarad et al., 2003; CitationEidi et al., 2005; CitationDrobiova et al., 2009). Moreover, garlic oil also showed hypoglycemic effect in diabetic patients, through increased insulin secretion as demonstrated in rats with streptozotocin-induced diabetes (CitationLiu et al., 2006).
Application of plant cell culture technologies have improved production of plant secondary metabolites contributing to medicinal value of the plant (CitationSchmeda-Hirschmanna et al., 2004; CitationNasim et al., 2010). In vitro cultures accelerate production of specific medicinal compounds at a rate similar or superior to that of intact plants by affecting the biosynthetic activity of cells. This can be controlled by regulating environmental factors, as well as artificial selection or induction of variant clones (CitationVanisree et al., 2004; CitationJunaid et al., 2008). Enhanced production of secondary metabolites including increased alliin production has been reported in plants grown under in situ condition (CitationBhagyalakshmi et al., 2005).
The leaf is the primary site for synthesis of cysteine sulfoxide (alliin) from where it gets translocated to other plant parts such as the bulb (CitationHughes et al., 2005; CitationNasim et al., 2009b). Earlier studies showed higher metabolite content including alliin in garlic cloves, but production and therapeutic efficiency of alliin in leaves have not been checked. Therefore, the present study was carried with an aim to: (i) compare the alliin (metabolite) production in leaves of plants grown under different culture conditions, that is, ex situ and in situ; (ii) compare the therapeutic efficiency (antidiabetic effect) of leaf extract obtained from plants grown under the two different culture conditions; and (iii) compare the efficacy of leaf extract from plants grown under different conditions with the drug glibenclamide as standard reference.
Materials and methods
Plant material
Local Indian garlic, Allium sativum L. cv. Yamuna Safed (Liliaceae), procured from the National Horticultural Research and Development Foundation, Nasik, Maharashtra, was used for the present study. Plant identity was confirmed and voucher specimen IC-375117 was deposited at the National Bureau of Plant Genetic Resources, New Delhi, India (CitationNasim et al., 2010). Plants grown under natural conditions (25 ± 5°C) in a herbal garden were taken as ex situ grown plants. The plants were grown in earthenware pots having sandy loam soil (pH 7.2). The plants grown on agar medium (CitationMurashige & Skoog, 1962), supplemented with 1 mg/L 6-benzylaminopurine (BAP) and 0.25 mg/L naphthalene acetic acid (NAA) at 25 ± 2°C in a culture room under a 16-h photoperiod provided by cool white fluorescent light (intensity 40 W/m2) for 15 weeks, were taken as in situ grown plants (CitationNasim et al., 2009b). Alliin production was noted in leaves of 2-month-old plants and further investigations were carried out at the same time (data not shown).
Phytochemical analysis
For high-performance thin-layer chromatography (HPTLC) analysis, 1 g dry leaf powder was extracted using 10 mL methanol–water (80:20, v/v) plus 0.05% formic acid (pH 3) following the modified method given by CitationArnault et al. (2003). An aliquot of 1 mL of 1 mg/L stock solution of alliin was taken and was further diluted with methanol to make the final volume to 10 mL (100 ng/mL). Chromatographic estimations were performed following CitationNasim et al. (2009b).
Animals
Healthy male albino rats (weight 200–300 g) procured from the Central Animal House facility, Jamia Hamdard were housed in clean cages under controlled environmental conditions (22–24°C; relative humidity 40–60%) as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India.
Induction of diabetes in rats
The induction of diabetes in rats was done following methods described previously (CitationMansour et al., 2002; CitationNasim et al., 2009a).
Preparation of garlic extracts and drug administration
For preparation of extract, 50 g leaves were homogenized in 75 mL of sterilized 0.9% saline solution in ice-cold conditions. The homogenized mixture was filtered three times through cheesecloth. The filtrate was centrifuged at 2000 g for 10 min and the volume of clear supernatant was made up to 100 mL with normal saline solution. The concentration of leaf preparation was found to be 500 mg/mL (0.5 g/mL), on the basis of the leaf weight. Aqueous extract obtained was stored at −20°C. Final dose administered corresponded to 0.5 g/kg body weight. The lower concentration was prepared according to the body weight of the rats by diluting the leaf extract with cold, sterile 0.9% saline (CitationNasim et al., 2009a). The present dose was decided on the basis of an earlier experiment that showed toxicity above the given value (data not shown).
Experimental design
In each experiment, 56 male rats were used. The rats were divided into seven groups (eight rats in each group). Treatment was given daily using intragastric tube for 5 weeks. Group I: Normal rats were administered 1 mL of sterile distilled water; Group II: Normal rats were administered 1 mL of aqueous leaf extract obtained from ex situ grown plants; Group III: Normal rats were administered with 1 mL of aqueous leaf extract obtained from in situ grown plants; Group IV: Alloxan-treated rats were administered with 1 mL distilled water; Group V: Alloxan-treated rats were administered with 1 mL aqueous leaf extract obtained from ex situ grown plants; Group VI: Alloxan-treated rats were administered with 1 mL aqueous leaf extract from in situ grown plants; Group VII: Alloxan-treated rats were administered glibenclamide orally (600 µg/kg body weight) dissolved in 1 mL water (CitationEidi et al., 2005).
Separation of serum and biochemical estimation
The separation of serum was done as described previously (CitationNasim et al., 2009a). Serum collected was analyzed for enzyme levels immediately or within 24 h after storing at 4°C. Non-hemolyzed serum was taken. Serum glucose, lipid profile (total cholesterol, low-density lipoprotein (LDL)- and very low-density lipoprotein (VLDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, total lipids, triglycerides), and serum enzyme levels namely alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were analyzed using assay kits (Span Diagnostic, Surat, India).
Histopathological studies
Rats from control and treated groups were perfused with 10% neutral formalin solution. The pancreas was removed immediately; paraffin sections (5-µm thickness) were made and stained by hematoxylin–eosin (H&E) stain. The cell density, cell size, and cell injury in the islets of Langerhans in the pancreas of diabetic rats was compared with normal pancreatic cells. The sections were observed under light microscope and photographs were taken. Settings for histopathological studies were done as described by CitationLuna (1960).
Statistical analysis
The data obtained from three independent experiments (three replicates each) were checked for the level of significance using Student’s t-test and ANOVA variance analysis (P ≤ 0.05). P-values under 0.05 were considered statistically significant (shown as *); P-values under 0.001 were considered highly significant (shown as ***); and those not significant are shown as NS.
Results and discussion
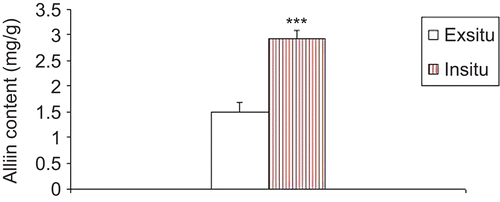
Leaves of plants grown under in situ condition show increased production of alliin in comparison with those raised under ex situ condition ( and ). HPTLC analysis showed the prominent band (Lane 3) depicting higher alliin production in leaf samples taken from plants grown under in situ condition, although a light band (Lane 4) was observed in leaf samples obtained from plants grown under ex situ condition depicting comparatively low alliin production (). Leaves from plants grown under in situ condition showed noted enhancement ~50% in alliin production (). Alliin accumulation depends upon plant variety and environmental conditions in which the plants are grown (CitationNasim et al., 2009a, Citation2010). Controlled growth and environmental conditions including light and temperature factors affect production of carbohydrates, thus influencing the production of alliin (CitationNasim et al., 2010). Cell culture conditions have proved to be advantageous over conventional cultivation of plants because: (i) useful compounds can be produced under controlled conditions independent of climatic changes or soil conditions and (ii) cultured cells would be free of microbes and insects (CitationSchmeda-Hirschmanna et al., 2004; CitationVanisree et al., 2004; CitationNasim et al., 2010).
Figure 1. HPTLC fingerprint (allilin) obtained from leaf extract of ex situ and in situ raised plants. Track assignment: 1–2: Reference substance (allilin) with RF sample; 3: in situ leaves extract; 4: ex situ leaves extract.
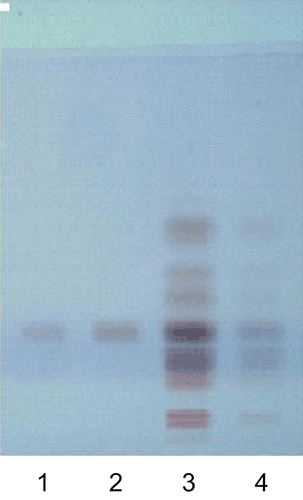
Figure 2. Allilin content of leaves from plants raised under ex situ and in situ conditions. Each value represents Mean ± SEM. ***P<0.001 denotes level of significance. P-values are highly significant at P<0.01.
Alloxan-induced diabetic rats showed a decrease in liver and body weight as compared with control (). Administration of leaf extract did not affect body and liver weight in normal rats (Groups I, II, and III). The average body weight of diabetic control rats (Group IV) was significantly low as compared with the control rats (normal, Group I). The diabetic rats treated with aqueous leaf extract (dose of 0.5 g/kg body weight) from ex situ and in situ grown plants showed gain in mean body weight (Groups V and VI) as compared with diabetic control rats (Group IV). The gain in body weight was significant in rats administered with leaf extract from plants grown under in situ conditions (Group VI). The body weight of diabetic rats administered with leaf extract from plants grown under ex situ and in situ conditions noted an increase of 13% and 22.7% (Groups V and VI) in comparison with diabetic control rats (Group IV). The body weight of diabetic rats also noted an increase after glibenclamide administration (Group VII). The mean liver weight of diabetic control rats (Group IV) was reduced considerably when compared with normal control rats (normal) (Group I). The administration of leaf extract from plants grown under ex situ and in situ conditions increased the mean liver weight in diabetic rats (Groups V and VI). Leaf extract from plants grown under ex situ and in situ conditions was effective in altering the liver weight of diabetic rats in comparison with glibenclamide (). The liver weight of diabetic rats administered glibenclamide (Group VII) showed a small gain, although rats administered leaf extract from plants grown in ex situ condition showed an increase in liver weight by 6%, and rats administered with leaf extract from plants grown in in situ condition showed an increase in liver weight by 10%. Alloxan acts as a cytotoxic agent that affects organs/metabolism that lead to significant decline in the growth rate (CitationKhalil, 2004). The decrease in liver weight in the diabetic animals results from decrease in soluble protein content due to decrease in protein synthesis in cytosol (CitationShalini et al., 2004). The action of alloxan in the pancreas is preceded by its rapid uptake by the β cells that evoke a sudden rise in insulin secretion in the presence or absence of glucose (CitationSzkudelski, 2001). Active metabolites present in the leaf extract possess antioxidant activity and thiol-containing proteins that possess hypoglycemic properties that protect functional organs (CitationRabinkov et al., 1998). Plants grown under in situ condition show high levels of sulfur compounds (alliin and its derivatives) in the leaf extract that might contribute to higher therapeutic potency.
Table 1. Body and liver weight of normal and alloxan-induced diabetic rats under different treatments.
The fundamental mechanism underlying hyperglycemia in diabetes involves overproduction (excessive hepatic glycogenolysis and gluconeogenesis) as well as decreased utilization of glucose by the tissue (CitationSheela & Augusti, 1992). A significant elevation (~75%) in serum glucose was observed in diabetic controls (Group IV) as compared with normal rats (Group I). The administration of leaf extract from plants grown under ex situ and in situ conditions did not affect serum glucose levels in normal rats. The administration of leaf extract from plants grown under ex situ and in situ conditions and glibenclamide brought decline in serum glucose toward the normal values (), though results were significant in rats administered with leaf extract (plants grown in ex situ and in situ conditions) in comparison with rats treated with glibenclamide. Diabetic rats administered with aqueous leaf extract showed 35% and 50% reduction in serum glucose levels in rats administered with leaf extract (plants grown in ex situ and in situ conditions), while ~27% decrease was noted in glibenclamide-treated group (). Leaf extract obtained from plants grown under in situ condition was found to be effective in reducing serum glucose levels than ex situ grown plant. This is probably due to higher production of bioactive compounds, S-allyl cysteine sulfoxide, allicin, di(2-propenyl) disulfide, or 2-propenyl propyl disulfide, though leaf extract was more effective as compared with glibenclamide (CitationSangeetha & Quine, 2008). The mechanism of hypoglycemic action probably involves direct or indirect stimulation of insulin secretion (CitationEidi et al., 2005; CitationNasim et al., 2009a). It is suggested that disulfide compounds have the effect of sparing insulin from –SH inactivation by reacting with endogenous thiol-containing molecules such as cysteine, glutathione, and serum albumins (CitationEidi et al., 2005). It may be due to restoration of delayed insulin response or inhibition of intestinal absorption of glucose or increased secretion of insulin from existing β-cells or its release from bound insulin (CitationEidi et al., 2005).
Table 2. Serum glucose and lipid profile of normal and diabetic rats measured under different experimental conditions.
The administration of aqueous leaf extract obtained from plants raised under different culture conditions affected the lipid profile of rats . The administration of leaf extract from plants grown under ex situ and in situ conditions did not affect level of total cholesterol, total lipids, triglycerides, and fractions of cholesterol (HDL-, LDL-, and VLDL-cholesterol) in normal rats (Groups I–III) (). Level of total cholesterol, total lipids, triglycerides, and fractions of cholesterol (LDL- and VLDL-cholesterol) were significantly higher in diabetic rats (Group IV) as compared with normal control rats (Group I). HDL-cholesterol did not exhibit any significant change. Aqueous leaf extract decreased the values of serum lipid profile (except HDL-cholesterol) in diabetic rats. Diabetic rats treated with leaf extract from ex situ and in situ grown plants showed better recovery in serum lipid profile than the glibenclamide drug; reduction was more significant in rats administered with leaf extract from plants grown under in situ condition. A significant reduction of total cholesterol (8.6%), total lipids (4.9%), triglycerides (~1%), LDL-cholesterol (8.4%), and VLDL-cholesterol (4.1%) was noted in rats treated with leaf extract obtained from plants grown under in situ condition in comparison with those treated with leaf extract obtained from plants grown under ex situ condition (). However, HDL-cholesterol value increased by about 6.5%. Epidemiological studies showed that supplementation of leaf extract decreases serum cholesterol, hepatic cholesterol, and/or triglyceride levels in animals (CitationYeh & Yeh, 1994), though the mechanism by which garlic does so is not fully elucidated. Studies have demonstrated that garlic-supplemented diets decreased activities of various lipogenic enzymes such as FAS, G6PDH, and the cholesterogenic enzyme, HMG-CoA reductase (CitationSheela & Augusti, 1995); therefore, it is speculated that the lipid-lowering effects of leaf extracts may result from decreased lipogenesis and cholesterogenesis.
Serum enzymes levels increased in diabetic rats (Group IV) as compared with normal rats (Group I) (). The administration of leaf extract from plants grown under ex situ and in situ conditions did not affect ALP, AST, and ALT activities in normal rats. The administration of leaf extract and glibenclamide brought the serum enzymes levels significantly toward the normal values in diabetic rats. Diabetic rats administered with garlic leaf extract (both from ex situ and in situ grown plants) showed 8–11%, 14–26%, and 17–25% reduction in ALP, AST, and ALT activities in comparison with diabetic control (). The garlic leaf extract (ex situ and in situ conditions) was more effective in reducing the levels of enzymes in comparison with glibenclamide. The results were promising in rats administered with leaf extracts from plants grown under in situ condition as they possess higher levels of alliin. The decline in enzyme levels shows the normalizing effect of garlic leaf extract on hepatocellular damage and suppression of gluconeogenesis. The increase in enzyme levels is mainly due to leakage of enzymes into bloodstream as result of alloxan toxicity that leads to liver damage (CitationEl-Demerdash et al., 2005). The increase in enzyme levels in serum indicates liver destruction. After treatment with aqueous leaf extract (both from in situ and ex situ grown plants), enzyme levels were restored to normal by reducing their induction in diabetes.
Table 3. Serum enzyme levels of normal and diabetic rats measured under different conditions.
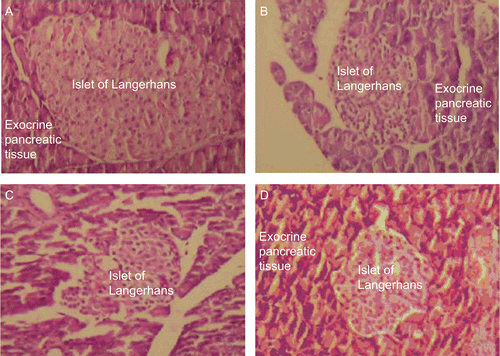
Microscopic studies indicated reduction in cell size and cell injury in islets of Langerhans in pancreas of diabetic rats as compared with normal pancreatic cells observed in control ( and ). Histopathological study confirmed destruction of β-cells in diabetic rats. Diabetic rats administered with leaf extract (both from ex situ and in situ grown plants) showed less cell damage and recovery of β-cells in pancreas ( and ). The size and recovery/revival rates of β-cells were found to be better in rats administered with extract obtained from plants grown under in situ condition than normal rats (). However, higher recovery of pancreatic cells in rats treated with leaf extract from plants grown under in situ condition indicated high regenerative capacity (CitationAbdel et al., 1997).
Figure 3. Light micrographs of rat pancreas under different conditions. A Control rats showing normal pancreatic cells. B Alloxan induced diabetic rats showing change in morphology of pancreatic cells. C Diabetic rats treated with leaf extract from ex situ raised plants showing recovery of injured pancreatic cells D Diabetic rats treated with leaf extract from in situ raised plants showing recovery of injured pancreatic cells.
Conclusion
Conclusively, leaf extracts from in situ grown plants show higher production of alliin contributing to higher therapeutic potency (reducing serum glucose, serum enzyme levels, and revival of pancreatic cells) as compared with ex situ grown plants and drug glibenclamide. The studies suggest that environmental factors influence production and therapeutic potential of alliin. Further research is required to find the factors or mechanism responsible for enhancing the therapeutic value.
Declaration of interest
We would like to thank the Dean of the Faculty of Science, Jamia Hamdard, for financial support during the course of this study. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdel MA, El-Feki M, Salah E. (1997). Effect of Nigella sativa, fish oil and gliclazide on alloxan diabetic rats, biochemical and histopathological studies. J Egyptian–German Soc Zoo 23:237–265.
- Arnault JP, Christides N, Mandon T, Haffner R, Kahane J, Auger J. (2003). High-performance ion-pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J Chromat A 991:69–75.
- Baluchnejadmojarad T, Roghani M, Homayounfar H, Hosseini M. (2003). Beneficial effect of aqueous garlic extract on the vascular reactivity of streptozotocin-diabetic rats. J Ethnopharmacol 85:139–144.
- Bhagyalakshmi N, Thimmaraju R, Venkatachalam L, Murthy KN, Sreedhar RV. (2005). Nutraceutical applications of garlic and the intervention of biotechnology. Crit Rev Food Sci Nutr 45:607–621.
- Drobiova H, Thomson M, Al-Qattan K, Peltonen-Shalaby R, Al-Amin Z, Ali M. (2009). Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase method. Evid Based Complement Alternat Med, eCAM:1–7.
- Eidi A, Eidi M, Esmaeili E. (2005). Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 13:624–629.
- El-Demerdash FM, Yousef MI, El-Naga NI. (2005). Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol 43:57–63.
- Hughes J, Tregova A, Tomsett AB, Jones MG, Cosstick R, Collin HA. (2005). Synthesis of the flavour precursor, alliin, in garlic tissue cultures. Phytochemistry 66:187–194.
- Jones MG, Collin HA, Tregova IA, Trueman L, Brown R, Cosstick J, Hughes J, Milne MC, Wilkinson AB, Tomsett, Thomas B. (2007). The biochemical and physiological genesis of alliin in garlic. Med Arom Plant Sci Biotech 1:21–24.
- Junaid A, Mujib A, Nasim SA, Sharma MP. (2008). Screening of vincristine yield in ex vitro and in vitro somatic embryo derived plantlets of Catharanthus roseus L. (G) Don. Scientia Hort 119:325–329.
- Khalil EAM. (2004). Biochemical and histopathological studies on the influence of aqueous extract of fenugreek seed (Trigonella foenum graecum) on alloxan diabetic male rats. Egypt J Hosp Med 15:83–94.
- Liu CT, Wong PL, Lii CK, Hse H, Sheen LY. (2006). Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol 44:1377–1384.
- Luna LG. (1960). Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. New York, London: McGraw-Hill.
- Mansour HA, Newairy AS, Yousef MI, Sheweita SA. (2002). Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 170:221–228.
- Murashige T, Skoog F. (1962). A revised medium of rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 16:473–497.
- Nasim SA, Dhir B, Rashmi K, Samar F, Mahmooduzzafar, Mujib A. (2010). Alliin production in various tissues and organs of Allium sativum grown under normal and sulphur-supplemented in vitro conditions. Plant Cell Tissue Org Cult 101:59–63.
- Nasim SA, Dhir B, Samar F, Rashmi K, Mahmooduzzafa, Mujib A. (2009a). Sulphur treatment alters the therapeutic potency of alliin obtained from garlic leaf extract. Food Chem Toxicol 47:888–892.
- Nasim SA, Mujib A, Rashmi K, Samar F, Junaid A, Mahmooduzzafar. (2009b). Improved alliin yield in somatic embryos of Allium sativum L. (cv. Yamuna safed) as analyzed by HPTLC. Acta Biol Hung 60:441–454.
- Rabinkov A, Miron T, Konstantinovski L, Wilchek M, Mirelman D, Weiner L. (1998). The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim Biophys Acta 1379:233–244.
- Sangeetha T, Quine SD. (2008). Protective effect of S-allyl cysteine sulphoxide (alliin) on glycoproteins and hematology in isoproterenol induced myocardial infarction in male Wistar rats. J Appl Toxicol 28:710–716.
- Schmeda-Hirschmanna GM, Jordan A, Gerth D, Wilken E, Hormazabal, Tapia A. (2004). Secondary metabolite content in Fabiana imbricata plants and in vitro cultures. Z Naturforsch 59c:48–54.
- Shalini T, Siddiqui MR, Baquer NZ. (2004). Trigonella foenum graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Mol Cellular Biochem 266:151–159.
- Sheela CG, Augusti KT. (1992). Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol 30:523–526.
- Sheela CG, Augusti KT. (1995). Effects of S-allyl cysteine sulfoxide isolated from Allium sativum Linn and gugulipid on some enzymes and fecal excretions of bile acids and sterols in cholesterol fed rats. Indian J Exp Biol 33:749–751.
- Szkudelski T. (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546.
- Thomson ZM, Al-Amin KK, Al-Qattan LH, Ali SM. (2007). Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metabol 15:108–115.
- Vanisree M, Chen-Yue L, Shu-Fung L, Satish MN, Chien YL, Hsin-Sheng T. (2004). Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin 45:1–22.
- Yeh YY, Yeh SM. (1994). Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triacylglycerol synthesis. Lipids 29:189–193.
