Abstract
Context and Objective: We used the wing somatic assay in Drosophila melanogaster to test the hypothesis that two phytoextracts from Cecropia obtusifolia Bertol (Cecropiaceae) and Equisetum myriochaetum Schlecht. et Cham (Equisetaceae), which are used in folk medicine to treat type 2 diabetes mellitus, could detoxify the mutagen hydrogen peroxide.
Materials and methods: Third instar larvae from standard (ST) and high-bioactivation (HB) crosses were chronically exposed to different concentrations of the phytoextracts. Hydrogen peroxide was used to induce oxidative stress and was chronically tested in both crosses. Catalase activity was measured in larvae of both strains 48 h after treatment with hydrogen peroxide. A pretreatment protocol was devised to test the antimutagenic potency of the medicinal extracts.
Results: The present study showed that neither of the phytoextracts were genotoxic in Drosophila. Interestingly, the antioxidant enzyme activity levels were different between the larvae. Hydrogen peroxide resulted in a significant genotoxic effect in the ST cross, whereas a detoxification of hydrogen peroxide was found in the HB cross. Thus, catalase was stimulated in the HB cross, which was indicative of a cellular defense mechanism mounted against a xenobiotic hazard. We found that the percentage of inhibition of spots produced by E. myriochaetum was much higher than that induced by Cecropia obtusifolia.
Discussion and conclusions: These results are in agreement with the uses of these phytoextracts in traditional medicine. Indeed, the lack of genotoxicity and the antimutagenic activity observed for both phytoextracts validates their use as a therapeutic modality to treat diabetic patients. Moreover, these extracts are suitable for consumption as teas and/or phytomedicines.
Introduction
There are estimated to be around 500 species of medicinal plants with hypoglycemic effects that Mexican people use to treat type 2 diabetes mellitus (CitationAndrade-Cetto & Heinrich, 2005). A wide variety of compounds have been isolated from these medicinal plants (e.g., alkaloids, glycosides, terpenes, and flavonoids). Some of these have been biotransformed to produce metabolites that can either react with DNA or be conjugated and excreted. In Mexican folk medicine, two plants with hypoglycemic properties, Cecropia obtusifolia and Equisetum myriochaetum, have commonly been used to treat type 2 diabetes as infusions drinks during the day. Previously, our group showed that the extracts of the plants did not produce any significant antigenotoxic effects in somatic cells of Drosophila melanogaster after acute treatments (CitationTéllez et al., 2007; CitationToledo et al., 2008). The main constituents of the C. obtusifolia extracts were flavonoids and chlorogenic acid (CitationAndrade-Cetto & Wiedenfeld, 2001). Flavonoids were also found to be the main constituent of the E. myriochaetum extract (CitationWiedenfeld et al., 2000).
Many of the complications of diabetes, including retinopathy and atherosclerotic vascular disease, have been linked to oxidative stress (CitationBaynes, 1991). Oxidative stress results from an excess of reactive oxygen species, which can produce oxidative DNA damage (CitationBurcham, 1999). Reactive oxygen species, including oxygen ions, free radicals, and peroxides, are formed as a natural byproduct of the normal metabolism of oxygen. They play important roles in cell signaling and are believed to play a pivotal role in aging and the pathogenesis of a number of degenerative diseases, such as diabetes (CitationVendemiale et al., 1999; CitationAllen & Tresini, 2000). Cells can normally defend themselves against damage by reactive oxygen species with three basic antioxidant enzymes: (1) superoxide dismutases, which convert superoxides to oxygen and hydrogen peroxide; (2) catalase, which prevents free hydroxyl radical formation by breaking down hydrogen peroxide into oxygen and water; and (3) peroxidases, which catalyze an analogous reaction in which hydrogen peroxide is reduced to water by a reductant that acts as an electron donor, normally reduced thioredoxin or glutathione (CitationCorona & Robinson, 2006). Catalase is a widely distributed enzyme found in the peroxisomes of all aerobic organisms (CitationMueller et al., 1997). The level of expression of catalase has been shown to be related to the ability of the organism to contend with hydrogen peroxide stress (CitationDavies, 1995).
As mentioned above, many of the complications of diabetes mellitus are linked to oxidative stress. In addition to the known hypoglycemic effects of these plant extracts, their use in traditional medicine has suggested that they may also prevent some of the secondary effects of diabetes. Therefore, the present study was undertaken to test the hypothesis that the extracts from C. obtusifolia and E. myriochaetum could detoxify hydrogen peroxide.
Their genotoxicity and modulation of the effects induced by the oxidant were investigated with the wing somatic assay using the larvae of Drosophila melanogaster from standard (ST) and high-bioactivation (HB) crosses. Although the ST strain expressed basal levels of cytochrome P450 enzymes, the HB strain was characterized by an increased cytochrome P450-dependent bioactivation capacity for promutagens and procarcinogens (CitationGraf & van Schaik, 1992). Larvae from both crosses were chronically exposed with the phytoextracts. A pretreatment protocol was devised to test the antimutagenic potency of the medicinal extracts, and the antioxidant status of the larvae was determined by measuring their catalase activity.
Materials and methods
Plant materials and chemical compounds
Both plants were collected in the Mexican state of Hidalgo in September 2007. The plant material was identified by Prof. Andrade-Cetto, and the vouchers (IMSSM 14694 and IMSSM 14690) were deposited at the Medicinal plants Herbarium of the Mexican Institute for Social Security.
The aqueous extract of the leaves of Cecropia and the aerial part of Equisetum were prepared according to CitationWiedenfeld et al. (2000) and CitationAndrade-Cetto and Wiedenfeld (2001).
The components of the extracts were identified by high-performance liquid chromatography (HPLC). The extracts were run on a Nucleosil 60–30 C18 (Macherey & Nagel, Düren, Germany) column and eluted with H2O/MeOH/AcCN 70:15:15 at a rate of 4 mL/min. The samples were monitored by a Beckman System Gold DAD HPLC with 32 Karat software. The samples were lyophilized after the HPLC procedure. Hydrogen peroxide (30% solution [w/w]; CAS 7722-84-1) was purchased from Sigma-Aldrich Química (México, D.F.).
Toxicity and concentrations used
The concentrations of the phytotherapeutic extracts for C. obtusifolia were 1.66, 16.6, and 166 mg/mL (1.66 mg/ mL is the therapeutic dose used in the treatment of diabetic patients). For Equisetum, the concentrations assayed were 1.56, 15.6, 156, and 1560 µg/mL (1.56 µg/ mL is the therapeutic dose used in the treatment of diabetic patients).
The median lethal dose (LD50) for hydrogen peroxide was determined by treating third instar larvae with different concentrations of the genotoxin and measuring the survival of the adults.
Fly stocks and crosses
Two different stocks of flies were used, and both carried visible wing genetic markers on the third chromosome, multiple wing hairs (mwh, 3–0.3) and flare (flr3, 3–38):
flr3/In (3LR) TM3, ri pp sep l(3)89Aa bx34e e BdS (flr3/TM3, BdS)
mwh/mwh (mwh)
Two crosses were used, ST and HB. The ST cross was performed by mating flr3/TM3, BdS females with mwh/mwh males. The HB cross was performed by mating ORR/ORR; flr3/TM3, BdS females mated with mwh/mwh males. The ORR strain has chromosomes 1 and 2 from a dichloro-diphenyl-trichloroethane (DDT)-resistant Oregon line (ORR), which constitutively overexpresses Cyp genes (CitationGraf & van Schaik, 1992).
Somatic mutation and recombination test
Eggs from both crosses were collected over 8 h into culture bottles containing a solid agar base (5%, w/v) covered with a layer of live fermenting yeast supplemented with sucrose. After 72 (± 3) h, the larvae were washed from the culture bottles with a solution of 20% (w/v) sucrose and fed with the test compounds for the rest of the larval development stage (~48 h). For this chronic feeding, the larvae were put in ordinary media or in vials containing 0.85 g of Drosophila Instant Medium (formula 4–24; Carolina Biological Supply, Burlington, NC). We used a pretreatment protocol for the antimutagenic study. After 2-day-old larvae were exposed to hydrogen peroxide for 24 h, the larvae were washed and transferred to vials for chronic treatment (48 h) with the herbal extract. Concurrent negative controls were treated with solvent alone (distilled water). After eclosion, the adult flies were collected and stored in 70% (v/v) ethanol. The wings of the trans-heterozygous flies from both crosses were mounted on slides and coded before scoring (at a magnification of 400X) for the presence of cell clones showing mutant wing hairs. Mutant clones were classified into three types: (1) small single spots, which consisted of one or two mwh or flr3 cells; (2) large single spots, which consisted of three or more cells; and (3) twin spots, which consisted of adjacent mwh and flr3 cells. Single spots were produced by somatic point mutations, deletions and mitotic recombination occurring between the two markers. Twin spots were produced by mitotic recombination occurring between the proximal marker flr and the centromere of chromosome 3 (CitationGraf et al., 1984). To evaluate the genotoxic effects, the frequencies of spots per wing in a treated series were compared with those of the concurrently processed negative control series.
Analysis of the data
Statistical comparisons were made with a computer program written by Zordan (unpublished), which used the χ2-test for proportions followed by a multiple-decision procedure (CitationFrei & Würgler, 1988, Citation1995). Statistical analyses were performed for single, large, twin, and total number of spots recovered.
The percent inhibition induced by the phytoextracts was calculated using the control-corrected frequencies of clone formation per 105 cells (CitationAbraham, 1994) according to the following formula:
Determination of catalase activity
We measured catalase activity using a modified version of a previously described method (CitationBeers & Sizer, 1952). Briefly, eggs from both crosses were collected over 8 h into culture bottles containing a solid agar base (5%, w/v) covered with a layer of live fermenting yeast supplemented with sucrose. After 72 (± 3) h, the larvae were washed from the culture bottles with a solution of 20% (w/v) sucrose followed by three washes with distilled water. The larvae were treated with 0.2 M hydrogen peroxide for different incubation times (0, 3, 6, 18, 24, 30, 42, and 48 h). In each 1.5 mL Eppendorf tube, 0.5 g of larvae was macerated with a pestle in 0.5 mL of 50 mM phosphate-buffered saline (PBS) (pH 7.0). The cells were centrifuged at 4°C for 25 min at 16,873g. The supernatant was recovered and used as the crude extract. A portion (1–50 µL) of the crude extract was placed in a 3 mL quartz cuvette and mixed with 1.9 mL of the assay mixture (100 mM PBS [pH 7.0] and 1:10−5 Triton-X 100). Immediately before the measurements were made, 100 µL of 500 mM H2O2 was added to the cuvette, which was vigorously shaken and placed in the spectrophotometer cell. The catalase activity was followed by measuring the hydrogen peroxide decay at 240 nm in a Beckman Coulter DU640 spectrophotometer for 3 min. Catalase-specific activity was calculated by the rate of decomposition of hydrogen peroxide, which was proportional to the reduction of the absorbance at 240 nm. The catalase activities of Drosophila extracts were normalized to total cellular protein in the lysate and expressed as units per mg of protein. The protein content was quantified using the Quick Start Bradford protein assay from Bio-Rad. All data points are expressed as the mean value and standard error of two independent experiments. Data were analyzed with the parametric Student’s t-test, and statistical significance was set at P < 0.05.
In vitro catalase activity assay
Purified catalase (EC 1.11.1.6) from human erythrocytes was purchased from Sigma Chemicals (C - 3556, St. Louis, MO). The sample containing catalase (1:1000) was incubated at room temperature with each phytoextract. Catalase activity was assayed by measuring the rate of the decrease in hydrogen peroxide absorbance at 240 nm.
Results
Plant composition
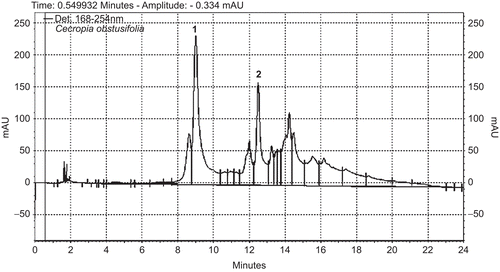
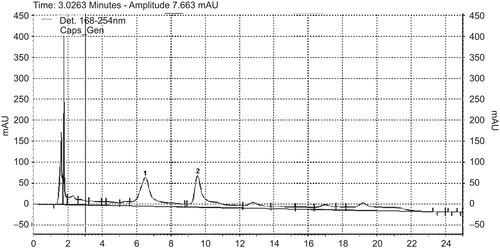
The HPLC chromatograms of both plants presented a similar composition to previously reported data; the main compounds of the extract of Cecropia obtusifolia were chlorogenic acid and isoorientin (). The main components of the Equisetum myriochaetum extract were kaempferol-3-O-sophoroside, kaempferol-3,7-di-O-β-glucoside, kaempferol-3-O-sophoroside-4'-O-β-glucoside and caffeoyl-methylate-4-β-glucopuranoside ().
Genotoxic studies
The wing somatic test was performed three times in independent chronic experiments. The data from each experiment were not heterogeneous, which was shown by a Kruskal–Wallis test at P < 0.05; therefore, the data were pooled for statistical analysis. The results of the genotoxicity assays for the phytoextracts of C. obtusifolia and E. myriochaetum are shown in . Even with increasing concentrations of the phytoextracts, the medicinal plants did not induce any genotoxic effects on either type of spot in the ST or HB crosses. shows the pooled data recorded after the larvae of ST and HB crosses were exposed to hydrogen peroxide. Although the results were negative for larvae of the HB cross, the frequency of spots per wing (small and total spots) increased significantly with genotoxic hydrogen peroxide concentrations from 0.1 to 0.4 M in larvae from the ST cross. Thus, hydrogen peroxide was clearly genotoxic in the ST cross.
Table 1. Frequency and number of spots/wing obtained after chronic exposure of trans-heterozygous larvae of Drosophila melanogaster to different concentrations of the phytoextracts tested.
Table 2. Fly spot data obtained after chronic exposure of trans-heterozygous larvae of Drosophila melanogaster to different concentrations of hydrogen peroxide.
Antimutagenic studies
shows the results of larvae pretreated with hydrogen peroxide 24 h prior to the phytoextracts. The percent inhibition produced by E. myriochaetum in the ST and HB crosses was 75 and 23%, respectively. The oxidative damage produced by hydrogen peroxide was significantly reduced in the ST cross by E. myriochaetum Cecropia obtusifolia produced 35% inhibition in the ST cross and 31% in the HB cross. An antimutagenic effect of both extracts was observed in the frequency of total spots per wing for all combined treatments compared with the effects of hydrogen peroxide alone.
Table 3. Effect of the phytoextracts on the genotoxicity of hydrogen peroxide in the pretreatment protocol.
Catalase activity
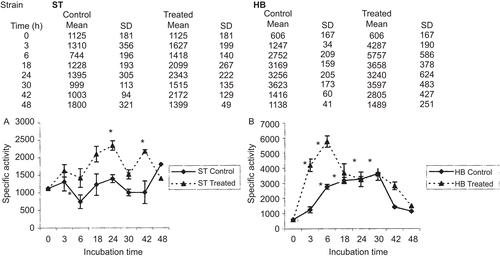
To determine the antioxidant status of the larvae, catalase activity was measured as nanomoles of oxidized hydrogen peroxide/min/mg of total protein. The addition of H2O2 to the larvae caused changes in the levels of catalase activity. In the larvae of the ST cross, the treated cells showed an increase in catalase activity of 1.45- and 1.26-times the level of the controls at 3 and 6 h, respectively. The greatest induction was observed at 18, 24, and 42 h, which resulted in increases of between 1.87- and 2.08-times the level of activity observed in the control larvae. At 48 h, the level decreased to 1.24-times the value for the control larvae. With the exception of a transient significant peak in catalase activity at 3 and 6 h of 7- and 9.5-times the value for the control larvae (measured as the levels of oxidized hydrogen peroxide), respectively, the level of activity in the larvae of the HB cross remained constant and nonsignificant after 30 h compared with the activity of unexposed cells. At 42 and 48 h, the level decreased to between 4.63- and 2.46-times the value of the control larvae, respectively ().
In vitro catalase activity assay
We found that neither phytoextract was able to degrade hydrogen peroxide by itself. Furthermore, neither extract inhibited catalase activity in vitro (data not shown).
Discussion
Previous studies have also shown that the extract of C. obtusifolia, with the same composition that we found, exerted a hypoglycemic effect on type 2 diabetic patients (CitationRevilla et al., 2007). Furthermore, we have previously shown that C. obtusifolia plant extract exerts its hypoglycemic effect by inhibiting the glucose 6-phosphatase system and preventing gluconeogenesis (CitationAndrade-Cetto & Cárdenaz, 2010). The composition that we determined for E myriochaetum was in agreement with previous studies, which demonstrated that this extract was able to control sugar blood levels in type 2 diabetic patients (CitationRevilla et al., 2002).
Cytochrome P450 enzymes from all organisms participate in the detoxification/activation of xenobiotics. Detoxification pathways have evolved to act in the metabolism of the potentially toxic chemical compounds that an organism may encounter in its environment (CitationWilloughby et al., 2006). Insect P450s are implicated in the oxidative–reductive metabolism of several types of endogenous and exogenous substrates, such as hormones, fatty acids, insecticides, herbicides, and plant toxins (CitationFeyereisen, 1999). In Drosophila melanogaster, insecticide-resistant strains constitutively overexpress metabolic Cyp genes; thus, they have higher P450 bioactivation capacities than susceptible strains. These differences could partially explain the results obtained in the present study. Neither medicinal extract was able to induce somatic mutations or mitotic recombination but an antimutagenic effect induced by E. myriochaetum was observed in the ST cross. The lack of genotoxic activity observed for the extracts were consistent with the results obtained in other studies. Indeed, acute treatment of standard Drosophila larvae with basal biotransformation activity (ST cross) or a variant with increased CYP450-dependent bioactivation capacity (HB cross) did not result in genotoxicity (CitationTéllez et al., 2007; CitationToledo et al., 2008).
It is well-established that the electrophilic compounds generated by hydrogen peroxide produce genetic damage (CitationCorona & Robinson, 2006). Furthermore, oxy-radicals derived from hydrogen peroxide can act either directly on the genome to cause chromosome damage (CitationVendemiale et al., 1999) or indirectly by modulating gene transcription (CitationRueff et al., 1986). Our results on the genotoxicity of hydrogen peroxide in the in vivo Drosophila assay, which was used to induce oxidative damage, indicated that the agent was able to induce genetic damage. It was clearly genotoxic in the ST cross, which was probably due to the production of electrophilic compounds. Hydrogen peroxide was not genotoxic in the HB cross, however, which might have been due to the increased metabolic activity of this cross leading to more efficient detoxification. No increases of twin spots were observed in either cross, which indicated that a recombinagenic activity was not induced. Furthermore, the similar frequencies of mwh clones observed at all concentrations tested in the ST cross suggested that hydrogen peroxide probably has mutagenic activity. The time-dependent catalase activity in the Drosophila ST larvae was similar to the results of an in vitro study by CitationShull et al. (1991) in tracheobronchial epithelial cells, these results were likely related to the role of catalase in the detoxification of hydrogen peroxide, which we observed in our experiments. We found that the HB cross had a more efficient antioxidant system than the ST cross. Indeed, its catalase activity was nearly six-times higher than the catalase activity of the ST cross in treated larvae and almost double in untreated larvae. To the best of our knowledge, this is the first report showing catalase activity in both the ST and HB crosses.
A pretreatment protocol was devised to test the antimutagenic potency of the medicinal extracts. Our results showed that Equisetum myriochaetum elicited a higher antimutagenic effect in the ST cross compared with the HB cross. The results obtained with both aqueous extracts confirmed the hypothesis that the two phytomedicinals could detoxify hydrogen peroxide after chronic exposure of third instar larvae of Drosophila melanogaster.
The antioxidant effects of both phytoextracts could be related to their total flavonoid content. These results are relevant because these phytoextracts are often used as herbal infusions, with or without biomedical medication, as an alternative to treat type 2 diabetes mellitus. We observed a lack of genotoxicity and an increase in antioxidant activity, which was measured as antimutagenic effects, after chronic treatment of larvae. These effects are important for diabetic patients because the phytoextracts could prevent some of the secondary effects of diabetes. Furthermore, these experiments validate the use of these phytoextracts in traditional medicine and support their manufacture as safe phytomedicines.
Conclusions
The present study has shown that neither of the phytoextracts tested were genotoxic to Drosophila. The activity of catalase was stimulated, which indicated a cellular defense mechanism against oxidative stress. Interestingly, hydrogen peroxide was genotoxic in larvae of the ST cross. The increased levels of catalase activity in the hydrogen peroxide-exposed larvae showed that catalase was responsible for the detoxification of hydrogen peroxide. The observed antimutagenic effects could be due to the flavonoid content of the phytoextracts, which could scavenge the reactive oxygen radicals produced by hydrogen peroxide.
The lack of genotoxicity and the increased antimutagenic activity of both phytoextracts after chronic treatment in flies suggested that the use of these plant extracts in traditional medicine for the management of diabetic patients is safe. In addition to the established hypoglycemic effect of these plants, we suggest that they can also prevent diabetic complications. More experiments are needed to further clarify how these extracts can prevent the development of diabetes-related complications.
Declaration of interest
This work was partially supported by the DGAPA, PAPIIT project IN228510 and CONACyT CB 079910.
References
- Abraham SK. (1994). Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis, 9, 383–386.
- Allen RG, Tresini M. (2000). Oxidative stress and gene regulation. Free Radic Biol Med, 28, 463–499.
- Andrade-Cetto A, Wiedenfeld H. (2001). Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J Ethnopharmacol, 78, 145–149.
- Andrade-Cetto A, Heinrich M. (2005). Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol, 99, 325–348.
- Andrade-Cetto A, Cárdenas R. (2010). Gluconeogenesis inhibition and phytochemical composition of two Cecropia species J Ethnopharmacol, 130, 93–97.
- Baynes JW. (1991). Role of oxidative stress in development of complications in diabetes. Diabetes, 40, 405–412.
- Beers RF Jr, Sizer IW. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem, 195, 133–140.
- Burcham PC. (1999). Internal hazards: Baseline DNA damage by endogenous products of normal metabolism. Mutat Res, 443, 11–36.
- Corona M, Robinson GE. (2006). Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect Mol Biol, 15, 687–701.
- Davies KJ. (1995). Oxidative stress: The paradox of aerobic life. Biochem Soc Symp, 61, 1–31.
- Feyereisen R. (1999). Insect P450 enzymes. Annu Rev Entomol, 44, 507–533.
- Frei H, Würgler FE. (1988). Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat Res, 203, 297–308.
- Frei H, Würgler FE. (1995). Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat Res, 334, 247–258.
- Graf U, van Schaik N. (1992). Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat Res, 271, 59–67.
- Graf U, Würgler FE, Katz AJ, Frei H, Juon H, Hall CB, Kale PG. (1984). Somatic mutation and recombination test in Drosophila melanogaster. Environ Mutagen, 6, 153–188.
- Mueller S, Riedel HD, Stremmel W. (1997). Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem, 245, 55–60.
- Revilla-Monsalve MC, Andrade-Cetto A, Islas S, Wiedenfeld, H. (2002). Hypoglycemic effect of Equisetum myriochaetum aerial parts on type 2 diabetic patients. J Ethnopharmacol, 81, 117–120.
- Revilla-Monsalve MC, Andrade-Cetto A, Palomino-Garibay MA, Wiedenfeld H, Islas-Andrade S. (2007). Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extracts on type 2 diabetic patients. J Ethnopharmacol, 111, 636–640.
- Rueff J, Laires A, Borba H, Chaveca T, Gomes MI, Halpern M. (1986). Genetic toxicology of flavonoids: The role of metabolic conditions in the induction of reverse mutation, SOS functions and sister-chromatid exchanges. Mutagenesis, 1, 179–183.
- Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT. (1991). Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem, 266, 24398–24403.
- Téllez MG, Rodríguez HB, Olivares GQ, Sortibrán AN, Cetto AA, Rodríguez-Arnaiz R. (2007). A phytotherapeutic extract of Equisetum myriochaetum is not genotoxic either in the in vivo wing somatic test of Drosophila or in the in vitro human micronucleus test. J Ethnopharmacol, 111, 182–189.
- Toledo VM, Tellez MG, Sortibrán AN, Andrade-Cetto A, Rodríguez-Arnaiz R. (2008). Genotoxicity testing of Cecropia obtusifoliaextracts in two in vivo assays: the wing somatic mutation and recombination test of Drosophila and the human cytokinesis-block micronucleus test. J Ethnopharmacol, 116, 58–63.
- Vendemiale G, Grattagliano I, Altomare E. (1999). An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res, 29, 49–55.
- Wiedenfeld H, Andrade Cetto A, Perez Amador C. (2000). Flavonol glycosides from Equisetum myriochaetum. Biochem Syst Ecol, 28, 395–397.
- Willoughby L, Chung H, Lumb C, Robin C, Batterham P, Daborn PJ. (2006). A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem Mol Biol, 36, 934–942.