Abstract
Context: Ligustrazine (Lig) is a compound isolated from the rhizome of Ligusticum chuanxiong Hort. (Umbelliferae) and has been reported to be effective for the treatment of a variety of vascular diseases.
Objective: The anti-atherosclerotic activities of Lig are evaluated in vivo for the first time in the present study.
Materials and methods: We gave rats a single injection of vitamin D3 and then fed them with an atherogenic diet for 6 weeks to induce atherosclerosis. Lig was simultaneously given to rats by gavage at the dose of 20 or 80 mg/kg in the therapy groups. Multiple approaches including spectrophotometry, hematoxylin and eosin (H&E) staining, and quantitative RT-PCR were applied to investigate the effects of Lig on blood parameters, aorta and liver histology, and gene expression. In addition, the solely effects of Lig on food intake, body weight gain, and taste preference were also evaluated.
Results: We found that two doses of Lig treatment decreased the total cholesterol levels by 65.2 and 76.7%, respectively, in the plasma. Triglyceride (by 53.2 and 77.9%) and low-density lipoprotein (by 71.2 and 79.0%) levels were also decreased. However, high-density lipoprotein level was slightly increased. The circulating endothelial cells were decreased by 42.2 and 60.0% in Lig-treated rats, indicating the attenuation of endothelial injury. In contrast, Lig restored the total antioxidant capacity and superoxide dismutase 1 (SOD1) activity while decreasing the MDA generation. Furthermore, Lig improved liver dysfunction by decreasing ALT (by 13.0 and 49.7%) and AST (by 10.7 and 14.3%) levels. Histological examinations revealed that Lig suppressed atherosclerotic plaque progression in the thoracic aorta and lipid accumulation in the liver. At the transcriptional level, Lig inhibited the induction of antioxidant genes both in aorta and in liver. Lig also suppressed the mRNA expression of the genes involved in the hepatic fatty acid oxidation. Finally, Lig had a minimum effect on food intake, body weight gain, and taste preference.
Discussion and conclusion: Our results suggest that Lig suppresses the development of atherosclerosis and hepatic lipid accumulation via the alleviation of oxidative stress and the improvement of dyslipidemia.
Introduction
Atherosclerosis is the condition in which an artery wall thickens as the result of a build-up of fatty materials such as cholesterol (CitationSeyama & Wachi, 2004). It severely threatens human health with universal incidence in the western lifestyle (CitationWoo et al., 1999). Development of atherosclerosis leads to many deleterious effects, including myocardial and cerebral infarction, gangrene, and loss of function in the extremities (CitationHansson, 2009). It has been recognized that atherosclerosis is a chronic inflammatory disease accompanied by overloaded oxidative stress (CitationSingh & Jialal, 2006). Among various risk factors, obesity and dyslipidemia play an important role in the pathogenesis of atherosclerosis (CitationMuniyappa et al., 2007). Obese patients are often seen to have higher plasma levels of low-density lipoprotein (LDL), which can be oxidized by vessel cells and converted into oxidized LDL (ox-LDL) (CitationIshigaki et al., 2009). The latter subsequently leads to a series of pathological changes including the occurrence of oxidative stress, inflammation and injury in the endothelium, as well as the migration and proliferation of vascular smooth muscle cells (VSMCs) (CitationIshigaki et al., 2009).
The rhizome of Ligusticum chuanxiong Hort. (Umbelliferae) has long been used by practitioners of traditional Chinese medicine in treating blood stasis and vital energy stagnation (CitationWong et al., 2003). The active ingredient of this herb has already been characterized and its chemical structure has been identified (CitationSutter and Wang, 1993) (). This compound, ligustrazine (Lig), has been reported to be effective for the treatment of a variety of vascular diseases, notably ischemic stroke and pulmonary hypertension secondary to chronic obstructive pulmonary disease (COPD) (CitationSun et al., 2008). The major therapeutic effects of Lig are likely to be via its action as a vasodilator (CitationPeng et al., 1996), although it has also been reported to have other effects, including inhibition of platelet aggregation (CitationWu et al., 1992), protection of endothelial cells against LDL-induced damage (CitationLi et al, 1994) and at high concentrations it acts as a free radical scavenger (CitationZhang et al., 1994).
Although the beneficial effects of Lig on vascular cells have been extensively studied in vitro, whether Lig has similar functions in vivo, particularly through the inhibition of oxidative stress, remains unknown. Therefore, this study was designed to evaluate the potential of Lig in the inhibition of oxidative damage and the reduction of the severity of atherosclerosis and fatty liver in hypercholesterolemic rats.
Materials and methods
Reagents
Lig was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (NICPBP). Saccharin and LiCl were purchased from Sigma (St. Louis, MO). Trizol reagent and monodispersed magnetizable particles (Dynabeads CELLection Pan Mouse IgG kit) were obtained from Invitrogen (Carlsbad, CA). SYBRGreen Master Mix was obtained from Applied Biosystems (Foster City, CA). Kits for cholesterol, triglyceride (TG), LDL, and high-density lipoprotein (HDL) quantification were obtained from Kangtai Clinical Reagent Corporation (Beijing, China). Kits for the measurement of total antioxidant capacity (T-AOC), alanine transaminase (ALT), and aspartate transaminase (AST) were obtained from Jiancheng Bioengineering Institute (Nanjing, China). Malondialdehyde (MDA) assay kit for lipid peroxidation and the enzymatic activity assay kit for superoxide dismutase 1 (SOD1) were obtained from Beyotime Biotechnology (Nantong, China). RECA-1 antibody was obtained from Novus Biologicals (Littleton, CO). All other reagents were of analytical grade.
Animal experiments
Male Sprague-Dawley rats (SPF grade, weighing around 200 g at the beginning of the study) were purchased from the Animal Center, Nanjing General Hospital of Nanjing Military Command, Nanjing, China. All animal-handling procedures were in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China and the approved guidelines set by the Laboratory Animal Care Committee at Nanjing Normal University. They were exposed to a 12 h light/dark cycle and had free access to drinking water. After 1 week of accustomization, the rats were divided into four groups, namely, control, atherosclerotic, low-dose Lig (L-Lig), and high-dose Lig (H-Lig) (n = 10 in each group). To induce atherosclerosis, rats of the latter three groups were intraperitoneally injected with vitamin D3 at the dose of 600,000 IU/kg and then were fed an atherogenic diet containing 3% cholesterol, 0.5% sodium cholate, 0.2% propyl thiouracil, 5% refined sugar, 10% lard, and 81.3% chow diet (CitationKitagawa et al., 1993). Rats of control group received an intraperitoneal injection with 0.9% saline solution and were maintained on normal rodent chow diet. At the same time, rats of L-Lig and H-Lig groups received Lig daily by gavage (20 and 80 mg/kg, respectively) and rats of control and atherosclerotic groups received equal amount of 0.9% saline solution in the same way. The doses of Lig were selected based on the previous report showing that Lig protects rats from brain ischemia (CitationChang et al., 2007). The whole experiment lasted for 6 weeks. Animals were bled periodically for measurement of plasma cholesterols and triglycerides during this period. We found that in atherosclerotic group, plasma levels of cholesterol and triglyceride significantly increased and reached the peak at the end of the study, suggesting the successful establishment of atherosclerosis. When the experiment was finished, rats were fasted overnight and anesthetized with the intravenous injection of 50 mg/kg sodium pentobarbital. Blood, thoracic aortas, and the livers were quickly collected.
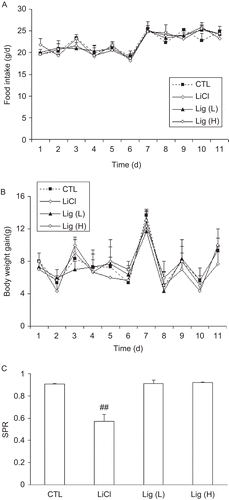
To evaluate the effects of Lig on food intake, body weight, and taste preference, we performed another parallel experiment. Rats were divided into four groups, namely, control, LiCl, L-Lig, and H-Lig (n = 6 in each group). All the rats were fed a standard rodent diet. Rats of L-Lig and H-Lig groups received Lig daily as described above and rats of control and LiCl groups received equal amount of 0.9% saline solution. At the same time, the rats were placed on a water restriction schedule, during which time they received two water bottles per cage from 08:30–09:00 h and from 14:00–19:00 h each day for 10 days. Food intake and body weight were measured daily. On Day 11, the rats received a single bottle of water containing 0.15% saccharin at 08:30 h. Bottle position in the cage was randomized. The saccharin water was removed 30 min later, and then the rats still received saline solution for control or Lig for Lig-treated groups. LiCl group received an i.p. injection of 0.6 mol/L LiCl dissolved in saline (3 mmol/kg) as a positive control. The rats were allowed to recover for 48 h, during which time they were still water-restricted. After 48 h, the rats received two bottles (one containing water and the other containing 0.15% saccharin water, with bottle position randomized) at 08:30 h. Fluid intake was measured from each bottle after 2 h. Data are expressed as saccharin preference ratios (SPR = volume of saccharin water consumed/total fluid volume consumed during the 2 h period).
Atherosclerosis quantification
Thoracic aortas were gently dissected free of adhering tissues, rinsed with ice-cold phosphate-buffered saline (PBS), immersion-fixed in 4% buffered paraformaldehyde, paraffin-embedded, and then cross-sectioned for routine hematoxylin and eosin (H&E) staining. To quantitate plaque size, images of three sections of each aorta vessel were acquired with a DP70 digital camera connected to a microscope (Olympus BX41), and lesion areas determined using Image Analysis System software (Leica) and expressed as a percentage of the total surface area covered by lesion on the thoracic aorta. The mean value of plaque cross-sectional areas from three blind-selected sections was used to estimate the extent of atherosclerosis for each animal.
Determination of plasma and liver parameters
Blood was obtained from vena cava and the plasma was isolated and stored at −80°C until analysis. The plasma levels of lipids including cholesterol, TG, LDL, and HDL were determined enzymatically using commercially available kits. The T-AOC values of the cells were measured with an analysis kit utilizing antioxidants in the samples to reduce Fe3+ to Fe2+, which was then chelated with porphyrin to produce a purple complex; this was quantified by measuring the absorbance at 550 nm. MDA generation and enzymatic activity of SOD1, ALT, and AST were measured with the use of corresponding commercial kits according to the manufacturer’s protocols. The hepatic levels of TG, LDL, and HDL were similarly measured after liver homogenization.
Counting of circulating endothelial cells
We used monodispersed magnetizable particles (Dynabeads CELLection Pan Mouse IgG kit) for the immunomagnetic isolation and quantification of circulating endothelial cells (CEC; CitationClarke et al., 2008). The 4.5 μm diameter polystyrene beads were coated with affinity-purified pan-anti-mouse immunoglobulin G1 covalently bound to the surface. The beads were washed, according to the instructions provided by the manufacturer, with a strong magnet (MPC6; Dynal MCP-6 Magnetic Particle Concentrator, Invitrogen Dynal AS, Oslo, Norway ) used to remove sodium azide. Typically, 100 μL of bead suspension was coated noncovalently with 10 μg/mL RECA-1, a pan-rat EL-specific monoclonal antibody diluted 1:10 in PBS–bovine serum albumin (BSA) (0.1%) by overnight incubation at 4°C with head-over-head agitation. After three washes with PBS–BSA to remove excess antibodies, the beads were resuspended in buffer until use. RECA-uncovered particles were used as a negative control. If the beads were stored for an extended period of time, 0.1% sodium azide was added to the buffer. Beads and target cells were incubated for 1.5 h at 4°C on a rotator. The amount of beads (4 × 108/mL) was in great excess of target cells (>2000 beads per target cell). Separation of beads and rosetted cells from the blood samples required a minimum exposure of 1 min to the magnet. Three washes were performed to completely remove nonrosetted cells. After the third wash, rosetted cells were recovered in a solution of acridine orange (a viral fluorescent dye at a final concentration of 5 μg/mL in PBS, 150 μL) and subjected to fluorescence microscopy (Olympus BX41).
Liver histology
The livers were isolated, fixed in 4% paraformaldehyde solution for 24 h in situ, processed for paraffin embedding, and cut into 7 µm transverse sections for routine H&E staining.
Real-time RT-PCR
Total RNA from thoracic aortas and livers was extracted by using Trizol reagent according to the manufacturer’s protocol. Two micrograms of total RNA was reverse-transcribed into complementary DNA and β-actin served as an internal control for total complementary DNA content. mRNA levels of interested genes were quantified by real-time RT-PCR using SYBRGreen Master Mix. The primer sequences are available upon request.
Data analysis
All data are presented as mean ± SEM. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. Calculations were performed using SPSS/Windows version 12.5S statistical package (SPSS, Chicago, IL). In all cases, P < 0.05 was taken as statistically significant.
Results
Blood analysis
The levels of plasma lipids including total cholesterol, TG, and LDL were significantly higher in atherosclerotic rats compared with that of control rats (). However, Lig treatment dose-dependently decreased all these variables. In contrast, Lig recovered the decreased level of HDL in atherosclerotic group. Atherosclerotic rats had more CEC and higher plasma levels of ALT and AST, indicating the endothelial and liver injuries, respectively. However, Lig treatment led to fewer CEC and lower ALT and AST plasma levels in rats, suggesting this compound improved endothelial and liver dysfunction. One of the hallmarks in atherosclerosis is the burdened oxidative stress. Indeed, we found that T-AOC and SOD1 activity was impaired in atherosclerotic rats and Lig treatment partially restored them. In parallel, MDA generation (an indicator of lipid peroxidation) was increased to 1.7-fold in atherosclerotic group and two doses of Lig treatment inhibited MDA levels by 12.8 and 23.8%, respectively. Finally, rats became significantly obese in atherosclerotic group. However, the gain of body weight became less with Lig treatment.
Table 1. Blood parameters.
Atherosclerosis assessment
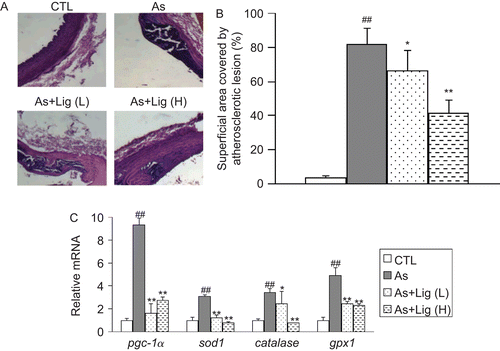
As shown in , the surface of the intima layer of thoracic aortas in control rats was smooth and glossy, but became rough and disordered while atherosclerosis developed. Also, there was a pathological thickening in the intima in atherosclerotic rats. These morphological changes were ameliorated by Lig treatment. In addition, rats fed with an atherogenic diet developed typical plaques characterized by the punctate and lamellar calcification under the endothelium. However, Lig treatment significantly inhibited the formation of atherosclerotic plaques. Quantification analysis indicated that the lesion area in atherosclerotic group was about 81.4% (). The extent of atherosclerosis in the thoracic aorta was reduced in the L-Lig and H-Lig groups by 18.9 and 49.0%, respectively, compared with the atherosclerotic group.
Figure 2. (A) Representative hematoxylin and eosin (H&E) staining for the thoracic aortas (400× magnification). (B) Quantification of lesion area. (C) mRNA quantification for the antioxidant genes in aorta. Data are expressed as mean ± SEM (for each group, n = 10). ##P < 0.01 compared with control (CTL) rats; *P < 0.05 and **P < 0.01 compared with atherosclerotic (As) rats.
Antioxidant gene expression in aorta
We next examined the mRNA expression of antioxidant genes in the aorta by using quantitative RT-PCR. We found that the expression of pgc-1α, sod1, catalase, and gpx-1 was higher in atherosclerotic group. Lig treatment significantly decreased the mRNA levels of all these genes ().
Liver histology, lipid profile, and gene expression
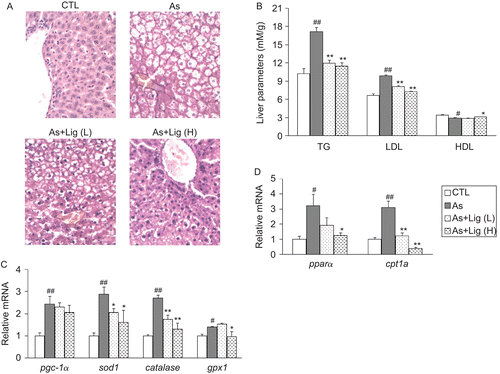
After 6 weeks of consuming the atherogenic diet, hepatic lipid accumulation with vacuolated hepatocytes was easily detected in rats, indicating the development of fatty liver. However, micro- and macrovascular fat droplets were much less in Lig-treated groups (). The levels of TG and LDL in liver homogenates were elevated in the atherosclerotic group, accompanied with the decrease of HDL level. And Lig treatment reversed these pathological changes (). For the antioxidant gene expression, we found similar to that observed in the aorta, the mRNA levels of sod1, catalase, and gpx-1 were all increased in atherosclerotic group. The mRNA expression of genes involved in the hepatic fatty acid oxidation (FAO) including pgc-1α, pparα, and cpt1a was also up-regulated in atherosclerotic group. In contrast, Lig treatment dose-dependently suppressed their expression ( and ).
Figure 3. (A) Representative hematoxylin and eosin (H&E) staining for the livers (400× magnification). (B) Lipid profile in the livers. (C) mRNA quantification for the antioxidant genes. (D) mRNA quantification for the genes in fatty acid oxidation. Data are expressed as mean ± SEM (for each group, n = 10). #P < 0.05 and ##P < 0.01 compared with control (CTL) rats; *P < 0.05 and **P < 0.01 compared with atherosclerotic (As) rats.
Food intake, body weight, and taste preference
To exclude the possibility that the observed effects of Lig might due to less food intake rather than its own anti-atherosclerotic activity, we performed experiments to evaluate the influence of Lig in food intake, body weight, and taste preference in normal rats. As shown in , food intake was comparable among all the four groups in an 11-day period, suggesting that Lig did not affect food intake. The body weight gain of rats receiving low dose of Lig was quite similar to that of the control rats, although high dose of Lig treatment led to a trend to decreased body weight but without significant difference (). Finally, rats showed a high saccharin preference and LiCl treatment significantly suppressed this preference, as expected. At the meantime, we did not find any effect of Lig on taste preference ().
Discussion
In the present study, our results demonstrated that Lig improved dyslipidemia and pathological changes in thoracic aorta and liver in atherosclerotic rats. Lig also decreased CEC number, ALT and AST levels although recovered T-AOC and SOD1 activity in blood. At the transcriptional level, Lig inhibited the induction of antioxidant genes both in aorta and in liver. Lig also suppressed the mRNA expression of the genes involved in the hepatic FAO.
It is well known that hyperlipidemia is the leading risk factor for atherosclerosis. Epidemiological investigations revealed a positive correlation between the severe degree of atherosclerosis and the plasma concentrations of cholesterol as well as LDL (CitationHolvoet et al., 2008). Elevated plasma levels of cholesterol or LDL–cholesterol also indicate increased incidence of atherosclerosis events (CitationGoldstein et al., 1973). In addition, it has been demonstrated that the clinical complications of atherosclerosis were suppressed and lifetime was prolonged when plasma lipids were lowered by hypocholesterolemic agents (CitationIllingworth & Bacon, 1987). On the other hand, plasma level of HDL is inversely correlated with the risk of coronary heart diseases including atherosclerosis (CitationGordon et al., 1977; CitationCastelli et al., 1986). Atherosclerosis can be modulated by raising the level of HDL (CitationGordon et al., 1989). In our study, we found that after the intake of atherogenic diet that contained high cholesterol, rats showed a significant increase of plasma lipids including cholesterol, TG, and LDL with a decrease of HDL when compared with that of the control group. However, Lig treatment significantly improved hyperlipidemia, suggesting that Lig has beneficial effects on the whole lipid profile and suppresses the initiation and progression of atherosclerosis.
The loss of adequate vascular function and endothelial damage are important in the pathogenesis of atherosclerosis and thus peripheral artery disease (PAD) (CitationDuprez & Cohn, 2007). Disruption of the endothelium and exposure of the blood constituents to a thrombogenic surface may in part account for the triad of the prothrombotic or hypercoagulable state seen in atherosclerosis, that is, abnormally turbulent blood flow, prothrombotic blood constituents (e.g., platelets and clotting factors), and abnormalities in endothelial physiology (CitationMakin et al., 2003). As a result, the pathological vascular remodeling including inflammatory cell recruitment, fibrosis, VSMC proliferation, neovascularization, and intraplaque hemorrhage is provoked and the formation of atherosclerotic plaques is accelerated. In the present study, we provided direct evidence to show that Lig antagonized the progression of atherosclerosis. First, Lig inhibited the pathological changes of the intima monolayer of thoracic aorta and decreased the lesion area. Second, Lig decreased the number of CEC, which is one of the hallmarks to index the endothelial damage. These results collectively indicated that Lig ameliorated the endothelial dysfunction. The effects of Lig on other vessel cells such as VSMCs remain unknown.
To date, the oxidative stress has been shown to play a critical role in atherogenesis. Growing evidence indicates that chronic and acute overproduction of reactive oxygen species (ROS) under pathophysiological conditions is integral in the development of cardiovascular diseases (CVD) including atherosclerosis (CitationVictor et al., 2009). ROS mediate various signaling pathways that underlie vascular inflammation in atherogenesis from the initiation of fatty streak development through lesion progression to ultimate plaque rupture (CitationPuddu et al., 2005). Various animal models of oxidative stress support the notion that ROS have a causal role in atherosclerosis and other CVD (CitationHsiai and Berliner, 2007). Human investigations also support the oxidative stress hypothesis of atherosclerosis (CitationSoccio et al., 2005). In response to oxidative stress, the vascular antioxidant system was activated in atherosclerotic lesions (CitationKobayashi et al., 2002) and in the liver (CitationKim et al., 2002). Coincided with the previous reports, we found the genes encoding the master regulator in the antioxidant system, pgc-1α (CitationSt-Pierre et al., 2006), and three antioxidant enzymes (sod1, catalase, and gpx-1) were all induced in aorta and in liver of atherosclerotic rats, together with the decreased T-AOC in the plasma, suggesting the burdened oxidative stress. However, the induction of these genes was inhibited although T-AOC was restored by Lig, suggesting that Lig acts as an antioxidant and attenuates oxidative stress. These effects of Lig may be achieved via two different mechanisms: (1) Lig directly scavenges the ROS, which has been shown in the previous study (CitationZhang et al., 1994)and (2) Lig inhibits the activation of nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase system, which is the main source of ROS generation (CitationRoberts et al., 2006). Further studies are required to elucidate the precise function of Lig in the whole oxidative stress aspect.
Liver lipid accumulation has been suggested to contribute to atherosclerosis (CitationLizardi-Cervera & Aguilar-Zapata, 2009) and therefore, its inhibition may also affect atherogenesis in rats. To eliminate the excess accumulation of lipids in the liver, FAO pathway, in which the transcriptional coactivator pgc-1α plays a dominant role (CitationSong et al., 2004), is activated (CitationHashimoto et al., 2000). We found that atherosclerotic rats developed fatty liver with the compensatory induction of hepatic FAO genes (pgc-1α, pparα, and cpt1a). The plasma levels of liver injury markers (ALT and AST) were also increased. Furthermore, both pathological examination and gene expression showed that Lig treatment reduced fatty liver in the rats. Lig can therefore protect against diet-induced liver damage, and consequently, atherogenesis. Considering that the inflammation always occurs during the development of atherosclerosis and fatty liver, it is noteworthy to determine if Lig also has anti-inflammatory property.
Finally and notably, Lig had a minimum effect on food intake, body weight gain, and taste preference. This observation excludes the possibility that Lig attenuates atherosclerosis development through less intake of cholesterol-enriched diet rather than its own anti-atherosclerotic activity. This property of Lig is also important to the clinical use because some drugs currently used in the therapy of atherosclerosis are easy to have the untoward effect on the gastrointestinal tract including nausea, enterorrhea, anepithymia, and thus decrease the quality of life.
Conclusion
The present study revealed for the first time that Lig inhibits atherosclerosis progression and hepatic lipid accumulation in vivo possibly through the attenuation of oxidative stress and the improvement of dyslipidemia. Of note, Lig has long been clinically used in China for the treatment of various CVD including diabetic nephrosis, atherosclerotic coronary heart disease, cerebral infarction, vertigo, and the neonates with hypoxic ischemic, and so on. In addition, this drug has been formally adopted by the Pharmacopoeia of the People’s Republic of China (2005 English Edition). Our findings further highlight the significance of Lig in the therapy of various CVD including atherosclerosis.
Acknowledgements
This work was supported by grants from the the National Science Foundation of China (30870928), the Research Fund for the Doctoral Program of Higher Education of China (20103207110007), the Fok Ying Tong Education Foundation (121022), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (09KJA180004), and the Opening Project of Jiangsu Key Laboratory for Molecular and Medical Biotechnology (MMB09KF05).
Declaration of interest
None.
References
- Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. (1986). Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA, 256, 2835–2838.
- Chang Y, Hsiao G, Chen SH, Chen YC, Lin JH, Lin KH, Chou DS, Sheu JR. (2007). Tetramethylpyrazine suppresses HIF-1alpha, TNF-alpha, and activated caspase-3 expression in middle cerebral artery occlusion-induced brain ischemia in rats. Acta Pharmacol Sin, 28, 327–333.
- Clarke LA, Shah V, Arrigoni F, Eleftheriou D, Hong Y, Halcox J, Klein N, Brogan PA. (2008). Quantitative detection of circulating endothelial cells in vasculitis: Comparison of flow cytometry and immunomagnetic bead extraction. J Thromb Haemost, 6, 1025–1032.
- Duprez DA, Cohn JN. (2007). Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep, 9, 139–144.
- Goldstein JL, Hazzard WR, Schrott HG, Bierman EL, Motulsky AG. (1973). Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J Clin Invest, 52, 1533–1543.
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA. (1989). High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation, 79, 8–15.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. (1977). High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med, 62, 707–714.
- Hansson GK. (2009). Inflammatory mechanisms in atherosclerosis. J Thromb Haemost, 7 (Suppl 1), 328–331.
- Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. (2000). Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem, 275, 28918–28928.
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR Jr. (2008). Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA, 299, 2287–2293.
- Hsiai T, Berliner JA. (2007). Oxidative stress as a regulator of murine atherosclerosis. Curr Drug Targets, 8, 1222–1229.
- Illingworth DR, Bacon S. (1987). Hypolipidemic effects of HMG-CoA reductase inhibitors in patients with hypercholesterolemia. Am J Cardiol, 60, 33G–42G.
- Ishigaki Y, Oka Y, Katagiri H. (2009). Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr Opin Lipidol, 20, 363–369.
- Kim JW, Kang KW, Oh GT, Song J, Kim ND, Pak YK. (2002). Induction of hepatic inducible nitric oxide synthase by cholesterol in vivo and in vitro. Exp Mol Med, 34, 137–144.
- Kitagawa S, Yamaguchi Y, Kunitomo M, Imaizumi N, Fujiwara M. (1993). Altered vasoconstrictor responsiveness in vitamin D-induced arteriosclerotic rat aortas. Jpn J Pharmacol, 61, 283–289.
- Kobayashi S, Inoue N, Azumi H, Seno T, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yokozaki H, Yokoyama M. (2002). Expressional changes of the vascular antioxidant system in atherosclerotic coronary arteries. J Atheroscler Thromb, 9, 184–190.
- Li YJ, Li YJ, Wu JX, Yu XJ, Yan YF. (1994). Protective effect of tetramethylpyrazine against damages of aortic endothelial cells elicited by low-density lipoproteins. Zhongguo Yao Li Xue Bao, 15, 407–410.
- Lizardi-Cervera J, Aguilar-Zapata D. (2009). Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol, 8 (Suppl 1), S40–S43.
- Makin AJ, Chung NA, Silverman SH, Lip GY. (2003). Thrombogenesis and endothelial damage/dysfunction in peripheral artery disease. Relationship to ethnicity and disease severity. Thromb Res, 111, 221–226.
- Muniyappa R, Montagnani M, Koh KK, Quon MJ. (2007). Cardiovascular actions of insulin. Endocr Rev, 28, 463–491.
- Peng W, Hucks D, Priest RM, Kan YM, Ward JP. (1996). Ligustrazine-induced endothelium-dependent relaxation in pulmonary arteries via an NO-mediated and exogenous l-arginine-dependent mechanism. Br J Pharmacol, 119, 1063–1071.
- Puddu GM, Cravero E, Arnone G, Muscari A, Puddu P. (2005). Molecular aspects of atherogenesis: New insights and unsolved questions. J Biomed Sci, 12, 839–853.
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. (2006). Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metab Clin Exp, 55, 928–934.
- Seyama Y, Wachi H. (2004). Atherosclerosis and matrix dystrophy. J Atheroscler Thromb, 11, 236–245.
- Singh U, Jialal I. (2006). Oxidative stress and atherosclerosis. Pathophysiology, 13, 129–142.
- Soccio M, Toniato E, Evangelista V, Carluccio M, De Caterina R. (2005). Oxidative stress and cardiovascular risk: The role of vascular NAD(P)H oxidase and its genetic variants. Eur J Clin Invest, 35, 305–314.
- Song S, Zhang Y, Ma K, Jackson-Hayes L, Lavrentyev EN, Cook GA, Elam MB, Park EA. (2004). Peroxisomal proliferator activated receptor gamma coactivator (PGC-1alpha) stimulates carnitine palmitoyltransferase I (CPT-Ialpha) through the first intron. Biochim Biophys Acta, 1679, 164–173.
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell, 127, 397–408.
- Sun Y, Jiang J, Zhang Z, Yu P, Wang L, Xu C, Liu W, Wang Y. (2008). Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. Bioorg Med Chem, 16, 8868–8874.
- Sutter MC, Wang YX. (1993). Recent cardiovascular drugs from Chinese medicinal plants. Cardiovasc Res, 27, 1891–1901.
- Victor VM, Rocha M, Solá E, Bañuls C, Garcia-Malpartida K, Hernández-Mijares A. (2009). Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des, 15, 2988–3002.
- Wong KL, Chan P, Huang WC, Yang TL, Liu IM, Lai TY, Tsai CC, Cheng JT. (2003). Effect of tetramethylpyrazine on potassium channels to lower calcium concentration in cultured aortic smooth muscle cells. Clin Exp Pharmacol Physiol, 30, 793–798.
- Woo KS, Chook P, Raitakari OT, McQuillan B, Feng JZ, Celermajer DS. (1999). Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol, 19, 2487–2493.
- Wu GX, Wu JC, Ma HT, Ruan CG. (1992). Inhibitory effects of tetramethylpyrazine on platelets during cardiopulmonary bypass and arterial thrombus formation in dogs. Zhongguo Yao Li Xue Bao, 13, 330–333.
- Zhang ZH, Yu SZ, Wang ZT, Zhao BL, Hou JW, Yang FJ, Xin WJ. (1994). Scavenging effects of tetramethylpyrazine on active oxygen free radicals. Zhongguo Yao Li Xue Bao, 15, 229–231.