Abstract
Context: Justicia gendarussa Burm (Acanthaceae) is a plant used to treat inflammatory diseases such as rheumatoid arthritis. However, the mechanism involved in the anti-inflammatory properties of this plant has not been studied well.
Objective: The in vitro anti-inflammatory activities of ethanol extract of Justicia gendarussa leaves (J-01) are studied here for the first time.
Materials and methods: The ethanol extract, J-01 was prepared from the leaves of Justicia gendarussa. The inhibitory effect of J-01 in nitric oxide (NO) production, inducible nitric oxide synthase (iNOS) and matrix metalloproteinase-9 (MMP-9) gene expressions were studied in lipopolysaccharide (LPS) stimulated macrophage cell line RAW 264.7.
Results: J-01 in a concentration dependent manner (200–50 μg/mL) attenuated NO production from macrophage stimulated with LPS (1 μg/mL). Further, J-01 significantly suppressed iNOS mRNA expression in these cells. J-01 has also downregulated the MMP-9 gene expression in LPS stimulated macrophage.
Discussion and conclusion: The modulatory function of J-01 in inhibiting NO, iNOS, and MMP-9 as obtained from the present in vitro studies provide first scientific evidence to support the anti-inflammatory properties of Justicia gendarussa. This plant may have potential use in the management of inflammatory conditions such as arthritis.
Introduction
A major percent of global population is severely affected by various crippling inflammatory disorders such as rheumatoid arthritis. These inflammatory diseases are treated with analgesics and anti-inflammatory drugs such as non-steroid anti-inflammatory drugs.These drugs have been reported to cause deleterious effects in humans. On the other hand, disease modifying anti-rheumatoid drugs is often required to inhibit or halt the underlying immune process to prevent long-term damage. In this context traditional herbal medicine has potential use in management of inflammatory diseases (CitationMajithia & Geraci, 2007). Justicia gendarussa Burm F (Acanthaceae) is native to China and distributed widely across India, Sri Lanka, and Malaysia. Various reports have shown that Justicia leaves are used as a traditional herbal medicine in the treatment of rheumatism, fever, muscle pain, respiratory disorders and digestive ailments (CitationSastri, 1959; CitationJayasinghe, 1994). Major active components of J. gendarussa are the flavonoids vitexin, apigenin and also sitosterols, alkaloids and reducing sugars (CitationWahi et al., 1974).
A few reports showed the anti-inflammatory properties of J.gendarussa. Antioxidant, immunosuppressive and anti-angiogenic properties of this plant have also been reported (CitationArokiyaraj et al., 2007; CitationMrunthunjaya & Hukkeri, 2007; CitationPeriyanayagam et al., 2009). A recent study showed the anti-arthritic properties of this plant using animal models (CitationPaval et al., 2009). However, it is not understood whether J. gendarussa extract will interfere with major inflammatory mediators which are known to evoke chronic inflammatory conditions. Many of these inflammatory mediators responsible for the pathogenesis of inflammatory conditions are produced by different cell types. Human monocytes and macrophages play a pivotal role in the onset and development of inflammatory diseases including arthritis and atherosclerosis and these cells release several pro-inflammatory mediators. Macrophages are known to produce nitric oxide (NO) in a variety of disease states, including rheumatoid arthritis, malaria, and vasculitis (CitationClancy et al., 1998). The importance of NO in the pathogenesis of arthritis is reported. Macrophages contribute to tissue destruction by the release of several proteases including matrix metalloproteinase enzymes (MMPs). Among these, MMP-9 plays an important role in tissue destruction and onset of inflammation through degradation of matrix proteins by proteolytic activation of cytokines/chemokines (CitationLee et al., 2009). The above information gave us impetus to explore whether J. gendarussa extract could regulate the inflammatory mediators responsible for the onset of inflammatory conditions. In the present study, therefore, we decided to evaluate whether ethanol extract of Justicia gendarussa leaves (J-01) could modulate the production of NO, inducible nitric oxide synthase (iNOS) and MMP-9 gene expression in lipopolysaccharide (LPS) stimulated macrophage RAW 264.7.
Materials and method
Materials
RAW 264.7 cells were obtained from National Center for Cell Science (NCCS), Pune. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dexamethasone, TRI Reagent, custom prepared oligonucleotides, N-1-napthylethylenediamine dihydrochloride, sulfanilamide, sodium nitrite, LPS, and sulforhodamine B (SRB) were obtained from Sigma Chemical Co (St Louis, MO). Penicillin and streptomycin were from Hi-media, Mumbai, India. Moloney murine leukemia virus (MMLV) reverse transcriptase, dNTP, and Taq DNA polymerase were from MBI Fermentas (Glen Burnie, MD).
Preparation of extract
Justicia gendarussa leaves were obtained from the experimental farm of Indian Institute of Spices Research (IISR), Calicut during the month of August 2008. The plant was identified and certified by Dr. K. V. Saji, Senior Scientist and a taxonomist and the voucher specimen has been deposited in the herbarium of IISR, Calicut. Ethanol extract of Justicia was prepared by extracting the dried leaves with 70% aqueous ethanol by cold maceration. The extraction was done for 72 h. The extract was filtered and dried under controlled temperature conditions and was stored in desiccator.
Cell culture and cytotoxicity assay
The mouse macrophage cell line RAW 264.7 (obtained from NCCS, Pune), was cultured in DMEM supplemented with 10% FBS, 100 IU penicillin and 100 µg streptomycin per mL at 37°C and 5% CO2. RAW264.7 cells at density of 2 × 105 mL were seeded in 96-well plates and incubated overnight. The cells were treated with different concentrations of J-01 in culture media containing 1% FBS and incubated for 24 h at 37°C and 5% CO2 to determine cytotoxicity of the extract. Cytotoxicity of the J-01 on RAW264.7 cells was determined by MTT assay as per our previous method (CitationMitra et al., 2008). In addition to the MTT assay, cell proliferation assay was also carried out using SRB (CitationVichai & Kirtikara, 2006). Non-toxic concentrations of J-01 were used for further experiments. Cells were seeded in 40 mm petriplates at a density of 2 × 105 cells/mL and incubated with/without LPS 1 μg/mL and different concentrations of J-01 in growth media containing 2% FBS for 24 h. Culture media were collected, centrifuged and used for determining nitrite. The cell pellet was stored at −80°C and was used for RNA isolation.
Determination of nitrite
Nitrite was determined spectrophotometrically by using the Griess reagent (0.1% N-1-naphthylene ethylenediamine hydrochloride, 1% sulfanilamide in 5% phosphoric acid (Sigma Chemical Co) as described previously (CitationChakravortty & Kumar, 1997). Absorbance was measured at 540 nm and nitrite concentration was determined using sodium nitrite as a standard. Each experiment was performed in triplicate and repeated at least three times.
RT-PCR
Isolation of RNA was carried out using TRI reagent and the RNA was subjected to DNase I treatment to get rid of any contaminating DNA in the samples as per our previous report (CitationMitra et al., 2008). Spectroscopic quantification was carried out by absorbance at A260/280. The RNA samples were also resolved on 1% denaturing agarose gel stained with ethidium bromide and photographed under UV light. RNA (1 μg/mL) was taken for first strand synthesis. RNA and oligo dT primers were annealed at 42°C and 5 × RT buffer, dNTPs were added and continued incubation for 42°C for 10 min followed by addition of MMLV reverse transcriptase and continued incubation for 1 h at 42°C. Two percent of the first strand product was taken for subsequent polymerase chain reaction (PCR) amplification. The primer sequence for iNOS gene was 5′-TCTTGGTCAAAGCTGTGCTC-3′ (sense) and 5′-CATTGCCAAACGTACTGGTC-3′ (antisense) as described previously (CitationPeng et al., 2000). The primer sequence for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was 5′-ACCACAGTCCATGCCATCAC-3′(sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense) as reported before (CitationKlopotek et al., 2006). The PCR conditions involved denaturation at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 40 s, 60°C for 1 min and extension at 72°C for 8 min. A final extension at 72°C for 8 min completed the PCR. MMP-9 gene amplification was carried out using mouse specific primers and the PCR conditions involved initial denaturation at 94°C for 3 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 1 min and extension at 72°C for 1 min. This was followed by final extension at 72°C for 10 min. The primer sequence for MMP-9 was 5′-CAAACCCTGCGTATTTC-3′ (sense) and 5′-AGAGTACTGCTTGCCCAGGA-3′ (antisense) as reported earlier (CitationKim et al., 2009). The PCR products were resolved on 1.5% agarose gel stained with ethidium bromide and photographed under UV. The molecular weight of the amplified cDNA was determined by comparison with a standard molecular weight marker (1 Kb ladder) and densitometry analysis was carried out for determining relative levels of specific mRNA in comparison with GAPDH RNA as described previously (CitationMitra et al., 2008).
Analysis of data
Data were analyzed to determine mean ± SD. Statistical analysis of the data was done by Student’s unpaired t-test using Graph pad prism software. P < 0.05 is considered significant.
Results
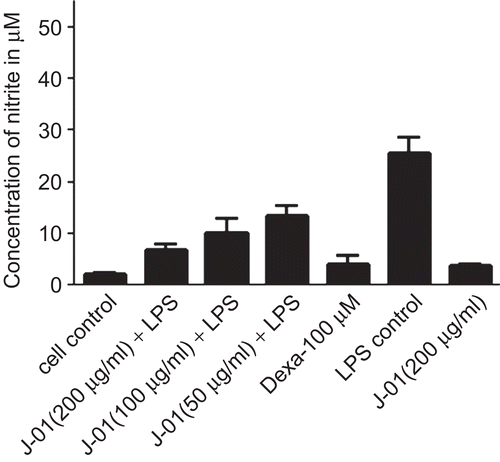
J-01 extract at different concentrations was used to determine its toxicity on RAW264.7 cells by MTT assay. Results showed that J-01 even at the highest concentration at 200 μg/mL did not exhibit any toxic effect to the cells (). Cell viability was also tested by SRB assay and results show that the viability of the cells was not affected by J-01 (). We tested whether J-01 at different concentrations (50–200 μg/mL) could regulate NO production from macrophage cells stimulated with LPS at 1 μg/mL. Results indicate a concentration dependent inhibition of NO production from macrophage cells by J-01 (P < 0.01, ). J-01 at 200, 100, and 50 μg/mL could inhibit 60.88, 47.88, and 40.05% NO production induced by LPS in RAW264.7 cells, respectively. J-01 itself did not show any direct effect on NO production from macrophage as inferred from the parallel studies carried out. The level of NO produced by cells when treated with J-01 alone without LPS is marginal and is not significant as compared to the untreated cells (). In the present study, dexamethasone, a standard drug also inhibited 85% NO production in LPS stimulated RAW264.7 cells.
Figure 1. Cytotoxicity of J-01 on RAW264.7 macrophage. RAW264.7 cells were incubated for 24 h with different con of J-01, ethanol extract of J. gendarussa. Cell viability was determined by (A) MTT assay and (B) SRB assay. Data are expressed as percentage of control. n = 3.
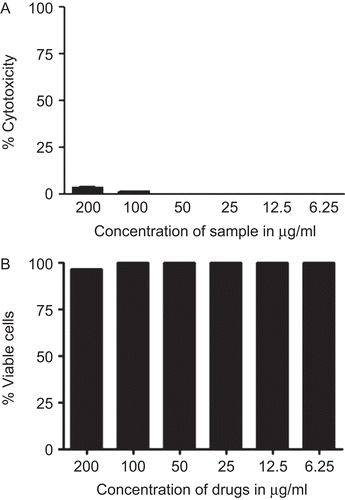
Figure 2. Effect of J-01 on LPS induced NO production from RAW264.7 cells. NO production was determined as nitrite accumulation in the medium after 24 h of drug treatment. Data represent µM of nitrite produced.*P < 0.01, n = 3.
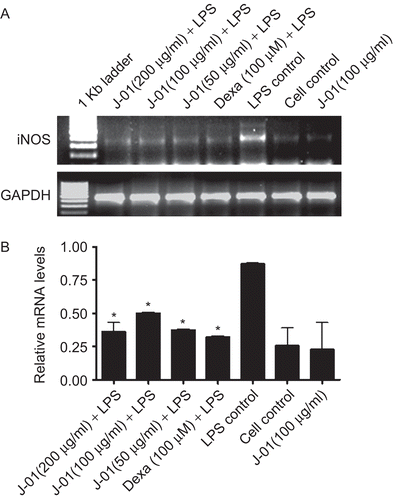
We further studied whether inhibitory effect of NO production by J-01 is targeted at the transcription of NO synthesis in the cells. Hence, we determined the status of iNOS gene expression pattern by RT-PCR using iNOS specific primers. iNOS gene expression was barely detectable in macrophage whereas the expression was markedly increased in cells stimulated with LPS at 24 h (). However, J-01 at all the tested concentrations has substantially down regulated iNOS gene expression induced by LPS ().
Figure 3. The effect of J-01 extract on iNOS gene expression. RAW264.7 cells were stimulated with or without LPS (1 μg/mL).RNA was isolated from drug treated and untreated cells and RT-PCR was performed using specific primers as described in the text (A) iNOS and GAPDH mRNA (B) relative level of iNOS gene expression normalized to GAPDH RNA and values depict arbitrary units. Data is representative of three experiments. *P < 0.05.
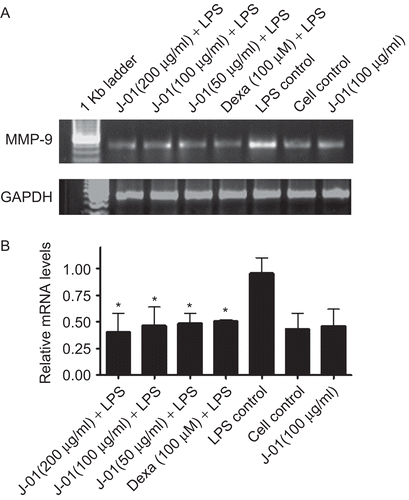
In the present study, the effect of J-01 on MMP-9 gene expression was also studied. The results showed that J-01 reduced the MMP-9 gene expression in macrophage induced by LPS. The gene expression levels in macrophage treated with J-01 alone without LPS did not have any effect of MMP-9 gene expression and the expression was similar to that of unstimulated cells (). Dexamethasone has also down regulated LPS evoked MMP-9 expression.
Figure 4. The effect of J-01 extract on MMP-9 gene expression. RAW264.7 cells were stimulated with or without LPS (1 μg/mL). RNA was isolated from drug treated and untreated cells and RT-PCR was performed using specific primers as described in the text. (A) MMP-9 and GAPDH mRNA (B) relative level of MMP-9 gene expression normalized to GAPDH RNA and values depict arbitrary units. Data is representative of three experiments. *P < 0.05.
Discussion and conclusion
NO is an endogenous free radical species which exhibits both signaling and cytotoxic activities. It functions in such diverse process as the regulation of blood flow, blood clotting, neurotransmission, and host defense (CitationJacobs & Ignarro, 2003). Increased iNOS gene expression and NO production are involved in the pathophysiology of several inflammatory conditions such as rheumatoid arthritis, asthma and inflammatory bowel disease. Enhanced NO production is observed in arthritic conditions both in rodent models and human (CitationMiysaka, 1997). It is interesting to infer from the present in vitro study that J-01 could significantly inhibit LPS induced NO production and iNOS gene expression in a dose dependent manner in macrophage cells. Macrophages are known to produce NO and one of the most prominent functions of NO is its participation in antimicrobial defense and in the manifestation of inflammation (CitationClancy et al., 1998; CitationKröncke et al., 1998). Production of NO by macrophage is regulated primarily through the tight control of iNOS gene expression, which is transcriptionally silent in the absence of activating stimuli (CitationJacobs & Ignarro, 2003). Therefore, the inhibitory effects of J-01 extract on NO and iNOS gene expression suggests one of the possible and important mechanisms responsible for the anti-inflammatory properties of J. gendarussa.
MMPs are induced during inflammatory responses and are important for immune regulation, angiogenesis, wound healing and tissue remodeling (CitationHo et al., 2008). Rheumatoid arthritis and arthrosclerosis are disorders of chronic inflammation and macrophages are central players in these pathological conditions (CitationStoll & Bendszus, 2006). As observed in the present study the inhibitory potency of J-01 in iNOS and MMP-9 gene expression were similar to that induced by dexamethasone in the cells. Considering the adverse effect of synthetic drugs, the use of potentially active herbal medicines appears promising in controlling many inflammatory conditions (CitationMajithia & Geraci, 2007). Although our studies were in progress, one recent study shows that J. gendarussa preparation could reduce experimentally induced arthritis in rats (CitationPaval et al., 2009) but whether J. gendarussa could exert any anti-inflammatory activities in these animals by inhibiting NO and MMP-9 activity was not understood. LPS is a known inducer of MMP-9 gene transcription (CitationHo et al., 2008). These results derived from our in vitro studies show that J. gendarussa specifically and strongly suppressed LPS-induced expression of MMP-9 gene in RAW264.7 cells. Dexamethasone, a well studied corticosteroid was taken as the standard drug which is reported as strong inhibitor of NO and MMP-9 induced by LPS stimulation (CitationKorhonen et al., 2002; CitationHarris et al., 2007) and similar findings were also observed in the present study.
The medicinal properties of Justicia gendarussa leaves in the treatment of chronic rheumatism, inflammation, fever, dyspepsia, eye diseases and bronchitis have been described in Ayurveda (CitationKirthikar & Basu, 2001). Justicia is reported to contain lignans, β-sitosterol, friedelin, lupeol and four simple o-substituted aromatic amines (CitationChakravarthy et al., 1982). As mentioned above J. gendarussa is endowed with potential anti-arthritic properties as derived from recent in vivo studies (CitationPaval et al., 2009). However, no studies were available till now using in vitro cell culture or cell based assays to suggest the anti-inflammatory properties of this plant. This is the first report to show that J-01, the ethanol extract of Justicia gendarussa has potent anti-inflammatory properties by inhibiting NO and MMP-9 expression which are known to play important roles in mediating inflammation.
Hence, it could be concluded that Justicia gendarussa is a non-toxic and pharmacologically important plant having potent inhibitory activity on NO production, iNOS and MMP-9 gene expression and suggests the potential application of this plant as herbal medicine in the management of inflammatory disorders such as arthritis. Our studies are underway to explore additional anti-inflammatory properties of this plant using various other inflammatory models.
Acknowledgements
The authors wish to thank Dr. V. A. Parthasarathy, Director, Indian Institute of Spices Research, Calicut, India for kind supply of Justicia gendarussa for the study. The authors thank Drs Dipshikha Chakravortty and Amit Lahari, Indian Institute of Science, Bangalore for helping in the densitometry scan.
Declaration of interest
The authors state that there is no conflict of interest in this study. The authors are responsible for the study and writing the present manuscript.
References
- Arokiyaraj S, Perinbam K, Agastian P, Balaraju K. (2007). Immunosuppressive effect of medicinal plants of Kolli hills on mitogen-stimulated proliferation of the human peripheral blood mononuclear cells in vitro. Indian J Pharmacol, 39, 180–183.
- Chakravortty D, Kumar KS. (1997). Induction of cell proliferation and collagen synthesis in human small intestinal lamina propria fibroblasts by lipopolysaccharide: Possible involvement of nitric oxide. Biochem Biophys Res Commun, 240, 458–463.
- Chakravarthy AK, Dastidar PG, Pakrash SC. (1982). Simple aromatic amines from Justicia gendarussa – 13C NMR spectra of the bases and their analogues. Tetrahedron, 38, 1797–1802.
- Clancy RM, Amin AR, Abramson SB. (1998). The role of nitric oxide in inflammation and immunity. Arthritis Rheum, 41, 1141–1151.
- Harris JE, Fernandez-Vilaseca M, Elkington PT, Horncastle DE, Graeber MB, Friedland JS. (2007). IFNgamma synergizes with IL-1beta to up-regulate MMP-9 secretion in a cellular model of central nervous system tuberculosis. Faseb J, 21, 356–365.
- Ho HH, Antoniv TT, Ji JD, Ivashkiv LB. (2008). Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-gamma via superinduction of ATF-3 and suppression of AP-1. J Immunol, 181, 5089–5097.
- Jacobs AT, Ignarro LJ. (2003). Cell density-enhanced expression of inducible nitric oxide synthase in murine macrophages mediated by interferon-beta. Nitric Oxide, 8, 222–230.
- Jayasinghe DM. (1994). Ayurveda Pharmacopeia. Department of Ayurveda, Colombo, Sri Lanka, 30–32.
- Kirthikar KR, Basu BD. (2001). Indian Medicinal Plants Vol. 8, Oriental Enterprises, Dehradun, India.
- Kim SE, Thanh Thuy TT, Lee JH, Ro JY, Bae YA, Kong Y, Ahn JY, Lee DS, Oh YM, Lee SD, Lee YS. (2009). Simvastatin inhibits induction of matrix metalloproteinase-9 in rat alveolar macrophages exposed to cigarette smoke extract. Exp Mol Med, 41, 277–287.
- Klopotek A, Hirche F, Eder K. (2006). PPAR gamma ligand troglitazone lowers cholesterol synthesis in HepG2 and Caco-2 cells via a reduced concentration of nuclear SREBP-2. Exp Biol Med (Maywood), 231, 1365–1372.
- Korhonen R, Lahti A, Hämäläinen M, Kankaanranta H, Moilanen E. (2002). Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol Pharmacol, 62, 698–704.
- Kröncke KD, Fehsel K, Kolb-Bachofen V. (1998). Inducible nitric oxide synthase in human diseases. Clin Exp Immunol, 113, 147–156.
- Lee YS, Lan Tran HT, Van Ta Q. (2009). Regulation of expression of matrix metalloproteinase-9 by JNK in Raw 264.7 cells: presence of inhibitory factor(s) suppressing MMP-9 induction in serum and conditioned media. Exp Mol Med, 41, 259–268.
- Majithia V, Geraci SA. (2007). Rheumatoid arthritis: Diagnosis and management. Am J Med, 120, 936–939.
- Mitra SK, Varma SR, Godavarthi A, Nandakumar KS. (2008). Liv.52 regulates ethanol induced PPARgamma and TNF alpha expression in HepG2 cells. Mol Cell Biochem, 315, 9–15.
- Miysaka, N. (1997). Nitric oxide production in rheumatoid arthritis. Mod Rheumatol, 7, 165–172.
- Mrunthunjaya K, Hukkeri VI. (2007). Antioxidant and free radical scavenging potential of J. gendarussa Burm. leaves in vitro. Natural product Sciences 3, 199–206.
- Paval J, Kaitheri SK, Potu BK, Govindan S, Kumar RS, Narayanan SN, Moorkoth S. (2009). Anti-arthritic potential of the plant Justicia gendarussa Burm F. Clinics (Sao Paulo), 64, 357–362.
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ Jr, Eisenstein TK. (2000). Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol, 68, 723–728.
- Periyanayagam K, Umamaheshwari B, Susheela L, Padmini M, Ismail M. (2009). Evaluation of anti angiogenic effect of the leaves of Justicia gendarussa (Burm F.) (Acanthaceae) by chrioallontoic membrane method. Am J Infect Dis, 5, 180–182.
- Sastri BN. (1959). Wealth of India: Raw Materials. Vol. V. Council of Scientific and Industrial Research, New Delhi, 3112–313.
- Stoll G, Bendszus M. (2006). Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke, 37, 1923–1932.
- Vichai V, Kirtikara K. (2006). Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc, 1, 1112–1116.
- Wahi SP, Wahi AK, Kapoor R. (1974). Chemical study of the leaf of Justicia gendarussa Burm. J Res Indian Med, 9, 65–66.
