Abstract
Context: Fructus Corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. (Cornaceae). Morroniside is an active constituent of Fructus Corni used in many traditional Chinese medicines (TCMs). This article describes a sensitive and specific assay for the quantitation of morroniside in rat plasma after oral administration of iridoid glycosides from Fructus Corni.
Materials and methods: In this article, back-propagation (BP) neural network method was fist developed for the prediction of pharmacokinetic (PK) parameters of morroniside in Fructus Corni.
Results: The results show that mean square error (MSE) of neural network model with 11 hidden neurons and 90% training data is 0.092.
Discussion and conclusion: This article provides a new method to calculate PK data, one do not need to figure out all the compartment parameters to acquire PK data of morroniside. Therefore, the BP neural network method would be useful for guiding the holistic PK study in consistence with the intrinsic theory and characteristics of TCM.
Introduction
Traditional Chinese medicine (TCM) has played an important role in the prevention and treatment of diseases in china for thousands of years (CitationWu et al., 2007; CitationWen et al., 2007; CitationQian et al., 2008). Fructus Corni, a well-known TCM herbal medicine with the Chinese name “Shanzhuyu,” was prepared from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. (Cornaceae) (CitationDing et al., 2008). Morroniside is the main active components in Fructus Corni. Pharmacological studies on morroniside show antioxidation and protective effects on rat mesangial cell proliferation (CitationWang et al., 2009). In this article, high-performance liquid chromatography (HPLC) method has been successfully applied to a pharmacokinetic (PK) study of the morroniside after oral administration of iridoid glycosides from Fructus Corni to rats.
Artificial neural networks (ANNs) are versatile computational tools that are well suited to recognize complex nonlinear relationships (CitationHunt & Deller, 2005; CitationBernardos & Vosniakos, 2007). Neural networks could be envisaged as a model-independent approach to PK and pharmacodynamic (PD) modeling, in that ANNs do not attempt to capture the intricacies of the in vivo characteristics but endeavor to generalize the association of the observed concentrations of active substance(s) in biofluid(s) with the detected PD effect as a function of time (and other variables where applicable). Previously, ANNs were successfully applied to a variety of interesting areas (CitationGevrey et al., 2003; CitationOlden et al., 2004; CitationZou et al., 2008). In this article, the application of the back-propagation (BP) neural network in the prediction of pharmacokintic parameters of morroniside in Fructus Corni is discussed in depth, and a comparison with the normal prediction methods is made.
Materials and methods
Materials and standards
The Fructus Corni was collected from Henan suppliers. Morroniside was prepared in our own laboratory, and its identity was verified by LC-MS, 1H NMR and 13C NMR. The purity of each standard compound was >98% as shown by HPLC analysis. Analytical grade acetonitrile was purchased from Merck (Darmstadt, Germany) and used without further purification. Deionized water was purified using the Milli-Q system (Millipore, Bedford, MA). Solvents of HPLC grade were used for all preparations. All other chemicals were of analytical grade and commercially available.
Blood sample processing
A volume of 0.3 ml blood sample was collected into heparinized tubes (final concentration about 20 IU/ml) at each time point from rat carotid catheter after administration. Blood samples were centrifuged for 5 min at 4500g under 4°C. The plasma were harvested and transferred into tubes in a 96-well plate format and frozen at −20°C until thawed for bioanalysis. The rat plasma samples were thawed to room temperature. The rat plasma (25 µl) were transferred into a microcentrifuge tube (1.7 ml) to precipitate the plasma protein by the acetonitrile. The upper layer was transferred to a clean tube after centrifugation at 5000 rpm for 10 min. Acetonitrile was evaporated to dryness under a gentle stream of nitrogen gas at room temperature. The obtained residue was redissolved in 100 µl acetonitrile and 20 µl aliquots of the supernatants were injected into the HPLC system for analysis.
HPLC analysis
Analyses were performed using an Agilent 1200 HPLC system (Agilent Technologies, Palo Alto, CA) with a diode array detector. The monitoring wavelength was set at 218 nm for gallic acid. An Agilent Zorbax Extend C18 column (250 × 4.6 mm, 5 µm) was used with a flow rate of 1.0 ml/min. Mobile phase was composed of (A) aqueous phosphoric acid (0.1%, v/v) and (B) acetonitrile phosphoric acid (0.1%, v/v) using a gradient elution of 2% B at 0–10 min, 2–5% B at 10–15 min, 5–15% B at 15–45 min, 15–25% B at 45–55 min, 25–90% B at 55–70 min, 90% B at 80 min. All samples were centrifuged at 15,000g for 10 min. The column temperature was maintained at 30°C.
PK study
To evaluate the suitability of the assay for PK studies, serial blood samples were collected at pro-dose, 5, 10, 15, 30, 45, 60, 90, 120, 180, 240 and 300 min after oral uptake of 20, 40 and 80 mg/kg body mass dose of iridoid glycosides from Fructus Corni. Each sample was immediately transferred to a heparinized glass tube and centrifuged at 1500g for 15 min at 8–10°C. The plasma was then transferred to another glass tube and stored at −20°C until analyzed.
For the absorbing area studies under ether anaesthesia, five rats were ligated the end of stomach sphincter, the passage at pylorus that opens into duodenum to prevent drug solution from entering the duodenum. Animals were oral administrated with iridoid glycosides 20, 40 and 80 mg/kg when they regained consciousness. Blood samples were obtained 15 min after gavaging. Plasma samples were separated and stored at −20°C until analyzed.
BP neural network modelling
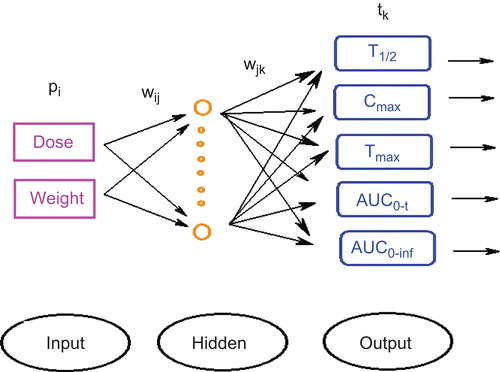
The BP neural network is also called an error BP network, which is composed of input, hidden and output layers. BP neural network, one of the multilayered feed forward structures, is the most commonly used and investigated neural network (CitationGoh, 1995; CitationRen & Gao, 2004; CitationXu & Yu, 2010). Each layer has several neurons. In this study, the input layer contains two neurons: dose and weight; while the output layer contains five neurons, which represent five PK parameters of morroniside in Fructus Corni: t1/2, Cmax, Tmax, AUC0–t and AUC0–inf. The BP neural network structure is shown in . The interconnected BP neurons receive signals from input neurons performing a simple processing and then pass signals to other neurons. The input neurons are each connected to the hidden nodes, which are in turn connected to the output neurons. The number of hidden neurons was selected using heuristics combined with a trial and error approach. Another important aspect of the networks is variable transformation. The tansig function was used as the input neuron transfer, while the purelin function was used as the output neuron transfer. Transformed variables were employed to calculate the MSE (mean square error) in separate experiments. The entire data set was scaled to lie in the range (−1,1), so that sensitivity can be adjust to at the same level. The output of the network was calculated each time, and difference between the predicted data and the observed data was propagated back to adjust the strengths of the connection weights.
Calculations and statistics
The plasma concentrations of morroniside were evaluated using the equation from the standard curves that were run with each batch of samples. Compartmental and non-compartment PK analysis of plasma concentrations-time data were carried out using the Drug and Statistics 2.0 software package to determine PK parameters. The observed value of Cmax was obtained from the observed data and the observed value of AUC0–t was calculated using the trapezoidal rule. The total AUC of these four active constituents was calculated by summing each AUC value. ANN analysis was performed using the software of MATLAB 7.1 (Mathworks Inc., Natick, MA,).
Results and discussion
Optimization of chromatographic conditions
Different mobile phase compositions were tested: (i) water-methanol; (ii) water-acetonitrile; (iii) aqueous phosphoric acid (0.1%, v/v)-acetonitrile phosphoric acid (0.1%, v/v); (iv) aqueous ammonium acetate (0.5%, v/v)-acetonitrile. As a result, the combination of aqueous phosphoric acid (0.1%, v/v)-acetonitrile phosphoric acid (0.1%, v/v) for the mobile phase gave the best separation. Furthermore, other chromatographic variables were also optimized, including the analytical column (Hanbon Hedera ODS-2, Hanbon Lichrospher C18 and Agilent-Zorbax Extend C18), the column temperature (20, 25 and 30°C) and the flow rate (0.8 ml/min and 1.0 ml/min). The optimal separation was achieved on an Agilent Zorbax Extend C18 column (250 × 4.6 mm, 5 µl) at a column temperature of 30°C with a flow rate of 1.0 ml/min.
Application to PKs
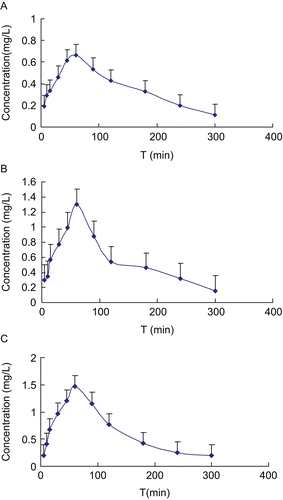
The method was used to determine the plasma concentration of morroniside in rats after oral administration of 20, 40 and 80 mg/kg iridoid glycosides. The dosages for Cmax were 0.7642 ± 0.0845, 1.300 ± 0.3439, and 200.77 ± 32.12 mg/l, respectively, for AUC(0–t), they were 109.0716 ± 14.6384, 166.014 ± 30.3033, and 182.6015 ± 40.2990 mg/h/l, respectively. The PK parameters are listed in and the mean plasma concentration–time profile is illustrated in . Results of the study are consistent with the works by CitationLi et al. (2007) and with other previously published studies.
Table 1. Pharmacokinetic parameters of oral administration iridoid glycosides of Fructus Corni. (mean ± SD, n = 5).
Application of the BP network model to PK parameters prediction of morroniside
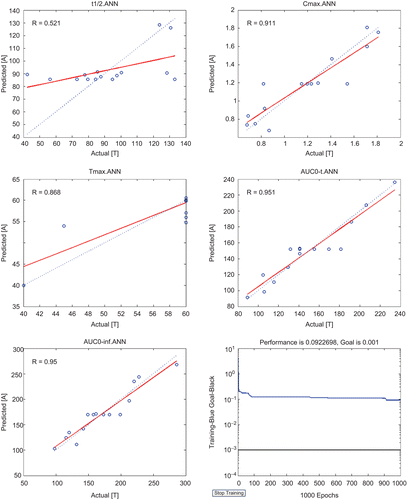
Determined by heuristics combined with a trial and error approach, with the test range of neurons from 3 to 12, the results show that the number was 11, when the least MSE 0.092 was approached. The final results with their respective coefficient of regression (R) values were shown in . These relationships appear to show significant correlation (R ranges from 0.521 to 0.951) and some of them have good predictive performance. Further investigation shows that dose emerged as the primary determinant of the variability in AUC0–inf, Cmax and AUC0–t, while weight was also an important parameter during prediction. Three major conclusions were drawn from above analysis: (i) Area under curve and maximum concentration are highly co-related with dose and weight. PK parameters prediction of simple neural networks containing certain architecture was acceptable. (ii) Half life and time to maximal plasma concentration were not related with dose and weight; they were determined by the properties of themselves. (iii) It provides a new method to calculate PK data, one do not need to figure out all the compartment parameters to acquire pk data of morroniside.
Conclusion
A simple, rapid and specific HPLC method has been established for investigating PKs of morroniside after oral administration of iridoid glycosides from Fructus Corni to rats. Furthermore, this article demonstrates the BP neural network has been successfully applied to predict PK parameters of morroniside. This study will help in rationalizing the design of clinical trails and reducing considerable cost of preclinical experiments. Besides, it provides a promising approach to investigate and explain the PK data of TCM in a new way. In addition, it proves that ANN is a valuable modelling tool that can be used to explain the relationships between the dosing regimen and PK parameters, which may also be helpful in drug discovery and development. This study would be useful for guiding the holistic PK study in consistence with the intrinsic theory and characteristics of TCM.
Declaration of interest
The authors are grateful to the financial support of the National Natural Science Foundation of China (No. 30873438) and Science and technology of Chinese medicine of Zhejiang province (No. 2009CB008).
References
- Bernardos PG, Vosniakos GC. (2007). Optimizing feedforward artificial neural network architecture. Eng Appl Artif Intel, 20, 365–382.
- Ding X, Wang MY, Yu ZL, Hu W, Cai BC. (2008). [Studies on separation, appraisal and the biological activity of 5-HMF in Cornus officinalis]. Zhongguo Zhong Yao Za Zhi, 33, 392–6, 484.
- Gevrey M, Dimopoulos I, Lek S. (2003). Review and comparison of methods to study the contribution of variables in artificial neural network models. Ecol Modell, 160, 249–264.
- Goh ATC. (1995). Back-propagation neural networks for modelling complex systems. Artif Intell Eng, 9, 143–151.
- Hunt SD, Deller JR Jr. (2005). Selective training of feedforward artificial neural networks using matrix perturbation theory. Neural Netw, 8, 931–944.
- Li X, Wang Q, Zhang X, Sheng X, Zhou Y, Li M, Jing X, Li D, Zhang L. (2007). HPLC study of pharmacokinetics and tissue distribution of morroniside in rats. J Pharm Biomed Anal, 45, 349–355.
- Olden JD, Joy MK, Death RG. (2004). An accurate comparison of methods for quantifying variable importance in artificial neural networks using simulated data. Ecol Modell, 178, 389–397.
- Qian ZM, Qin SJ, Yi L, Li HJ, Li P, Wen XD. (2008). Binding study of Flos Lonicerae Japonicae with bovine serum albumin using centrifugal ultrafiltration and liquid chromatography. Biomed Chromatogr, 22, 202–206.
- Ren S, Gao L. (2004). Wavelet packet transform and artificial neural network applied to simultaneous kinetic multicomponent determination. Anal Bioanal Chem, 378, 1392–1398.
- Wang W, Sun F, An Y, Ai H, Zhang L, Huang W, Li L. (2009). Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol, 613, 19–23.
- Wen XD, Qi LW, Chen J, Song Y, Yi L, Yang XW, Li P. (2007). Analysis of interaction property of bioactive components in Danggui Buxue Decoction with protein by microdialysis coupled with HPLC-DAD-MS. J Chromatogr B Analyt Technol Biomed Life Sci, 852, 598–604.
- Wu KM, Farrelly JG, Upton R, Chen J. (2007). Complexities of the herbal nomenclature system in traditional Chinese medicine (TCM): Lessons learned from the misuse of Aristolochia-related species and the importance of the pharmaceutical name during botanical drug product development. Phytomedicine, 14, 273–279.
- Xu H, Yu B. (2010). Automatic thesaurus construction for spam filtering using revised back propagation neural network. Expert Syst Appl, 37, 18–23.
- Zou J, Han Y, So SS. (2008). Overview of artificial neural networks. Methods Mol Biol, 458, 15–23.