Abstract
Context: The growth and development of adipose tissue leading to obesity is suggested to depend on angiogenesis. Our previous study showed that Melissa officinalis L. (Labiatae), Morus alba L. (Moraceae), and Artemisia capillaris Thunb. (Compositae) are involved in the regulation of angiogenesis. We hypothesized that Ob-X, a mixture of three herbs, M. alba, M. officinalis, and A. capillaris, can regulate obesity.
Objective: To investigate the inhibitory effect of Ob-X on obesity in genetically obese ob/ob mice.
Materials and methods: The effect of Ob-X on angiogenesis was measured using a mouse Matrigel plug assay. The effects of Ob-X on obesity were investigated in ob/ob mice.
Results: Ob-X inhibited angiogenesis in a dose-dependent manner, as evidenced by decreased blood vessel density in a mouse matrigel plug assay. Administration of Ob-X to ob/ob mice for 5 weeks produced a significant reduction in body weight gain by 27% compared with control (12.1 ± 3.01 vs. 16.6 ± 2.24 g, respectively). Ob-X also significantly decreased visceral adipose tissue mass by 15% (0.87 ± 0.12 vs. 1.02 ± 0.15 g, respectively). The size of adipocytes in visceral adipose tissue was reduced by 46% in Ob-X-treated mice. Ob-X treatment inhibited hepatic lipid accumulation and significantly decreased circulating glucose levels compared with controls (197 ± 56.5 vs. 365 ± 115 mg/dL, respectively).
Discussion and conclusion: These results suggest that Ob-X, which has an anti-angiogenic activity, reduces body weight gain and visceral adipose tissue mass in genetically obese mice, providing evidence that obesity can be prevented by angiogenesis inhibitors.
Introduction
Obesity is a threat to healthcare because it is rapidly increasing in prevalence and is associated with metabolic disorders such as dyslipidemia, atherosclerosis, and type 2 diabetes. Obesity is characterized by increased adipose tissue mass that results from both increased fat cell number (hyperplasia) and increased fat cell size (hypertrophy) (CitationCouillard et al., 2000). The excessive growth of body fat requires extensive modulations in adipose tissue structure, involving adipogenesis, angiogenesis, and remodeling of extracellular matrix (CitationCrandall et al., 1997).
Similar to neoplastic tissues, growing adipose tissue of adults can grow throughout life and is highly vascularized, whereas most adult tissues normally do not grow and the supporting vasculature is quiescent (CitationHobson & Denekamp, 1984). Each adipocyte in adipose tissue is nourished by an extensive capillary network, and activated endothelial cells in angiogenic vessels produce various growth factors and cytokines that communicate with adipocytes in a paracrine fashion to promote their growth and expansion (CitationCrandall et al., 1997; CitationSilverman et al., 1988; CitationBouloumié et al., 2002; CitationCao, 2007, Citation2010). It is suggested, therefore, that adipose tissue growth is angiogenesis-dependent and may be inhibited by angiogenesis inhibitors. This idea is supported by reports that treatment with angiogenesis inhibitors results in body weight loss and adipose tissue reduction, showing that adipose tissue mass is sensitive to angiogenesis inhibitors and can be regulated through the vasculature (CitationRupnick et al., 2002; CitationBråkenhielm et al., 2004; CitationNeels et al., 2004).
We screened anti-angiogenic activity of water extracts from medicinal herbs and plants, which have been used for a long period of time, and selected three herbal extracts Melissa officinalis L. (Labiatae; lemon balm), Morus alba L. (Moraceae; white mulberry), and Artemisia capillaris Thunb. (Compositae; injin) to make Ob-X (CitationKim et al., 2006). Based on the documented role of angiogenesis in adipose tissue growth and the anti-angiogenic potential of Ob-X, we hypothesized that growth and development of adipose tissue may be inhibited by Ob-X. In this study, we treated genetically induced obese ob/ob mice with Ob-X because these mice have body weights three times greater than normal mice and exhibit a 5-fold increase in body fat content (CitationHalaas et al., 1997; CitationFriedman & Halaas, 1998). Moreover, recessive mutations in the mouse obese (ob) gene result in obesity and diabetes in a syndrome resembling morbid human obesity (CitationColeman, 1978; CitationFriedman & Leibel, 1992). Accordingly, it seems that genetically induced obesity can be effectively regulated by Ob-X.
Here, we report that the anti-angiogenic herbal composition Ob-X reduces body weight gain and visceral adipose tissue mass, as well as adipocyte size in genetically obese ob/ob mice. In addition, hepatic lipid accumulation was inhibited and serum glucose levels were also decreased by Ob-X. These studies suggest that obesity is prevented by an angiogenesis inhibitor Ob-X.
Materials and methods
Preparation of Ob-X
Ob-X was prepared from the food grade of aqueous extracts of the three herbs, M. officinalis (Frutarom, Wadenswil, Switzerland), M. alba (Segae, FL; Buyeo, Korea), and A. capillaris (Segae, FL), as previously described (CitationLee et al., 2008). The quality of each herbal extract in Ob-X was controlled by standardization with reference compounds by high-pressure liquid chromatography. The corresponding reference compounds are rosmarinic acid (M. officinalis), 1-deoxynojirimycin (M. alba), and 6,7-dimethylesculetin (A. capillaris).
Mouse Matrigel plug assay
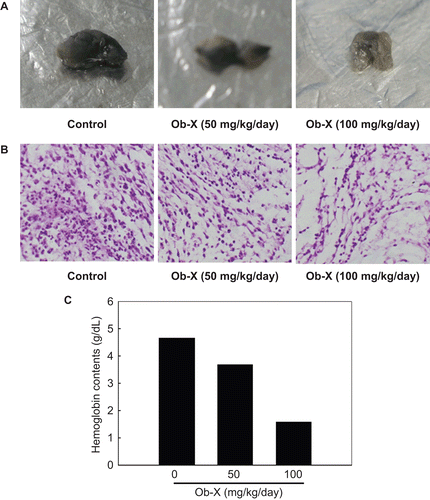
The in vivo anti-angiogenic activity of Ob-X was investigated in a mouse matrigel plug assay, which allows quantitative analysis of the extent of blood vessel invasion into matrigel plugs. A mouse matrigel plug assay was performed as described by CitationPassaniti et al. (1992) with some modifications. Matrigel (0.4 mL) was mixed with 20 ng of basic fibroblast growth factor (bFGF), 10 ng of vascular endothelial growth factor (VEGF), and 140 μg of heparin at 4°C, and injected subcutaneously into the abdominal midline of 6-week-old male C57BL/6 mice, where the plug formed a solid gel. For oral administration, 50 or 100 mg/kg/day of Ob-X was administered orally twice a day for 4 days. After 5 days, mice were killed and the matrigel plug was removed and processed for histological analysis and determination of hemoglobin concentrations.
For hematoxylin and eosin (H&E) staining, plugs were fixed in 10% phosphate-buffered formalin for 1 day and processed in a routine manner for paraffin sections. Plug sections (5 μm) were cut and stained with H&E for microscopic examination. To quantify the formation of new blood vessels, plugs were homogenized and hemoglobin concentrations were measured using a Drabkin reagent kit 525 (Sigma, St. Louis, MO).
Animal study
Six-week-old male ob/ob (C57BL/6J-Lepob) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and divided into two groups: control and Ob-X-treated (n = 8/group). We used ob/ob mice in this study because they rapidly accumulate adipose tissue. Mice received daily oral administration of saline or Ob-X at a dose of 0.5 mg/mouse/day for 5 weeks. Body weights were measured daily by a person blind to each treatment group. After a 12 h fast on the last day of the study, the animals were sacrificed by cervical dislocation. Mesenteric fat pads and liver tissues were weighed and prepared for histological analysis. Blood was collected from the retroorbital sinus into tubes, and serum was separated and stored at −80°C until analysis. Serum glucose levels were measured using an automatic blood chemical analyzer (CIBA Corning, Oberlin, OH). All animal experiments were approved by the Institutional Animal Care and Use Committees of Mokwon University, and followed National Research Council Guidelines.
Histological analysis
The right lobe of the liver was fixed in 10% phosphate-buffered formalin for 1 day and processed in a routine manner for paraffin sections. Tissue sections (5 μm) were cut and stained with osmium tetroxide for microscopic examination. Adipose tissue was cryosected and stained with osmium tetroxide. To quantify adipocyte size and areas of hepatic lipid vacuoles, the stained sections were analyzed using the Image-Pro Plus analysis system (Media Cybernetics, Bethesda, MD).
Statistical analysis
Unless otherwise indicated, all values are expressed as mean ± SD. All data were analyzed by the unpaired Student’s t-test for statistically significant differences between groups.
Results
Effects of Ob-X on in vivo angiogenic activity
As shown in , plugs removed from the control mice revealed that VEGF and bFGF stimulated neovascularization of implanted Matrigel plugs, although plugs from Ob-X treated mice revealed that the angiogenic response induced by VEGF and bFGF was greatly reduced in a dose-dependent manner ( and ). The measurement of hemoglobin contents further confirmed these observations, and Ob-X at doses of 50 and 100 mg/kg/day resulted in 21% and 66% reductions of hemoglobin contents, respectively, relative to control (). These results indicate that Ob-X can inhibit angiogenesis in vivo.
Effects of Ob-X on body weight gain, visceral adipose tissue mass, and adipocyte size in obese ob/ob mice
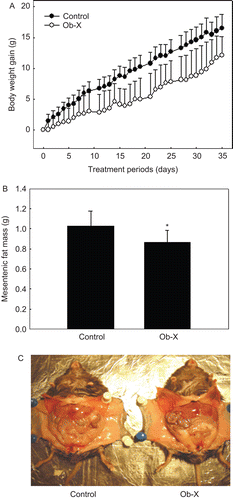
Because Ob-X showed anti-angiogenic activity, we investigated if Ob-X reduces body weight gain and adipose tissue mass in genetically obese ob/ob mice. When Ob-X was administered to ob/ob mice at a dose of 0.5 mg/mouse per day for 35 days, body weight gain of obese mice treated with Ob-X began to decrease significantly from Day 11 after start of treatment, and decreased by 27% at the end of the experiment (Day 35) compared with controls (12.1 ± 3.01 vs. 16.6 ± 2.24 g, respectively) (). Treated mice had 15% lower mesenteric fat mass (0.87 ± 0.12 vs. 1.02 ± 0.15 g, respectively) (). The overall visceral fat mass of Ob-X-treated mice was reduced in comparison with that of controls (). In addition, liver, kidney, and heart weights were not changed by Ob-X treatment (data not shown).
Figure 2. Effects of Ob-X on body weight gain and visceral adipose tissue mass in genetically obese ob/ob mice. Ob/ob mice (n = 8/group) received daily oral administration of saline (control group) or Ob-X at a dose of 0.5 mg/mouse/day (Ob-X group) for 5 weeks. All values are expressed as the mean ± SD. (A) Body weight gains at the end of the treatment period were statistically significant between the control group and the Ob-X group, (P < 0.05). (B) Mesenteric fat mass was measured. *Significantly different from control group, P < 0.05. (C) Visceral fat mass was apparently leaner in the Ob-X group than in the control group.
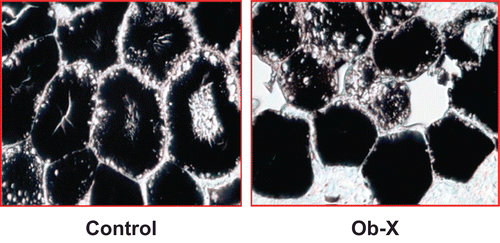
As shown in the , adipocyte size of Ob-X-treated mice was considerably decreased by 46% compared with controls. Thus, Ob-X can effectively improve obesity through reductions in adipocyte size and adipose tissue mass.
Figure 3. Light microscopic analysis of adipocytes in visceral adipose tissue of ob/ob mice. Adipose tissues were derived from mice receiving daily oral administration of saline (control group) or Ob-X at a dose of 0.5 mg/mouse/day (Ob-X group) for 5 weeks. Representative photographs of osmium tetroxide-stained sections of adipose tissues (original magnification 400×).
Effects of Ob-X on hepatic lipid accumulation in obese ob/ob mice
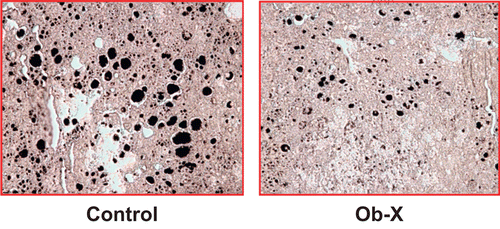
The black lipid vacuoles in the liver were substantially decreased in Ob-X-treated mice compared with control mice, as shown in . The area of lipid vacuoles was remarkably reduced by 83%, showing that Ob-X suppressed fat accumulation in the liver.
Figure 4. Effects of Ob-X on hepatic lipid accumulation in genetically obese ob/ob mice. Livers were derived from mice receiving daily oral administration of saline (control group) or Ob-X at a dose of 0.5 mg/mouse/day (Ob-X group) for 5 weeks. Representative photographs of osmium tetroxide-stained sections of livers (original magnification 200×).
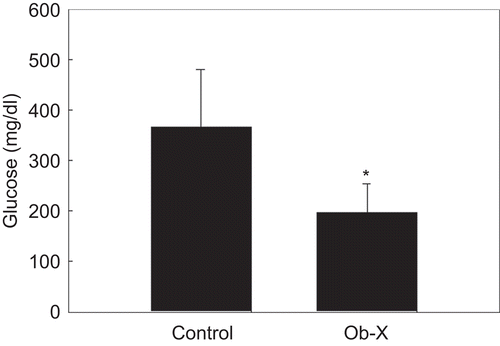
Effects of Ob-X on serum levels of glucose in obese ob/ob mice
Consistent with reductions in visceral fat mass and adipocyte size, circulating glucose levels were significantly decreased by Ob-X. The serum glucose levels of Ob-X-treated mice were also significantly decreased by 46% compared with controls (197 ± 56.5 vs. 365 ± 115 mg/dL, respectively) ().
Figure 5. Effects of Ob-X on serum glucose levels in genetically obese ob/ob mice. Ob/ob mice (n = 8/group) received daily oral administration of saline (control group) or Ob-X at a dose of 0.5 mg/mouse/day (Ob-X group) for 5 weeks. All values are expressed as the mean ± SD. *Significantly different vs. control group, P < 0.05.
Discussion
Angiogenesis is crucial for all tissue growth, expansion, and repair (CitationFolkman, 1995). Adipose tissue is probably the most highly vascularized tissue in the body, as each adipocyte is surrounded by capillaries and the angiogenic vessels supply nutrients to adipocytes (CitationCao, 2007). Accumulating evidence shows that capillary endothelial cells communicate with adipocytes via paracrine signaling pathways, extracellular components, and direct cell–cell interactions (CitationBouloumié et al., 2002; CitationRupnick et al., 2002; CitationBråkenhielm et al., 2004). Indeed, angiogenesis has been shown to have a crucial role in the modulation of adipogenesis and obesity (CitationCao, 2007; CitationLijnen, 2008). Modulators of angiogenesis affect the expansion and metabolism of fat mass by regulating the growth and remodeling of the adipose tissue vasculature (CitationCao, 2010). Different angiogenesis inhibitors significantly reduced body weight and adipose tissue mass (CitationRupnick et al., 2002), and newly formed adipose tissue depends on continued angiogenesis for further growth (CitationLiu & Meydani, 2003), strongly indicating a role of angiogenesis in adipose tissue growth. In this study, Ob-X reduced the formation of new blood vessels induced by the angiogenic factors VEGF and bFGF in a mouse Matrigel plug assay. Plugs from Ob-X-treated mice exhibited decreased blood vessel density and hemoglobin contents in a dose-dependent manner. These results suggest that Ob-X has the ability to inhibit angiogenesis and may reduce fat mass.
Body weight gain and adipose tissue mass of Ob-X-treated ob/ob mice were significantly less than those of controls, supporting the hypothesis that Ob-X can reduce adipose tissue growth due to its inhibition of angiogenesis. Ob-X treatment for 5 weeks significantly decreased body weight gain by 27%. Although these effects were lower than the results showing a body weight loss after administration of the potent inhibitor of angiogenesis TNP-470 to ob/ob mice (CitationLijnen et al., 2002), this difference may be due to differences in angiogenesis inhibitor as well as percent body fat of animals.
While Ob-X was administered to 5-week-old ob/ob mice, TNP-470 was administered to 8-week-old ob/ob/mice. Since 8-week-old ob/ob mice have a greater fat content than 5-week-old ob/ob mice, they would be expected to exhibit greater weight loss. Our data are supported by other results indicating that body weight gain and adipose tissue mass of obese animals were reduced significantly by several kinds of angiogenesis inhibitors (CitationRupnick et al., 2002; CitationLijnen, 2008). In agreement with reductions in body weight gain, Ob-X significantly reduced mesenteric fat mass by 15% in obese ob/ob mice. Our previous study showed that treatment of high-fat diet-induced obese mice with Ob-X for 12 weeks reduced visceral fat mass by 38% (CitationKim et al., 2010). CitationLijnen et al. (2002) also reported that administration of TNP-470 for 12 weeks induced decreases in visceral fat mass in high-fat diet-fed mice. Similarly, our human study showed that 1.5 g of Ob-X per day for 12 weeks reduced visceral fat area by 9.5% (from 81.5 ± 4.40 cm2 to 73.8 ± 4.72 cm2; P < 0.01, unpublished data).
There is a strong association between visceral adiposity and insulin resistance (CitationKissebah, 1997; CitationOkuno et al., 1998; Citationde Souza et al., 2001). Since visceral fat mass was significantly decreased by Ob-X in this study, Ob-X may have an important role in alleviating insulin resistance and diabetes. In addition to visceral fat reduction, Ob-X inhibited adipocyte hypertrophy. The size of adipocytes in visceral adipose tissue was considerably smaller in Ob-X-treated mice than in controls. Morphometric analysis showed that Ob-X substantially reduced the size of adipocytes by 46%, eventually resulting in decreased adipose tissue mass and body weight gain.
Metabolic changes were also examined during Ob-X-induced weight loss. Ob-X suppressed fat accumulation in the liver. These results are consistent with our previous study showing that Ob-X treatment to high-fat diet-induced obese mice decreased triglyceride droplets, a process mediated through increased mRNA expression of enzymes for fatty acid β-oxidation in the liver (CitationLee et al., 2008). CitationRupnick et al. (2002) reported that TNP-470-treated animals preferentially used fatty acids as energy substrates during weight reduction. These data suggest that Ob-X-induced weight reduction, beginning with angiogenesis inhibition in adipose tissue, is followed by subsequent effects on energy substrates and energy metabolism. Ob-X treatment in obese ob/ob mice, who exhibited hyperglycemia, also significantly decreased glucose levels, suggesting that insulin resistance and diabetes may be regulated through angiogenesis inhibition.
In conclusion, these studies demonstrate that the anti-angiogenic herbal composition Ob-X reduces body weight gain and visceral fat mass in genetically obese ob/ob mice, without changes in weights of any other organs. These events may influence hepatic steatosis and circulating glucose levels. Thus, it seems that Ob-X, by specifically targeting adipose tissue, has the potential to prevent and treat human obesity and its related disorders.
Acknowledgement
This study was supported by (70007823) funded by MKE and Mid-career Researcher Program (No. 2009-0083990) and Female Scientist Program (No. 2010-0017313) through NRF grant funded by the MEST, Korea.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bouloumié A, Lolmède K, Sengenès C, Galitzky J, Lafontan M. (2002). Angiogenesis in adipose tissue. Ann Endocrinol (Paris) 63:91–95.
- Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. (2004). Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res 94:1579–1588.
- Cao Y. (2010). Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9:107–115.
- Cao Y. (2007). Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117:2362–2368.
- Coleman DL. (1978). Obese and diabetes: Two mutant genes causing diabetes–obesity syndromes in mice. Diabetologia 14:141–148.
- Couillard C, Mauriège P, Imbeault P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. (2000). Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord 24:782–788.
- Crandall DL, Hausman GJ, Kral JG. (1997). A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4:211–232.
- de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, Burkey BF. (2001). Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50:1863–1871.
- Folkman J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31.
- Friedman JM, Halaas JL. (1998). Leptin and the regulation of body weight in mammals. Nature 395:763–770.
- Friedman JM, Leibel RL. (1992). Tackling a weighty problem. Cell 69:217–220.
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. (1997). Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94:8878–8883.
- Hobson B, Denekamp J. (1984). Endothelial proliferation in tumours and normal tissues: Continuous labelling studies. Br J Cancer 49:405–413.
- Kim J, Park BY, Park EK, Lee HS, Halm JC, Bae KH, Kim MY. (2006). Screening of anti-angiogenic activity from plant extracts. Kor J Pharmacog 37:253–257.
- Kim MY, Park BY, Lee HS, Park EK, Hahm JC, Lee J, Hong Y, Choi S, Park D, Lee H, Yoon M. (2010). The anti-angiogenic herbal composition Ob-X inhibits adipose tissue growth in obese mice. Int J Obes (Lond) 34:820–830.
- Kissebah AH. (1997). Central obesity: measurement and metabolic effects. Diabetes Rev 5:8–20.
- Lee J, Chae K, Ha J, Park BY, Lee HS, Jeong S, Kim MY, Yoon M. (2008). Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J Ethnopharmacol 115:263–270.
- Lijnen HR. (2008). Angiogenesis and obesity. Cardiovasc Res 78:286–293.
- Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. (2002). Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler Thromb Vasc Biol 22:374–379.
- Liu L, Meydani M. (2003). Angiogenesis inhibitors may regulate adiposity. Nutr Rev 61:384–387.
- Neels JG, Thinnes T, Loskutoff DJ. (2004). Angiogenesis in an in vivo model of adipose tissue development. FASEB J 18:983–985.
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T. (1998). Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101:1354–1361.
- Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. (1992). A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 67:519–528.
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. (2002). Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA 99:10730–10735.
- Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. (1988). Angiogenic activity of adipose tissue. Biochem Biophys Res Commun 153:347–352.