Abstract
Context: The present study describes the spasmogenic and spasmolytic activities of Daphne oleoides Schreb. (Thymelaeaceae), exploring the possible underlying pharmacological mechanisms.
Aim: Pharmacological investigation of Daphne oleoides to provide evidence for its therapeutic application in gastrointestinal motility disorders.
Materials and methods: Methanol crude extract of Daphne oleoides (Do.Cr) was studied in gastrointestinal isolated tissues.
Results: In spontaneously contracting rabbit jejunum preparations, Do.Cr at 0.3–3.0 mg/mL caused moderate stimulation, followed by relaxant effect at the next higher concentrations (5.0–10 mg/mL). In presence of atropine, spasmogenic effect was blocked and the relaxation was emerged, suggesting that the spasmogenic effect of Daphne oleoides is mediated through activation of muscarinic receptors. When tested against the high K+ (80 mM)-induced contractions, Do.Cr (0.3–5.0 mg/mL), like verapamil, inhibited the induced contractions, suggesting Ca++ channel blockade (CCB) effect. The CCB effect was further confirmed when pre-treatment of the tissue with Do.Cr shifted the Ca++ concentration-response curves to the right, similar to that caused by verapamil.
Discussion and conclusion: These results indicate that Daphne oleoides exhibits gut excitatory and inhibitory effects, occurred via cholinergic and Ca++ antagonistic pathways, respectively.
Introduction
The genus Daphne (Thymelaeaceae) consists of 70 species found in Europe, Mediterranean region, temperate and subtropical Asia, Malaysia, Philippine Islands, North Africa, and Australia (CitationNasir & Ali, 1971). It is represented by three species in Pakistan, i.e., Daphne oleoides Schreb., Daphne papyracea Wall., and Daphne retusa Hemsl. (CitationZhuang et al., 1983). Plants of the genus Daphne are widely used in traditional medicine as an abortificient (Ronlan & Wickberk, 1970). A tincture named “Mezereum” prepared from the ethanol extract of the bark of Daphne mezereum is useful against various skin diseases and neuralgia (CitationWangner et al., 1987). The plant also showed stimulating effect in vitro on human and gramcycles and the tumour promoting potential which may be induced by the known diterpene esters (CitationBernhard et al., 1990). Daphne mezereum has found to contain antileukemic diterpenoid (CitationKupchan & Baxter, 1975). The root of Daphne odora is called “Ruixanggen” in China. It is used in treatment of snake bite and neuralgic pain (CitationWatt, 1963). The methanol extract of Daphne papyracea possesses hypotensive and tranquillizing properties, and flowers of Daphne genkwa have been used as diuretic and in cancer therapy for centuries in Chinese folklore (CitationSugi & Nagashio, 1977). The same extract also exhibited in vivo inhibitory activity against P-338 lymphocytic leukaemia in mice and “genkwadaphnin” isolated from the same source showed antileukemic activity (CitationKasai et al., 1981). Daphne oleoides Schreb. (Thymelaeaceae) is a small multibranched shrub found in the Western Himalayas, from Garhwal Westward to Murree, occurring at an altitude of 3000 to 9000 feet, locally known as “Laighonai.” It is known to contain daphneticin-4″-O-α-d-glucopyranoside, daphnetin-8-O-β-d-glucopyranoside, 4-ethoxybenzoic acid, 4-hydroxybenzoic acid, grantioidin, phenolic acids, terpenoids, and undecyl 2,3-dimethoxycinnamate (CitationNisar-ullah et al., 1999). Daphne oleoides has been used in various traditional healing systems of the world. Its roots and aerial parts have been used in Turkish folk medicine for the treatment of lumbago fever as well as in rheumatic pain (CitationYesilada et al., 1995). Various extracts and fractions obtained from whole plant of Daphne oleoides have been reported to show potent inhibitory activity against macrophage-derived cytokines, interleukin 1α (IL-1α), interleukin 1β (IL-1β), and tumor necrosis factor (TNF-α; CitationYesilada et al., 1997). Root of this plant is purgative and the bark and leaves are given in cutaneous affections (CitationWatt, 1972). Infusion of leaves is also used in gonorrhea and applied to abscesses (CitationBaquar, 1989). Daphne oleoides has also been used against malaria and rheumatism in folk medicinal usage of plants in Turkey (CitationTabata et al., 1993; CitationFujita et al., 1995). In this investigation, we provide evidence that Daphne oleoides exhibit gut stimulatory and relaxant effects, mediated through muscarinic receptors stimulation and Ca++ antagonist mechanisms, respectively.
Materials and methods
Plant material and preparation of extract
The whole plant of Daphne oleoides was collected from Banda Piran, Siran valley, 2900 m above the sea level, District Mansehra, October 2007. It was identified by Prof. Dr. Manzoor Ahmad, plant Taxonomist, Department of Botany, Government Degree College, Abbotabad, NWFP, Pakistan, where voucher specimen (D-0016) has been deposited in herbarium. Plant material was shade dried and ground to powder (1 kg). The extraction was made by using cold percolation method by extracting with methanol (2.5 L) at room temperature for seven days. The extraction was repeated thrice. The combined extract was evaporated to obtain greenish mass, i.e., crude extract of Daphne oleoides (Do.Cr), yielding ~ 3%. Do.Cr was solubilized in distilled water.
Chemicals
Acetylcholine perchlorate (ACh), atropine sulphate, potassium chloride, and verapamil hydrochloride were purchased from Sigma Chemicals Co, St Louis, MO. Chemicals used for making physiological salt solutions were potassium chloride (Sigma Chemicals Co, St Louis, MO), calcium chloride, glucose, magnesium chloride, sodium bicarbonate, sodium dihydorgen phosphate (Merck, Darmstadt, Germany), and sodium chloride from BDH Laboratory supplies, Poole, England. The chemicals used in phytochemical analysis include acetic anhydride, aluminum chloride (Sigma Chemical Co, St Louis, MO), chloroform, hydrochloric acid, methanol, and petroleum ether (BDH Laboratory supplies, Poole, England). All the chemicals used were of analytical grade available.
Animals
Adult rabbits (1.5–2.0 kg) of local breed and either sex were housed at the Animal House of the Aga Khan University, maintained at 23–25°C and were given a standard diet and tap water. Animals had free access to water, but food was withdrawn 24 h prior to experiment. Rabbits were sacrificed by blow on back of the head. Experiments performed complied with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996), approved by the Ethical Committee of the Aga Khan University.
Phytochemical screening
Preliminary analysis of the plant extract for various phytochemical classes was carried out following the reported methods (CitationKhan & Gilani, 2009). Plant material treated with petroleum ether and subsequently extracted with chloroform was noted for green to pink or pink to purple color after reaction with acetic anhydride and hydrochloric acid in succession to detect sterols and terpenes, respectively. Appearance of yellow color with aluminum chloride reagent detects flavonoids. The observation of yellow florescence under UV light on filter paper impregnated with the vapors from boiling extract indicates the presence of coumarins.
Rabbit jejunum
The jejunum was dissected out by opening the rabbit abdomen, immersed in Tyrode’s solution and cleaned of mesenteries (CitationGilani et al., 2009). Each segment of about 2-cm length was suspended in a 10 mL tissue bath containing Tyrode’s solution, maintained at 37°C and aerated with a mixture of 95% oxygen and 5% carbon dioxide (carbogen). The composition of the Tyrode’s solution in mM was KCl 2.68, NaCl 136.9, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, CaCl2 1.8, and glucose 5.55. Intestinal responses were recorded isotonically using Bioscience transducers and a Harvard oscillograph. Each tissue was allowed to equilibrate for at least 30 min before the addition of any drug and then stabilized with a sub-maximal concentration of ACh (0.3 µM) with a 3-min interval until constant responses were recorded. The contractile effect of the test material was assessed as percent of the maximum effect (CitationGilani et al., 2005a) produced by the control drug, ACh (1 µM).
Determination of Ca++ antagonist effect
For the elucidation of Ca++ channel blockade (CCB) activity, high K+ (80 mM) was used to depolarize the preparations as described by CitationFarre et al. (1991). K+ was added to the tissue bath, which produced a sustained contraction. Test materials were then added in a cumulative fashion to obtain concentration-dependent inhibitory responses (CitationVan Rossum, 1963). To confirm the Ca++ antagonist effect of the test substance, the tissue was allowed to stabilize in normal Tyrode’s solution, which was then replaced with Ca++-free Tyrode’s solution containing EDTA (0.1 mM) for 30 min in order to remove Ca++ from the tissues (CitationGilani et al., 2005b). This solution was further replaced with K+-rich and Ca++-free Tyrode’s solution of the following composition (mM): KCl 50, NaCl 91.04, MgCl2 1.05, NaHCO3 11.90, NaH2PO4 0.42, glucose 5.55, and EDTA 0.1. Following an incubation period of 30 min, control concentration-response curves (CRCs) of Ca++ were obtained. When the control Ca++ CRCs were found super-imposable (usually after two cycles), the tissue was pretreated with the plant extract for 60 min to test the possible CCB effect. The CRCs of Ca++ were constructed in the presence of different concentrations of the test material.
Statistical analysis
Data expressed as mean ± standard error of mean (SEM, n = number of experiments) and the median effective concentrations (EC50 values) with 95% confidence intervals (CI). CRCs were analyzed by non-linear regression using GraphPad program (GraphPAD, San Diego, CA).
Results
Phytochemical analysis
Do.Cr was tested positive for the presence of flavonoids, coumarins, sterols, and terpenes.
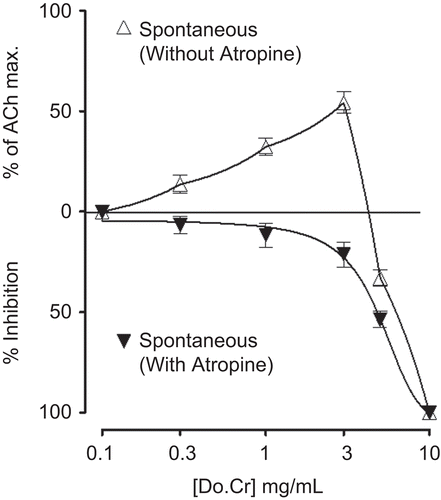
Effect on spontaneous contractions
Do.Cr at lower concentrations (0.3–3.0 mg/mL) caused contractile effect on spontaneous contractions of rabbit jejunum. The efficacy of the stimulant effect was 13.6 ± 4.5, 32.3 ± 4.3, and 54.3 ± 5.3% (mean ± SEM; n = 3) at 0.3, 1.0, and 3.0 mg/mL, respectively, compared to ACh maximum contraction. The next higher concentrations (5.0–10 mg/mL) did not cause further increase in contraction, instead caused relaxation of spontaneous contractions (). The spasmogenic effect was abolished in the presence of atropine (0.3 µM) and the spasmolytic effect was observed with EC50 value of 5.1 mg/mL (4.1–7.0, 95% CI, n = 3) as shown in .
Figure 1. Concentration-response curves of the crude extract of Daphne oleoides (Do.Cr) on spontaneous contractions of isolated rabbit jejunum preparations in the absence and presence of atropine (0.3 µM). The spasmogenic responses are expressed as percent of the acetylcholine maximum (ACh Max.). Values shown are mean ± SEM, n = 3.
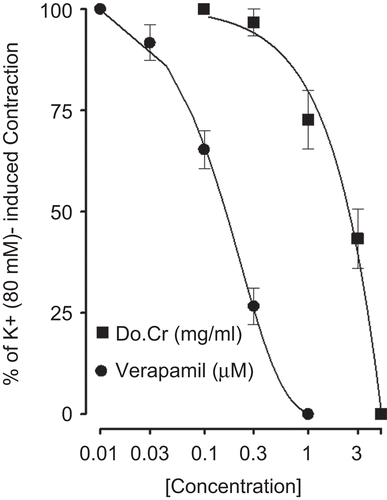
Effect on high K+-induced contractions
When tested against high K+ (80 mM)-induced contractions, Do.Cr caused relaxation () with EC50 value of 2.6 mg/mL (1.5–4.4, n = 3). Verapamil, used as positive control, inhibited the K+ (80 mM)-induced contractions, with EC50 values of 0.15 µM (0.1–0.22, n = 3) as shown in .
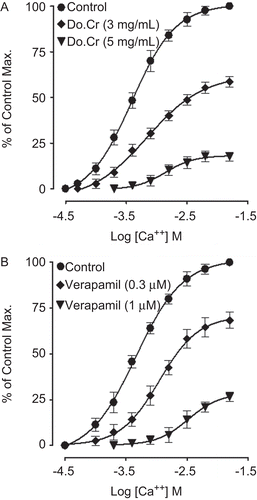
Effect on Ca++-curves
Do.Cr concentration-dependently (3.0–5.0 mg/mL, n = 3) shifted the Ca++ CRCs to the right (), like that caused by verapamil at 0.3–1.0 µM (n = 4), as shown in .
Discussion
In spontaneously contracting rabbit jejunum tissues, Daphne oleoides initially produced excitatory effect, like that caused by ACh. Pretreatment of the tissues with atropine, a muscarinic receptor antagonist (CitationDelmendo et al., 1989), abolished the stimulatory effect of plant extract, which indicates that the Daphne oleoides causes gut stimulation via cholinergic pathway. ACh is a neurotransmitter released by the parasympathetic nervous system, mediates its action in gut by activation of M3 receptor subtypes and hence plays an important physiological role to regulate the peristaltic movements of the gut (CitationBrown & Taylor, 1996; CitationGhayur & Gilani, 2005a). The observed spasmogenic effect of Daphne oleoides explains its medicinal use as laxative agent in the hypo-motility disorder of the gut, i.e., constipation. The contractile effect was followed by relaxation at next higher concentrations of the extract, indicating the co-existence of spasmolytic constituent(s). The combination of gut inhibitory component(s) with stimulatory effect in Daphne oleoides is probably meant by nature not to allow the spasmogenic effect going beyond the therapeutic limits, where it could have caused abdominal cramps, as is the case with chemical drugs used in constipation, particularly at high doses (CitationGhayur & Gilani, 2005b).
In earlier studies, we observed that the antispasmodic effect of medicinal plants is usually mediated through blockade of Ca++ channels (CitationGilani et al., 2005c,d, Citation2007) To elucidate whether the spasmolytic effect of Daphne oleoides is also mediated via similar mechanism, its extract was tested on high K+-induced contractions. High K+ (> 30 mM) is known to cause smooth muscle contractions through opening of voltage-dependent L-type Ca++ channels, thus allowing influx of extracellular Ca++ causing a contractile effect (CitationBolton, 1979; CitationPietrobon & Hess, 1990; CitationKaraki et al., 1997) and the substance causing inhibition of high K+-induced contraction is considered as inhibitor of Ca++ influx (CitationGodfraind et al., 1986; CitationOkmura et al., 1993). Do.Cr, like the verapamil, a standard Ca++ antagonist (CitationFleckenstein, 1977), relaxed the high K+-induced contractions, indicating its CCB action. The Ca++ antagonist effect of Daphne oleoides was further confirmed when it shifted the Ca++ CRCs to the right, like that caused by verapamil, used as positive control. Ca++ channel blockers have been shown to be beneficial in the gut disorders, resulting from the hyperactivity, such as abdominal cramps and diarrhoea (CitationPasricha, 2006). Hence, possessing CCB effect makes the Daphne oleoides a useful therapeutic herbal candidate for the remedy of such conditions.
Phytochemical analysis of the Daphne oleoides extract revealed the presence of flavonoids, coumarins, sterols, and terpenes. The observed cholinergic and CCB effects of the extract may be due to the presence of terpenes and flavonoids, as such phytochemicals have been known to exhibit cholinomimetic and Ca++ antagonist activities, respectively (CitationPerry, 1996; CitationRevuelta et al., 1997; CitationBurns et al., 2002).
Conclusions
This study indicates that Daphne oleoides possess gut modulatory (stimulatory and inhibitory) effects, mediated through cholinergic and Ca++ antagonist mechanisms, respectively.
Acknowledgement
This study was supported by funds made available by the Higher Education Commission of Pakistan under the Scheme of Distinguished National Professor Research Allowance.
Declaration of interest
The authors report no declarations of interest.
References
- Baquar SR. (1989). Medicinal and Poisonous Plants of Pakistan. Karachi, Pakistan: Printas, 161.
- Bernhard K, Andreas N, Hildebert W. (1990). Triumbellin, a tricoumarin rhamnopyranoside from Daphne mezereum. Phytochemistry, 29, 3633–3637.
- Bolton TB. (1979). Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev, 59, 606–718.
- Brown JH, Taylor P. (1996). Muscarinic receptor agonists and antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman AG. (Eds.), Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th edn. New York, USA: McGraw-Hill, 141–59.
- Burns A, Byrne J, Ballard C, Holmes C. (2002). Sensory stimulation in dementia. BMJ, 325, 1312–1313.
- Delmendo RE, Michel AD, Whiting RL. (1989). Affinity of muscarinic receptor antagonists for three putative muscarinic receptor binding sites. Br J Pharmacol, 96, 457–464.
- Farre AJ, Colombo M, Fort M, Gutierrez B. (1991). Differential effects of various Ca2+ antagonists. Gen Pharmacol, 22, 177–181.
- Fleckenstein A. (1977). Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol, 17, 149–166.
- Fujita T, Sezik E, Tabata M, Yesilada E, Honda G, Takeda Y, Tanaka T, Takaishi Y. (1995). Traditional medicine in Turkey. VII. Folk medicine in middle and West Black Sea regions. Econ Bot, 49, 406–422.
- Ghayur MN, Gilani AH. (2005a). Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci, 50, 1889–1897.
- Ghayur MN, Gilani AH. (2005b). Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci, 50, 1889–1897.
- Gilani AH, Bashir S, Janbaz KH, Khan A. (2005). Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J Ethnopharmacol, 96, 585–589.
- Gilani AH, Bukhari IA, Khan RA, Khan AU, Ullah F, Ahmad VU. (2005). Cholinomimetic and calcium channel blocking activities of Carthamus oxycantha. Phytother Res, 19, 679–683.
- Gilani AH, Bashir S, Janbaz KH, Shah AJ. (2005). Presence of cholinergic and calcium channel blocking activities explains the traditional use of Hibiscus rosasinensis in constipation and diarrhoea. J Ethnopharmacol, 102, 289–294.
- Gilani AH, Shah AJ, Ghayur MN, Majeed K. (2005). Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci, 76, 3089–3105.
- Gilani AH, Bashir S, Khan AU. (2007). Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J Ethnopharmacol, 114, 393–399.
- Gilani AH, Mehmood MH, Janbaz KH, Khan A, Saeed SA. (2009). Gut modulatory and antiplatelet activities of Viscum cruciatum. Pharm Biol, 47, 955–961.
- Godfraind T, Miller R, Wibo M. (1986). Calcium antagonism and calcium entry blockade. Pharmacol Rev, 38, 321–416.
- Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. (1997). Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev, 49, 157–230.
- Kasai R, Kuo-hsiung L, Huan-Chang H. (1981). Genkwadaphnin, a potent antileukemic diterpene from Daphne genkwa. Phytochemistry, 20, 2592–2594.
- Khan A, Gilani AH. (2009). Antidiarrheal, antisecretory and bronchodilatory activities of Hypericum perforatum. Pharm Biol, 47, 962–967.
- Kupchan SM, Baxter RL. (1975). Mezerein: antileukemic principle isolated from Daphne mezereum L. Science, 187, 652–653.
- Nasir E, Ali SI. (1971). Flora of West Pakistan. Karachi, Pakistan: Feroz Sons, 1–12.
- National Research Council (1996). Guide for the Care and Use of Laboratory Animals. Washington, USA: National Academy Press, 1–7.
- Nisar-ullah Ahmed, S, Mohammed P, Rabnawaz H, Malik A. (1999). Chemical constituents of Daphne oleoides. Fitoterapia, 70, 214–215.
- Okumura K, Ichihara K, Nagasaka M, Oda N, Tajima K. (1993). Calcium entry blocking activities of MPC-1304 and of its enantiomers and metabolites. Eur J Pharmacol, 235, 69–74.
- Pasricha PJ. (2006). Treatment of disorders of bowel motility and water flux; antimemetics; agents used in biliary and pancreatic disease. In: Brunton LL, Lazo JS, Parker KL, Gilman’s, A.G. (Eds.), Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 11th edn. New York, USA: McGraw-Hill, 983–1008.
- Perry N. (1996). Cholinergic transmitter activities in European herbs: Potential in dementia therapy. Int J Geriatr Psych, 11, 1063–1069.
- Pietrobon D, Hess P. (1990). Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature, 346, 651–655.
- Revuelta MP, Cantabrana B, Hidalgo A. (1997). Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. Gen Pharmacol, 29, 847–857.
- Ronlán A, Wickberg B. (1970). The structure of mezerein, a major toxic principle of Daphne mezereum L. Tetrahedron Lett, 49, 4261–4264.
- Sugi M, Nagashio Y. (1977). Cancer Therapy in Modern China. Tokyo, Japan: Shizensha, 67–89.
- Tabata M, Honda G, Sezik E, Yesilada E. (1993). A Report on Traditional Medicine and Medicinal Plants in Turkey. Kyoto, Japan: Kyoto University, 33–59.
- Van Rossum JM. (1963). Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther, 143, 299–330.
- Wangner H, Jurice K, Bauer R, Kreher B. (1987). Immunologische in vitro and in vivo untersuchungen von arzneipräparaten. Z Phytother, 8, 180–183.
- Watt G. (1963). Analytical Microbiology. New York, USA: Academic Press, 125.
- Watt G. (1972). Dictionary of the Economic Products of India. Dehli, India: Cosmo Publications, 26.
- Yesilada E, Honda G, Sezik E, Tabata M, Fujita T, Tanaka T, Takeda Y, Takaishi Y. (1995). Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J Ethnopharmacol, 46, 133–152.
- Yesilada E, Ustün O, Sezik E, Takaishi Y, Ono Y, Honda G. (1997). Inhibitory effects of Turkish folk remedies on inflammatory cytokines: Interleukin-1alpha, interleukin-1beta and tumor necrosis factor alpha. J Ethnopharmacol, 58, 59–73.
- Zhuang LG, Otto S, Hildert W. (1983). Daphneticin, a coumarinolignoid from Daphne tangutica. Phytochemistry, 22, 617–619.