Abstract
Context: Tagetes patula L. is one of the French marigold group of the Asteraceae family. It is recognized in folklore for its medicinal and pesticidal properties.
Objective: In search of more effective, but non-toxic compounds with antioxidative potential led to the bioassay guided isolation studies on the extracts of T. patula.
Materials and methods: The bioassay on Tagetes patula flowers were carried out guided by in vitro antioxidant activity using DPPH assay. A minor but proven plant constituent methyl protocatechuate (1) was isolated by column chromatography, while patuletin (2) and patulitrin (3) obtained in bulk by employing solvent partition of methanol extract. Derivatization of patuletin into benzoyl, cinnamoyl and methyl was conducted to establish the structure activity relationship (SAR). Analgesic activity of compound 2 was evaluated using acetic acid-induced writhing test and hot-plate test in mice. The toxicity of methanol extract and compound 2 were also determined.
Results: Polar extracts, fractions and phases demonstrated better antioxidant activity. The synthetic methyl protocatechuate (1) showed IC50 value of 2.8 ± 0.2 μg/mL, whereas patuletin (2) (IC50 = 4.3 ± 0.25 µg/mL) was comparable to quercetin and rutin but significantly better than patulitrin (3) (IC50 = 10.17 ± 1.16 µg/mL). Toxicity test for the methanol extract and compound 2 did not elicit any behavioral changes or cause mortality in mice. Compound 2 also demonstrated mild analgesic property.
Discussion and conclusion: These findings demonstrate that the plant polar extracts and fractions possess significant antioxidant property with non-toxic effect. Compound 1 is a genuine plant constituent of T. patula.
Introduction
Tagetes patula L., locally known as “jafri”, a member of the French marigold group of the Asteraceae (or Compositae) family, is native of Mexico and other warmer parts of America (CitationChadha, 1976) and an ornamental plant across the globe. It is recognized in folklore for its medicinal properties to treat colic, constipation, diarrhea, stomach ailments, as well as rheumatism and as eye lotions (CitationChadha, 1976). Furthermore, the plant, especially the flowers, has been known to possess antimicrobial, antiseptic, blood purifying, diuretic, fly repellent (CitationChadha, 1976), hepatoprotective (Vasilenko et al., 1990), pesticidal, and nematicidal properties (CitationVasudevan et al., 1997). Many chemical constituents have been isolated from different parts of this plant (CitationTarpo & Cucu, 1961; CitationKoloshina et al., 1978; CitationVasudevan et al., 1997; CitationGarg et al., 1999; CitationFaizi & Naz, 2002; CitationSzarka et al., 2008), those exhibiting biological activities include flavonoids and their glycosides (CitationKoloshina et al., 1978; CitationVasudevan et al., 1997; CitationFaizi et al., 2008), steroids and triterpenes (CitationKasprzyk & Kozierowska, 1966; CitationVasudevan et al., 1997), polyacetylene (CitationVasudevan et al., 1997), and xanthophyll and helenien with beneficial effect in certain functioning of the retina (CitationTarpo & Cucu, 1961; CitationGarg et al., 1999), while its thiophene constituents possess nematicidal and insecticidal activities (CitationVasudevan et al., 1997). Moreover, phenol carboxylic acids, flavonoids and carotenoids have previously been reported to be present in Tagetae (CitationVasudevan et al., 1997; CitationGarg et al., 1999; CitationMashkováka et al., 2003); the latter two are the major classes of pigments in the plant. Carotenoid pigments from T. patula are used in food coloring (CitationVasudevan et al., 1997), and extracts containing flavonoids from T. patula have recently been reported as a potential dye for cotton and wool yarn (CitationGuinot et al., 2008).
Phenolic compounds including flavonoids and phenolic acids and their esters are common plant constituents that are known for antioxidant activities and may prevent cancer, cardiovascular and other diseases associated with free radicals. The radical scavenging abilities of these compounds depend greatly on the number and arrangement of phenolic hydroxyl groups (CitationRice-Evans et al., 1996). More than 8000 different flavonoids have been reported that are ubiquitously found in plants, notably in the pigments of barks, flowers, leaves, rinds, and seeds. In general, they are a group of plant polyphenols, having diverse pharmacological effects such as antiallergic, anticancer, anti-HIV, anti-inflammatory, antioxidant, antitumor, and antiviral activities (CitationWang et al., 1989; CitationNarayana et al., 2001; CitationTang et al., 2003; CitationLameira et al., 2006; CitationKale et al., 2008; CitationArroo et al., 2009). Indeed, fruits, vegetables and popular beverages such as wine, tea, and coffee, are the main dietary sources of flavonoids (CitationNarayana et al., 2001; CitationAndersen & Markham, 2006). Thus, antioxidant activity of flavonoids has attracted much attention as a possible dietary preventive therapy against many ailments including cardiovascular and neurodegenerative diseases (CitationNarayana et al., 2001; CitationCrozier et al., 2009). The antioxidative properties of flavonoids play an important role in the stability of food products as well as in the antioxidative defense mechanism of biological systems (CitationAndersen & Markham, 2006). Additionally, it has been postulated that flavonoids exert beneficial effects on human normal cells at relatively low concentrations, whereas their toxic effects were evident at higher concentrations (CitationMatsuo et al., 2005).
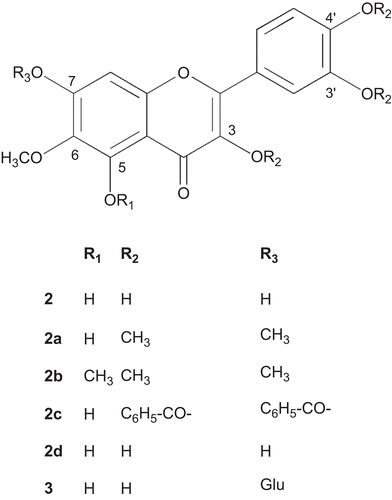
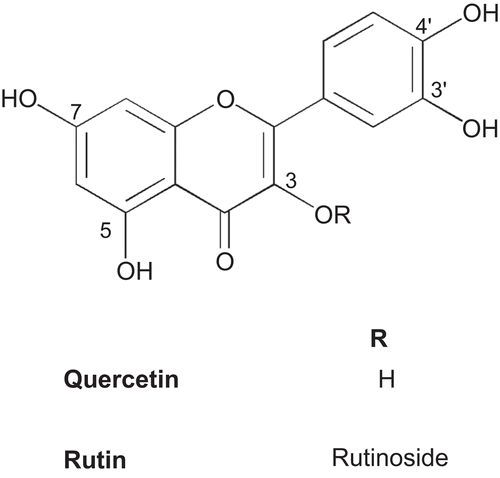
In the search of more effective but non-toxic compounds with antioxidative potential led to the bioassay guided isolation studies on the extracts of T. patula, which culminated in the evidence of methyl protocatechuate (1) as a genuine plant metabolite () and a simple and efficient process for the isolation of rare flavonoid, patuletin (6-methoxy quercetin) (2) (), and its glucoside, patulitrin (3) () (CitationVasudevan et al, 1997; CitationGarg et al., 1999; CitationPark et al., 2000). Patuletin was found to be the antioxidant principle of the plant. In addition, derivatization of patuletin including benzoylation, cinnamoylation, and methylation was conducted in order to examine the structure activity relationship (SAR) of patuletin and its derivatives. Moreover, the antioxidant and analgesic activities of the extracts, fractions and isolated phenolic compounds along with the toxicity of patuletin were also determined.
Materials and methods
General methods
UV (in MeOH) spectra were recorded on a Hitachi U-3200; λmax (log ε in nm), IR spectra (in CHCl3) FTIR-8900 (Shimadzu, Japan), vmax in cm−1, the EI-MS and HR-EIMS spectra on a MAT 312 (Finnigan, Germany) and JMS HX-110 spectrometer (Jeol, Japan). The 1H and 13C NMR spectra were run on Bruker AV-300, AV- 400 and AV-500 instruments (Bruker–Biospin, Germany) operating at 300, 400 and 500 MHz, and 75, 100 and 125 MHz, respectively. The GC-MS system was operated on a JSM 600H (Jeol, Japan) with Agilent 6890N USA (EI mode; ionization potential, 70 eV; column DS-6 (0.32 mm × 30 m); column temperature 50°-250°C (rate of temperature increases 5°C/min), carrier gas He, flow rate 1.8 mL/min, split ratio 1:30). GC was run on a 17-A-Shimadzu (FID mode, column: fused silica capillary column SPB-5 (30 m × 0.53 mm × 0.5 µm, Spelco, San Quirino PN, Italy), carrier N2). The chemical shifts (δ) and coupling constant (J) were shown in ppm and Hz. Silica gel HF 60254 was used for vacuum liquid chromatography (VLC). Silica gel 9385 was used for flash column chromatography (FCC). All the chemicals and solvents used were of analytical grade.
Plant material
Flowers of T. patula were collected during 2000 and 2003 from Karachi University Campus, identified by Rubina Dawar of the Department of Botany, University of Karachi, and a voucher specimen (no. 67280) was deposited in the herbarium.
Animals and reagents
NMRI mice (22-28 g) were obtained from the animal house facility of the International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan. Animals were housed ten per cage under standard environmental conditions with 12 h light and dark period, having free access to food and water. Ethical principles established in 1979 for the use of laboratory animals at the service of mankind Lyon, France were followed and the work was approval by the institutional animal use committee.. Aspirin (Reckitt and Colman, Karachi, Pakistan), 1,1-diphenyl-2-picryl-hydrazyl (DPPH), acetic acid, quercetin, rutin, and all other reagents used were of analytical grade (Sigma Chemicals, St. Louis, MO). For the analgesic in vivo assays, the samples were dissolved in DMSO (10%). For derivatization, acetic anhydride, benzoic anhydride, cinnamoyl chloride, dimethyl sulfate and protocatechuic acid were purchased from Merck (Hohenbrunn, Germany), and potassium carbonate (K2CO3) was obtained from BDH Chemicals, Poole, England.
Bioassay guided isolation of methyl protocatechuate (1) and flavonoids (2) and (3)
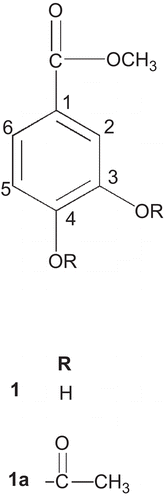
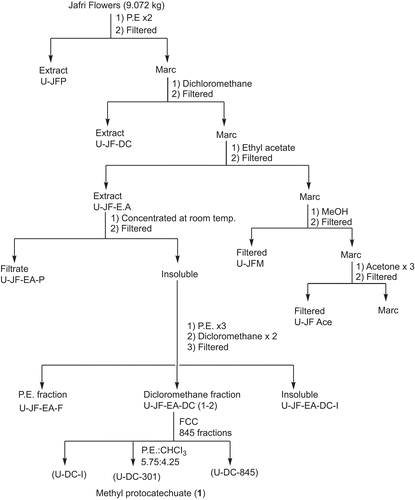
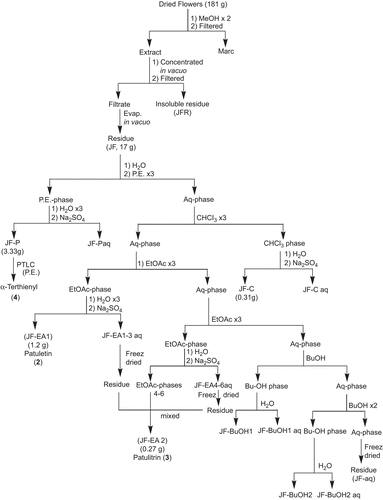
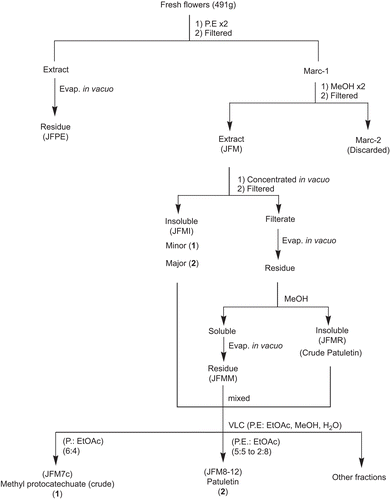
The fresh, uncrushed, orange red flowers of T. patula (491 g) were extracted twice with petroleum ether (PE) affording P. E extract (JFPE) and then by methanol, at room temperature (). After in vacuo concentration of combined methanol extracts (JFM), an insoluble matter settled down which was filtered, furnishing the powdery matter (JFMI), and filtrate. The filtrate was freed of the solvent in vacuo and the residue obtained was treated with methanol, furnishing methanol soluble (JFMM) and insoluble (JFMMI) fractions. These three fractions (JFMI, JFMM and JFMMI) showed one major spot of patuletin (2) on Thin Layer Chromatography (TLC), while JFMI also displayed an additional minor component, i.e., methyl protocatechuate (1). All the fractions were pooled together (17.68 g) and subjected to vacuum liquid chromatography (silica gel HF 60254, PE, EtOAc, MeOH, H2O) affording 27 fractions. Two different sets of fractions, i.e., 7c and 7d, and 8 to 12 were collected and combined accordingly to provide JFM7c, and JFM8-12 fractions, respectively. The spectral studies revealed their structures as methyl protocatechuate (1) () (CitationMiyazawa et al., 2003), and patuletin (2) () (CitationFaizi et al., 2010), respectively ().
Scheme 1. Bioassay guided isolation of antioxidant principles (1) and (2) from flowers of T. patula.
In order to prove that methyl protocatechuate (1) is a genuine constituent of the plant and not an artifact, the T. patula flowers (9.072 kg) were extracted sequentially with petroleum ether, dichloromethane (DC), ethyl acetate (EtOAc), methanol (MeOH), acetone and acetone:water (7:3) (). The EtOAc extract, showed deposition of yellow powder on concentration, after few days at room temperature. It was separated by filtration and washed with P.E. (three-times) followed by DC (twice) affording P.E (U-JF-EA-P) and DC (U-JF-EA-DC) soluble fractions. The DC fractions were pooled together on the basis of TLC profile and then subjected to flash column chromatography (silica gel 9385, PE, PE:CHCl3, EtOAc:CHCl3, EtOAc) affording 845 fractions. The fraction no. 301 (PE:CHCl3, 5.75:4.25) showed a single spot on TLC (Rf = 0.47, silica gel 60F254, CHCl3:MeOH, 9:1), the spectral studies revealed its structure as methyl protocatechuate (1) () which was further confirmed by preparation of 3,4-diacetyl derivative (1a) () (CitationWilliams, 1965; CitationParmar et al., 1997).
In order to obtain bulk quantities of patuletin for the preparation of its derivatives, the dried orange red flowers of T. patula (181 g) were extracted twice with methanol (). The combined methanol extract was concentrated in vacuo and kept overnight at room temperature, affording a brownish insoluble matter (JFR) that turned black on exposure to light and air. This was separated by filtration. The filtrate on evaporation of the solvent furnished a residue (JF, 17 g) that was partitioned between distilled water and PE, the aqueous phase was extracted successively with chloroform (three times), ethyl acetate (six times) and butanol (three times). Each phase (PE, CHCl3, EtOAc and BuOH) was washed with water, dried over anhydrous sodium sulfate and evaporated under reduced pressure that provided respective residues, JF-P, JF-C, JF-EA and JF-BuOH. Since, the first three EtOAc phases (JFEA-1-3) showed a single spot on TLC they were pooled resulting in a fraction JF-EA1 (1.2 g) which was identified as patuletin (compound 2, 0.55%). The remaining three EtOAc phases and water washings of all the six EtOAc phases appeared similar on TLC; therefore, they were combined to yield the fraction JF-EA2 (0.27 g), spectral studies elucidated it as patulitrin (compound 3, 0.11%) (). It has also been previously isolated from T. patula (CitationVasudevan et al., 1997; CitationGarg et al., 1999; CitationPark et al., 2000). The TLC of the first butanol phase (JF-BuOH1) also contained compound 3 while the next (JF-BuOH2) demonstrated a different TLC profile. The aqueous phase was freeze dried to JF-aq. The initial PE phase (JF-P) showed a prominent spot on TLC that was separated through PTLC (silica gel HF 60254, P.E.) and identified through spectral studies as α-terthienyl (compound 4) ().
Methyl protocatechuate (compound 1)
Methyl protocatechuate (1) () was prepared using conventional methods (5% methanolic HCl) (CitationMiyazawa et al., 2003). Electron impact mass spectrometry (EI-MS) (70 ev) m/z (rel.int.): M+ 168 (63), 137 (100), 109 (34); High-resolution EI-MS (HR-EIMS): M+ 168.0423 which is in accord with the molecular formula of C8H8O4, calc. 168.0420; 137.0239 with a fragment C7H5O3, calc. 137.0243; 109.0290 with a fragment C6H5O2, calc. 109.0291. 1H-NMR (300 MHz, C3D6O): δ 3.79 (s, 3H, COOCH3), δ 7.49 (d, J = 2.1 Hz, 1H, H-2), δ 6.88 (d, J = 8.4 Hz, 1H, H-5), δ 7.43 (dd, J = 2.1, 8.4 Hz, 1H, H-6), 8.42 (br. s, 2-OH) disappeared upon D2O shake; 13C-NMR (100 MHz, C3D6O): δ 51.86 (OCH3), δ 167.06 (C = O), δ 122.87 (C-1), δ 117.4 (C-2), δ 145.57 (C-3), δ 150.72 (C-4), δ 115. 76 (C-5), δ 123.31 (C-6).
3,4-Diacetyl, methyl protocatechuate (compound 1a)
Methyl protocatechuate (0.0026 g) was treated with acetic anhydride (1 mL) at room temperature for 3 days. The reaction mixture on workup afforded a product, which showed a single spot on TLC (Rf = 0.50, silica gel 60F254, P. E: EtOAc, 7:3). Spectroscopic studies (EIMS, Ultraviolet (UV), Infrared (IR), Proton nulear magnetic resonance 1H-NMR, two-dimentional nulear magnetic resonance (2D-NMR) confirmed its structure as 3, 4-diacetyl, methyl protocatechuate (1a) (). C12H12O6, EI-MS (70ev) m/z (rel. int.): M+ 252 (4.28), 221 (2.45), 210 (26.44), 168 (100), 149 (1.88), 137 (1.62), 109 (7.48), 79 (7.29); IR (CHCl3) λmax 2922, 2852, 1775, 1729, 1202, 1370; UV(MeOH) λmax (log ϵ) 207, 227, 226, 286; 1H-NMR (300 MHz, C3D6O): δ 3.89 (s, 3H,-COOCH3), δ 2.3 (s, 6H, 2-OCOCH3), δ 7.84 (d, J = 2.1 Hz, 1H, H-2), δ 7.38 (d, J = 8.4 Hz, 1H, H-5), δ 7.92 (dd, J = 8.4, 2.1 Hz, 1H, H-6). 13C-NMR (75 MHz, C3D6O): δ 129.28 (C-1); δ 125.55 (C-2); δ 143.31 (C-3); δ 147.22 (C-4); δ 124.68 (C-5); δ 128.38 (C-6); ester group: δ 166.03 (C = O); δ 52.66 (-OCH3); acetyl groups: δ 168.52, 168.82 [2(-C = O)]; δ 20.43, 20.43 [2(-COCH3)].
3,3′,4′,7-Tetramethyl patuletin (2a) and 3,3′,4′,5,7-pentamethyl patuletin (compound 2b)
The derivatives were prepared as described by CitationWang et al. (1989). 1H-NMR (400 MHz), CDCl3,: δ 6.48 (s, 1H, H-8), δ 7.68 (d, J = 2.02 Hz, 1H, H-2′), δ 6.97 (d, J = 8.6 Hz, 1H, H-5′), δ 7.71 (dd, J = 2.02 Hz, 8.6 Hz, 1H, H-6′), δ 3.84 (s, 3H, 6-OCH3), δ 3.9 (s, 3H, 3-OCH3), δ 3.94 (s, 3H, 3′-OCH3), δ 3.95 (s, 3H, 4′-OCH3), δ 3.96 (s, 3H, 7-OCH3), δ 12.58 (s, 5-OH) (disappeared upon D2O addition).
3,3′,4′,7-Tetrabenzoyl patuletin (2c) and 3,3′,4′,7-tetracinnamoyl patuletin (compound 2d)
The derivatives were prepared using method described earlier (CitationFaizi et al., 2008; Citation2010).
DPPH free radical scavenging assay
This method is based on the scavenging property of stable DPPH free radicals (CitationMensor et al., 2001). Reaction mixture containing extracts, fractions and pure compounds in methanol (2-500 µg/mL) and DPPH ethanolic solution (300 µM) were left at room temperature for a period of 30 min. Absorbance was measured at 517 nm on a UV-VIS spectrophotometer (Shimadzu UV-2200) and percentage inhibition of DPPH free radical after sample treatment was calculated. The IC50 values obtained graphically represents the concentration of sample required to scavenge 50% of free radicals.
Acetic acid-induced writhing in mice
The writhing test was performed on albino mice (CitationKoster et al., 1959). Animals (22-28 g) were treated with vehicle (10% DMSO, 10 mL/kg) or methanol extract (100 mg/kg) or pure compound patuletin (0.0033-10 mg/kg) or standard drug aspirin (10-100 mg/kg) intraperitoneally. After 30 min, acetic acid (0.9% acetic acid in saline) was administered i.p. (10 mL/kg) and the number of writhes was noted for a period of 20 min. The number of writhes in each treated group was compared with the control (DMSO-treated) group and percentage inhibition of writhes calculated.
Hot-plate test
The hot-plate was employed to determine the latency of responses (licking of the paw or jumping) as described earlier (CitationEddy & Leimbach, 1953). Mice (22-28 g) were placed on a metal plate heated to a temperature of 50 ± 0.05°C. The mice were treated either with DMSO (10%, 10 mL/kg, control) or methanol extract (100 mg/kg) or pure compound patuletin (10 mg/kg) intraperitoneally. The response time was noted at 0, 30, 60, 90 and 120 min.
Toxicity test
The acute toxic effect was determined using albino mice (22-28 g) of either sex treated with methanol extract (1 and 2 g/kg) and patuletin (50 and 100 mg/kg) intraperitoneally. Behavioral changes and mortality, if any were observed for 7 days.
Statistical analysis
The results are represented as mean ± standard error of mean. The difference between control and test samples were estimated by one-way analysis of variance and considered significant when p <0.05 as compared to control.
Results and discussion
The methanol extract (JFM) derived from T. patula flowers exhibited antioxidant activity (IC50 = 39.67 ± 1.33 µg/mL) that was significantly greater than the JFPE (IC50 = >>200) () and was fractionated further into insoluble part (JFMI), methanol soluble fraction (JFMM) and methanol insoluble fraction (JFMMI) (). All three fractions exhibited one major spot of patuletin (2), whereas JFMI also contained methyl protocatechuate (1) as a minor spot that was confirmed on TLC chromatogram. JFMI demonstrated significant antioxidant activity with IC50 value of 7.2 ± 0.3 µg/mL, which was nine times lower than the JFMM (IC50 = 64.67 ± 2.9 µg/mL) and it may be related to the presence of 1, which was absent in the other two fractions of the series. On the basis of TLC profile these fractions were combined and subjected to vacuum liquid chromatography that afforded methyl protocatechuate (JFM7c) (1) and JFM 8-12 fractions, containing pure patuletin (6-methoxy quercetin, 2). The corresponding IC50 values for antioxidant activity of 1 and 2 were 13.17 ± 2.6 µg/mL and 4.3 ± 0.25 µg/mL (). Since, JFM7c was not obtained in sufficient quantities to be chromatographed for the purification of compound 1, therefore, it was prepared from the authentic protocatechuic acid. The synthetic methyl protocatechuate displayed IC50 value of 2.8 ± 0.2 µg/mL, as opposed to 13.17 ± 2.6 µg/mL observed in our natural product (JFM7c), as shown in and . This discrepancy of ~10 fold in IC50 values between them could probably be related to the impurities or to the presence of minor interfering compounds, i.e., terpenoids and steroids residing in JFM7C and probably masking its antioxidant potential. In the literature, substantial antioxidant activity of compound 1 in lipid peroxidative thiobarbituric acid (TBA) assay (CitationTsuda et al., 1994) and its mechanism of free radical scavenging properties in DPPH assay (CitationSaito et al., 2004; CitationSaito & Kawabata, 2005) has been previously reported without any mention of its IC50 values.
Table 1. IC50 values of antioxidant activity of different extracts and fractions from T. patula flowers following different extraction schemes using DPPH assay.
Table 2. Antioxidant activity of 1, 2, 3, quercetin and rutin using DPPH assay.
Protocatechuic acid has been isolated earlier from T. patula (CitationMashkováka et al., 2003) and it might have been esterified in our , during the extraction process with methanol. To confirm this possibility was designed for reisolation of 1 from the flowers without using methanol as a solvent. The structure of 1 obtained, was confirmed through preparation of derivative, diacetate (1a), thereby confirming that 1 is present in the plant and is not an artifact. The diacetate (1a) has been reported previously (CitationWilliams, 1965; CitationParmar et al., 1997).
Another prominent antioxidant was a major flavonoid, patuletin (2) which was not in sufficient quantities; therefore for its isolation in larger quantities a different approach was employed () in which conventional chromatographic methods were excluded. The dried, orange red flowers of T. patula were extracted with methanol (JF) directly, and its antioxidant property was found to be dose-dependent with an IC50 value of 28.0 ± 3.74 µg/mL. In comparison with the methanol extract JFM (), the JF demonstrated 1.4 times better antioxidant activity (). This is probably due to direct methanolic extraction from flowers displaying better antioxidant activity which apparently was lost after the defatting procedure with PE. Upon bioassay-directed fractionation of JF, five phases of different polarities were obtained. The non-polar PE phase (JF-P) containing α-terthienyl as a major component (CitationFaizi et al., 2008), failed to elicit considerable antioxidant activity; however, it was slightly better than JFPE which was obtained by the direct extraction of the flowers with petroleum ether (). The other components of JF-P were long chain hydrocarbons, long chain fatty acids and their methyl esters, and an aromatic acid. Likewise, JFPE were constituted of long chain fatty acids and their methyl esters, sterols, vitamins (tocopherols) and terpenoids, as characterized by GC/GC-MS analysis. It has been reported that fatty acids and tocopherols are antioxidative phytochemicals (CitationKirmizigül et al., 2007; CitationLi et al., 2007) and may be accountable for the antioxidant activity. The other non-polar phase, chloroform phase (JF-C) displayed the antioxidant activity with an IC50 value of 55.67 ± 3.38 µg/mL (), and consisted of triterpenes, acetylenes and flavonoid as identified by the TLC profile. On the other hand, the intermediate polar phases, JF-EA1 and JF-EA2 consisted of pure patuletin (2) and patulitrin (3), respectively, exhibited promising antioxidant activity with former being significantly (2.5 times) more potent. Patuletin has been isolated from the capitula of T. patula (CitationGarg et al., 1999) and it has also been reported earlier from the aerial parts of T. mendocina as DPPH radical scavenger (CitationSchmeda-Hirschmann et al., 2004). Previously, patuletin (2) and patulitrin (3) have been reported to have similar antioxidant activity with an IC50 value of about 9.0 µg/mL (CitationPark et al., 2000). This difference in the IC50 values could be related to the solvent employed to solubilize the samples, or the presence of impurities in partially purified compound. Additionally, the reactivity of the DPPH radical itself has been reported to be affected by solvents and the time of incubation with DPPH (CitationSaito et al., 2004).
Yet another intermediate polar phase, JF-BuOH2 containing patulitrin (3) and other flavonoid glucosides, displayed IC50 values (9.03 ± 0.08 µg/mL) comparable to that of purified compound 3 and similar to JFMI ( and ). Polar fractions of the plant extracts (i.e., ethyl acetate and butanol) have been linked with antioxidant activity (CitationMensor et al., 2001). In the current investigation, polar extracts (JFM and JF), a fraction (JFMI) and a polar phase (JF-BuOH2) demonstrated maximum antioxidant activity, as shown in , whereas the most polar aqueous phase (JF-aq) containing predominantly glycosidic compounds was devoid of antioxidant property, thereby strengthening the contention that polar compounds harboring phenolic compounds are better antioxidants.
With respect to the structure–activity relationship (SAR) for the antioxidant activity it was observed that the differences among flavonoids i.e. patuletin (2), quercetin and rutin are not significant (). These three flavonoids are structurally similar, with the exception of the methoxy group present at C-6 position (patuletin) () and glycosylation at 3-OH (rutin) (). On the other hand, a significant decline in activity of patulitrin (compound 3) as compared to compound 2 is possibly due to the glycosidation at 7-OH (, ). Therefore, it may be postulated that the glycosylation of potential −OH group in flavonoids accounts for lower antioxidant activities, whereas the methoxy group at C-6 position does not interfere with this activity. Based on antioxidant IC50 values of phenolic compounds from this plant in comparison with standards used, antioxidant potency order appears to be methyl protocatechuate (1) = patuletin (2) = quercetin = rutin > patulitrin (3). All these phenolic compounds possess catechol structure which has been established to be necessary for antioxidant activity (CitationRice-Evans et al., 1996).
The flavonoid-bearing methoxy group demonstrates other pharmacological activities; for example patuletin, a methoxy flavonol, has antispasmodic (CitationNarayana et al., 2001), cholagogic (CitationKoloshina et al., 1978), and hypotensive effects (CitationNarayana et al., 2001) and its glycosides possess anticataract activity (Rosler et al., 1984). The other methoxy flavones also possess a wide spectrum of biological properties, including antihepatotoxic (CitationKiso et al., 1982), cytotoxic (CitationDíaz et al., 2003), antiproliferative (CitationTang et al., 2003; CitationJahaniani et al., 2005) and antimicrobial (CitationWang et al., 1989).
The derivatives of patuletin, i.e., tetra- and penta-methylated patuletin 2a and 2b (CitationWang et al., 1989) and tetrabenzoyl and tetracinnamoyl patuletin 2c and 2d (CitationFaizi et al., 2008; Citation2010), that are known compounds were also prepared, and evaluated for antioxidant activity. All these compounds failed to exhibit antioxidant activity up to 500 µg/mL. This lack of antioxidant activity may be related to the substitution of all potential –OH groups with either methyl or benzoyl or cinnamoyl groups. However, further SAR study for patuletin and its derivatives (as mono, di, tri, tetra and penta substituted derivatives) will be important before reaching any conclusion.
Analgesic activity was evaluated chemically (using the acetic acid-induced writhing test), and thermally (using the hot-plate test in mice) that determines the peripheral and central nervous system mediated analgesic responses, respectively. In the preliminary experiment, the extract was tested at 100 mg/kg and it showed reduction (about 34%) in the number of writhes, whereas, compound 2 tested at five doses ranging from 0.0033-10.0 mg/kg demonstrated greater reduction (66%) at 0.33 mg/kg (). The IC50 of compound 2 was 130 times lower than aspirin suggesting that it is a potent analgesic compound. However, in the case of hot-plate experiments neither extract nor patuletin demonstrated analgesic effect, indicating that the analgesic properties residing in T. patula and its pure compound act at the peripheral system and independent of the central nervous system.
Table 3. Effect of patuletin and aspirin on acetic acid-induced writhing test in mice.
Furthermore, intraperitoneal administration of methanol extract (JF) at 2 g/kg and pure compound patuletin (2) at 100 mg/kg did not elicit any behavioral changes or cause mortality in mice, indicating that it is non-toxic.
Conclusion
In conclusion, polar extracts, fractions and methyl protocatechuate (1), patuletin (2) and patulitrin (3) isolated from T. patula flowers possess significant antioxidant property. Compound 1 was derived as a native constituent of the flowers and not an artifact, and when synthetically prepared demonstrated marked antioxidant activity. Flavonoids 2 and 3 were isolated in substantial amounts by simple solvent-solvent extraction method with exclusion of chromatographic techniques, and 2 was found to be non-toxic, having analgesic property.
Acknowledgment
This paper was first presented as a poster for the Tenth International Symposium on Natural Product Chemistry held in Karachi, Pakistan, 6-9 January 2006.
Declaration of interest
Two of the authors, Samina Bano and Lubna acknowledge the Higher Education Commission Islamabad, Pakistan for financial support for Project No. 20–161 entitled “Medicinal and pesticidal agents based on Tagetes species” through the National Research Program for Universities (NRPU). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andersen OM, Markham KR. (2006). Flavonoids, Chemistry, Biochemistry and Applications. Boca Raton: Taylor & Francis, pp 1–1273.
- Arroo RRJ, Androutsopoulos V, Beresford K, Ruparelia K, Surichan S, Wilsher N, Potter GA. (2009). Phytoestrogens as natural prodrugs in cancer prevention: Dietary flavonoids. Phytochem Rev, 8, 375–386.
- Chadha YR. (1976). The Wealth of India, Vol.10. New Delhi, India: Publications and Information Directorate, Council for Scientific and Industrial Research, 109–112.
- Crozier A, Jaganath IB, Clifford MN. (2009). Dietary phenolics: Chemistry, bioavailability and effects on health. J Nat Prod, 66, 865–867.
- Díaz F, Chávez D, Lee D, Mi Q, Chai H-B, Tan GT, Kardono LBS, Riswan S, Fairchild CR, Wild R, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. (2003). Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo. J Nat Prod, 66, 865–867.
- Eddy NB, Leimbach D. (1953). Synthetic analgesics II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther, 107, 385–393.
- Faizi S, Naz A. (2002). Jafrine, a novel and labile β-carboline alkaloid from the flowers of Tagetes patula. Tetrahedron, 58, 6185–6197.
- Faizi S, Siddiqi H, Bano S, Naz A, Lubna Mazhar, K, Nasim S, Riaz T, Kamal S, Ahmad A, Khan SA (2008). Antibacterial and antifungal activities of different parts of Tagetes patula: Preparation of patuletin derivatives. Pharm Biol, 46, 309–320.
- Faizi S, Siddiqi H, Naz A, Bano S, Lubna. (2010). Specific deuteration in patuletin and related flavonoids via keto-enol tautomerism: Solvent- and temperature-dependent 1H-NMR studies. Helv Chim Acta, 93, 466–481.
- Garg SN, Charles R, Kumar S. (1999). A new acyclic monoterpene glucoside from the capitula of Tagetes patula. Fitoterapia, 70, 472–474.
- Guinot P, Gargadennec A, Valette G, Fruchier A, Andary C. (2008). Primary flavonoids in marigold dye: Extraction, structure and involvement in the dyeing process. Phytochem Anal, 19, 46–51.
- Jahaniani F, Ebrahimi SA, Rahbar-Roshandel N, Mahmoudian M. (2005). Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyi and a potential anti-cancer agent. Phytochemistry, 66, 1581–1592.
- Kale A, Gawande S, Kotwal S. (2008). Cancer phytotherapeutics: Role for flavonoids at the cellular level. Phytother Res, 22, 567–577.
- Kasprzyk Z, Kozierowska T. (1966). Distribution of sterols and triterpenic alcohols in plants of the Compositae family. Bull Acad Pol Sci Ser Sci Biol, 14, 645–649.
- Kirmizigül S, Böke N, Sümbül H, Göktürk RS, Arda N. (2007). Essential fatty acid components and antioxidant activities of eight Cephalaria species from southwestern Anatoli. Pure Appl Chem, 79, 2297–2304.
- Kiso Y, Sasaki K, Oshima Y, Hikino H. (1982). Structure of arcapillin, an antihepatotoxic principle of Artemisia capillaris herbs. Heterocycles, 19, 1615–1617.
- Koloshina NA, Pasechnik Ikh Zinchenko, TV. (1978). Flavonoids. U.S.S.R. 741,880 (Cl. A61K35/78), 25 July 1980. Chem Abstr, 93, 155840t.
- Koster R, Anderson M, De Beer EJ. (1959). Acetic acid for analgesic screening. J Federation Proc 18, 412–414.
- Lameira J, Medeiros IG, Reis M, Santos AS, Alves CN. (2006). Structure-activity relationship study of flavone compounds with anti-HIV-1 integrase activity: A density functional theory study. Bioorg Med Chem, 14, 7105–7112.
- Li L, Tsao R, Yang R, Kramer JKG, Hernandez M. (2007). Fatty acid profiles, tocopherol contents, and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.). J Agric Food Chem, 55, 1164–1169.
- Mashkováka SP, Golovko EA, Grigoryuk IP. (2003). Phenolcarboxylic acids of species of marigold (Tagetes L.). Dopovidi Natsional’noi Akademii Nauk Ukraini, 5, 158–161.
- Matsuo M, Sasaki N, Saga K, Kaneko T. (2005). Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull, 28, 253–259.
- Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res, 15, 127–130.
- Miyazawa M, Oshima T, Koshio K, Itsuzaki Y, Anzai J. (2003). Tyrosinase inhibitor from black rice bran. J Agric Food Chem, 51, 6953–6956.
- Narayana KR, Reddy MS, Chaluvadi Mr Krishna, DR. (2001). Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol, 33, 2–16.
- Park EJ, Kim Y, Kim J. (2000). Acylated flavonol glycosides from the flower of Inula britannica. J Nat Prod, 63, 34–36.
- Parmar VS, Kumar A, Bisht KS, Mukherjee S, Prasad AK, Sharma SK, Wengel J, Olsen CE. (1997). Novel chemoselective de-esterification of esters of polyacetoxy aromatic acids by lipases. Tetrahedron, 53, 2163–2176.
- Rice-Evans CA, Miller NJ, Paganga G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20, 933–956.
- Rosler K-HA Goodwin, RS, Mabry TJ, Varma SD, Norris J. (1984). Flavonoids with anti-cataract activity from Brickellia arguta. J Nat Prod, 47, 316–319.
- Saito S, Okamoto Y, Kawabata J. (2004). Effects of alcoholic solvents on antiradical abilities of protocatechuic acid and its alkyl esters. Biosci Biotechnol Biochem, 68, 1221–1227.
- Saito S, Kawabata J. (2005). Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron, 61, 8101–8108.
- Schmeda-Hirschmann G, Tapia A, Theoduloz C, Rodriguez J, Lopez S, Feresin GE. (2004). Free radical scavengers and antioxidants from Tagetes mendocina. Z Naturforsch C: J Biosc, 59, 345–353.
- Szarka S, Hêthelyi Ě, Kuzovkina IN., Lemberkovics Ě, Szõke Ě. (2008). GC-MS method development for the analyses of thiophenes from solvent extracts of Tagetes patula L. Chromatographia, 68, S63–S69
- Tang W, Hemm I, Bertram B. (2003). Recent development of antitumor agents from Chinese herbal medicines; Part 1. Low molecular compounds. Planta Med, 69, 97–108.
- Tarpo E, Cucu V. (1961). The flowers of Tagetes species as raw material for obtaining helenien. Pharmazie, 16, 486–488.
- Tsuda T, Watanabe M, Ohshima K, Yamamoto A, Kawakishi S, Osawa T. (1994). Antioxidative components isolated from the seeds of tamarind (Tamarindus indica L.). J Agric Food Chem, 42, 2671–2674.
- Vasilenko YuK Bogdanov, AN, Frolova LM, Frolov AV. (1990). Hepatoprotective properties of preparations from spreading marigold. Khim-Farm Zh, 24, 53–56.
- Vasudevan P, Kashyap S, Sharma S. (1997). Tagetes: A multipurpose plant. Bioresour Technol, 62, 29–35.
- Wang Y, Hamburger M, Gueho J, Hostettmann K. (1989). Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochemistry, 28, 2323–2327.
- Williams CM. (1965). Gas chromatography of the dihydroxybenzoic acids. Anal Biochem, 11, 224–229.