Abstract
Context: Panax ginseng C. A. Mey (Araliaceae) has been widely used in clinic for treatment of cardiovascular diseases in China. Ginsenoside Rb3 is the main chemical component of Panax ginseng.
Objective: The aim of this study was to evaluate the effect of ginsenoside Rb3 on myocardial ischemia-reperfusion injury in rats.
Methods: Sprague–Dawley rats were orally treated with Rb3 (5, 10 or 20 mg/kg) daily for 3 days followed by subjecting to left anterior descending coronary artery ligation for 30 min and reperfusion for 24 h.
Results: This study showed that ginsenoside Rb3 treatment resulted in a reduction in myocardial infarct size. Ginsenoside Rb3 significantly attenuated the changes of creatine kinase activity and lactate dehydrogenase activity. The cardioprotective effect of ginsenoside Rb3 was further confirmed by histopathological examination. Ginsenoside Rb3 alleviated the increase of malondialdehyde content and the decrease of superoxide dismutase activity in left ventricle. Treatment with ginsenoside Rb3 also decreased plasma endothelin and angiotensin II levels.
Conclusion: These findings suggested that ginsenoside Rb3 possesses the effect against myocardial IR injury and the underlying mechanism is related to its antioxidant activity and microcirculatory improvement.
Introduction
Ischemic heart diseases, especially acute myocardial ischemia, remain the leading cause of death in both developed and developing countries over the past quarter century. Reduction of mortality rate and prevention of myocardial infarction are of utmost importance. The prognosis of acute myocardial infarction has been improved dramatically with the development of highly successful approaches to restore blood flow by primary percutaneous coronary intervention and thrombolytic therapy to the ischemia tissue. Paradoxically, while coronary reperfusion improves the prognosis of acute myocardial infarction, it also leads to myocardial reperfusion injury by extending myocardial damage within the ischemic period (CitationBraunwald & Kloner, 1985). Therefore, myocardial ischemia-reperfusion injury is an important clinical problem. Intervention to alleviate reperfusion injury at the time of coronary recanalization has been considered to be promising strategy to further decrease infarct size and improve the prognosis after myocardial infarction.
Panax ginseng C. A. Mey (Araliaceae) has been widely used for clinical treatment of cardiovascular diseases in China. With the development of modern technology, more and more active principles have been isolated and purified from Panax ginseng. Up to now, about thirty different ginsenosides have been extracted and identified from the root of Panax ginseng. These ginsenosides are classified into three subclasses: panaxadiol, panaxatriol, and oleanic acid. Ginsenosides are considered to be the main bioactive constituents of Panax ginseng (CitationAttele et al., 1999). Previous study shows that ginseng and ginsenosides have protective roles in atherosclerotic plaque formation and various vascular injuries (CitationZhou et al., 2004). CitationWang et al. (2008) demonstrated that ginsenoside Rb1 had protective effects on myocardial ischemia and reperfusion injury by mediating the activation of the PI3K pathway and phosphorylation of protein kinase B. It is also reported that ginsenoside Re protects cardiomyocytes from oxidant injury by scavenging H2O2 and hydroxyl radicals (CitationXie et al., 2006). Recently, the study from our laboratory showed that ginsenoside Rb3 inhibited angiotensin II-induced VSMCs proliferation (CitationWang et al., 2010). However, the effect of ginsenoside Rb3 on myocardial IR animal model in vivo has not been investigated so far. Therefore, in the present study, we evaluate whether ginsenoside Rb3 could ameliorate myocardial IR injury in rats.
Materials and methods
Animals
The experiments were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, 1986) and were approved by the Local Ethics Committee. Sprague–Dawley rats weighing 230–260 g were obtained from the Experiment Animal Center of Norman Bethune University of Medical Science (Jilin, China). All the rats were housed in diurnal lighting conditions (12 h/12 h) and allowed free access to food and water for 7 days before the experiment.
Preparation of extract from Panax ginseng
Samples of Panax ginseng were collected from Tonghua country of Changchun city in Jilin Province during June 2008. Its identity was confirmed by Prof. Yanping Chen with authentic specimens retained at the herbarium, Jilin University. A voucher specimen was deposited in Department of Medicinal Chemistry, Jilin University. Dried Panax ginseng (3 kg) was cut into small pieces, pounded into powder and then was soaked with boiling water (1:12, w:w) for 60 min. After filtering the aqueous extracts were added to nonpolar macroporous resin (DM-130 Anhui, China) column and the impurities such as sugar were washed out by water. Then total saponin was eluted by 75% ethanol. The eluent was freeze-dried finally giving an amorphous powder. The residue was dissolved with 75% ethanol again and added acetone to it. The suspension was filtrated and the precipitate was subjected to a silica gel column chromatography (2 × 20 cm) and eluted with a stepwise gradient of butanol:ethyl acetate:H2O (4:1:5) and then underwent further chromatography on silica gel columns, employing the same eluent systems, to give a ginsenoside Rb3. The yield of the extract as a dried material was ~0.7% by weight of the original material. Further analysis by HPLC showed that the content of ginsenoside Rb3 in the resultant extract was 98% ().
Chemical reagents
Thiobarbituric acid, nitroblue tetrazolium and triphenyltetrazolium chloride were bought from Sigma Co. (St. Louis, MO). Carboxymethyl cellulose sodium salt (CMC-Na) was from Shanghai Jinshan Chemical Co., Ltd. (Shanghai, China). Creatine kinase (CK-MB) and lactate dehydrogenase (LDH) diagnostic biochemical assay kits were obtained from Biosino Biotechnology Company Ltd. (Beijing, China). Endothelin RIA kit was bought from Institute of Dong Ya Immunotechnology, Beijing, China. Angiotensin II RIA kit was obtained from Institute of the North Immune Reagents, Beijing, China. Other reagents were of commercially analytical grade.
Experimental protocols
Fifty rats were randomly divided into five groups with 10 animals in each group: control group (Con) and ischemia-reperfusion group, rats were orally administrated with 0.5% CMC-Na daily for 3 days, ginsenoside Rb3 groups, rats were orally administrated with Rb3 at dose of 5, 10 or 20 mg/kg, respectively, daily for 3 days. Myocardial ischemia-reperfusion model was produced by ligation of the left coronary artery for 30 min followed by reperfusion 24 h. Briefly, 2 h after last dose, rats were anesthetized with chloralose (300 mg/kg, intraperitoneally). Endotracheal intubation was performed under direct visualization. A thoracotomy was performed in the fourth intercostal space under sterile conditions. A 1.0 silk thread was passed around the left anterior descending coronary artery near its origin using a tapered needle. Both ends of the thread were passed through a 1 cm long polyethylene tube (outer diameter, 2.0 mm), which was used to ligate the coronary artery by pulling the thread. After 30 min of ischemia, reperfusion was established by loosening the thread for 24 h. Body temperature of the animals was measured by an electric thermometer placed in the rectum and maintained at 37°C by a heating pad placed under the rats. Sham-operated rats were subjected to the same procedures without left anterior descending coronary artery ligation.
Measurement of myocardial infarct size
At 24 h after reperfusion, the heart was harvested and the ventricle of heart was cut from the apex to the base into four transverse slices and incubated in 1% (w/v) triphenyltetrazolium chloride for 10 min. By this staining, normal myocardium appeared brick red and the infarct area was unstained. The slices were immediately fixed with 10% formalin for 24 h. The ischemic zone was dissected from nonischemic zone by the same lab assistant who was blind to the treatment. Myocardial infarct size was calculated as: weight of ischemic zone/total weight of the ventricle × 100% (CitationHaiyun et al., 2004).
Assay of the levels of CK-MB, LDH, endothelin and angiotensin II in serum
To measure the myocardial specific enzymes, including the activities of CK-MB and LDH, blood samples were collected at the end of experiment. The samples were left at room temperature for 1 h to allow complete clotting and then centrifugated at 1000g, 4°C, for 15 min. Then the serum was removed and stored at −80°C. The activities of CK-MB and LDH were analyzed using commercial kits by employing an automatic biochemical analyzer (COBAS-FARA, Germany). According to the manufacturer’s instructions, the levels of endothelin and angiotensin II in serum were determined, respectively.
Histopathological examination
For light microscopic evaluation, tissue sections from the left ventricles were fixed in phosphate buffered 10% formalin solution. Paraffin embedded specimens were cut into 5 μm thick sections and stained with hematoxylin-eosin. The sections were examined by an experienced observer who was blind to the treatment under light microscope and then photomicrographs were taken.
Assay of MDA and SOD in left ventricle
The preparation of left ventricles was as follows. Five hundred milligram of left ventricles was homogenized in nine volumes of ice-cold saline and centrifuged at 1000g, 4°C, for 15 min. The supernatants were removed and stored at −80°C for analysis. The amount of protein in supernatant was measured according to the previous method (CitationLowry et al., 1951) using bovine serum albumin as standard. The determination of malonyldialdehyde (MDA) content in left ventricles was performed according to the previous method (CitationOhkawa et al., 1979).Superoxide dismutase (SOD) activity was determined by the nitroblue tetrazolium reduction method (CitationMcCord & Fridovich, 1969).
Statistical analysis
All data were expressed as the means ± standard deviation (SD). Differences among groups were analyzed by one-way analysis of variance followed by least significant difference contrast. Values of p < 0.05 were considered statistically significant.
Results
Effects of ginsenoside Rb3 on myocardial infarct size and CK-MB, LDH activities
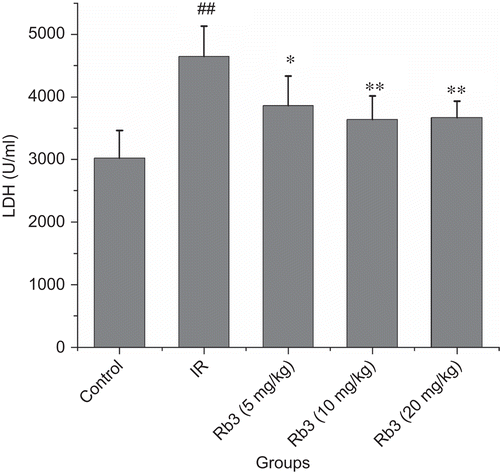
Myocardial ischemia-reperfusion caused a significant increase of myocardial infarct size. Treatment with ginsenoside Rb3 decreased the myocardial infarct size induced by ischemia-reperfusion ( and ). Myocardial ischemia-reperfusion also resulted in an augment in CK-MB and LDH activities. Ginsenoside Rb3 inhibited the increase of CK-MB and LDH activities ( and ).
Figure 2. Effects of ginsenoside Rb3 on myocardial infarct size in myocardial ischemia-reperfusion injury in rats. The myocardial infarct size was stained by triphenyltetrazolium chloride. (A) Control group. (B) Myocardial ischemia-reperfusion group. (C) Ginsenoside Rb3 (5 mg/kg) group. (D) Ginsenoside Rb3 (10 mg/kg) group. (E) Ginsenoside Rb3 (20 mg/kg) group.
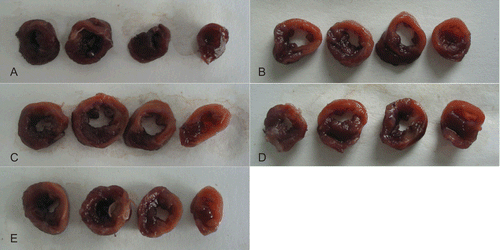
Figure 3. Effects of ginsenoside Rb3 on myocardial infarct size in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
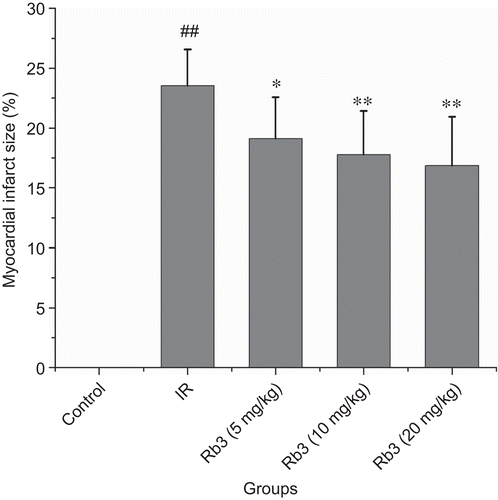
Figure 4. Effects of ginsenoside Rb3 on CK-MB activity in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
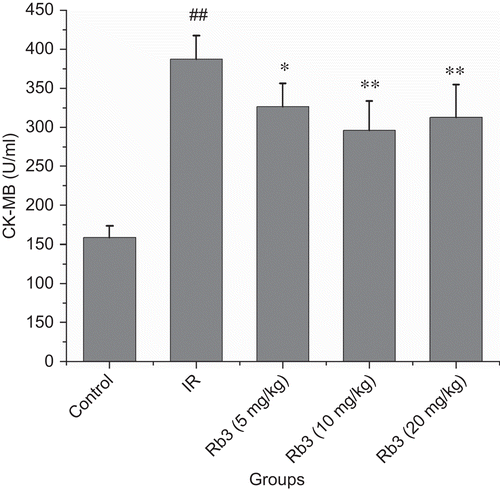
Figure 5. Effects of ginsenoside Rb3 on LDH activity in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
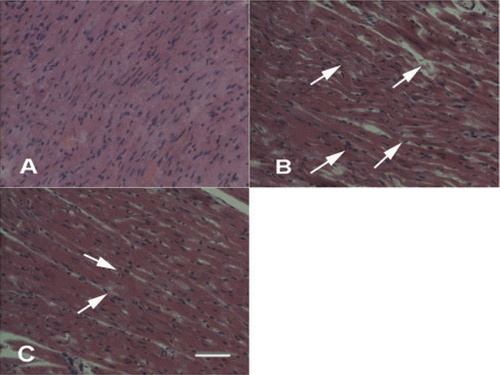
Effects of ginsenoside Rb3 on pathological changes in left ventricle
In control group, myocardium showed clear integrity of myocardial cell membrane and no inflammatory cell infiltration. Left ventricles from myocardial ischemia-reperfusion rats showed widespread myocardial structure disorder and subendocardial necrosis, which was related to infiltration with neutrophil granulocytes and myofibrillar fracture. The rats in ginsenoside Rb3 (5 or 10 mg/kg) groups exhibited mild necrosis and less infiltration of inflammatory cells. Left ventricles from ginsenoside Rb3 (20 mg/kg) showed nearly normal myofibrillar structure with few neutrophil granulocytes infiltration ().
Figure 6. Effects of ginsenoside Rb3 on pathological changes of left ventricle in myocardial ischemia-reperfusion injury in rats. (H&E, 400×). (A) Control group showed clear integrity of myocardial cell membrane, and no inflammatory cell infiltration. (B) Myocardial ischemia-reperfusion group showed widespread myocardial structure disorder and subendocardial necrosis, which is related to infiltration with neutrophil granulocytes and myofibrillar fracture. (C) Ginsenoside Rb3 group showed mild necrosis and less infiltration of inflammatory cells. Bar = 400 μm.
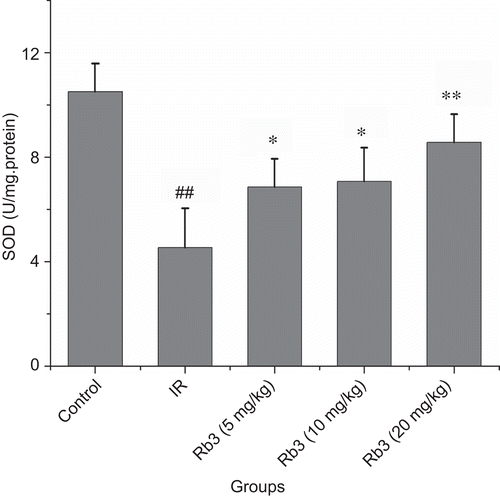
Effects of ginsenoside Rb3 on MDA and SOD in left ventricle
Compared with the control group, MDA content was significantly increased following myocardial ischemia-reperfusion. Administration of ginsenoside Rb3 at dose of 5, 10 or 20 mg/kg attenuated the increase of MDA content induced by myocardial ischemia-reperfusion (). Compared with the control, a significant reduction in the SOD activity in myocardial ischemia-reperfusion rats was found. In contrast, administration of ginsenoside Rb3 to myocardial ischemia-reperfusion animals partially prevented the reduction of SOD activity ().
Figure 7. Effects of ginsenoside Rb3 on MDA content in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
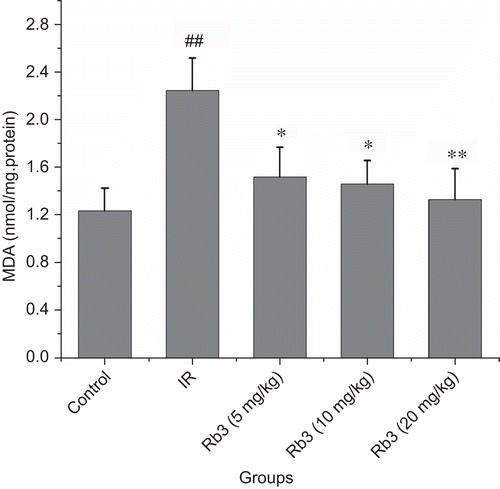
Figure 8. Effects of ginsenoside Rb3 on SOD activity in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
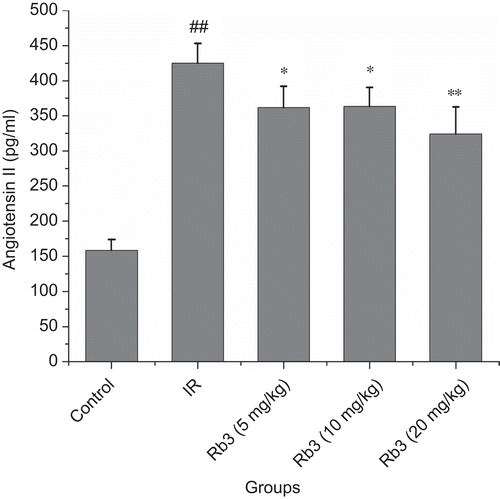
Effects of ginsenoside Rb3 on endothelin and angiotensin II levels in serum
As shows in and , the levels of endothelin and angiotensin II in myocardial ischemia-reperfusion rats significantly increased. Compared with myocardial ischemia-reperfusion group, the levels of endothelin and angiotensin II were decreased in ginsenoside Rb3 groups.
Figure 9. Effects of ginsenoside Rb3 on endothelin level in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
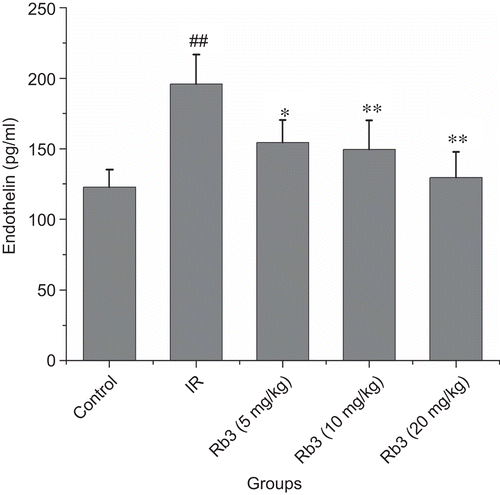
Figure 10. Effects of ginsenoside Rb3 on angiotensin II level in myocardial ischemia-reperfusion injury in rats. Data were expressed as the mean ± SD (n = 8–10). Statistical significances were determined using one-way analysis of variance (ANOVA) followed by the least significant difference test. ##p < 0.01 compared with control group; *p < 0.05, **p < 0.01 compared with myocardial ischemia-reperfusion group.
Discussion
In this study, we demonstrated that ginsenoside Rb3 effectively reduced myocardial infarct size in myocardial ischemia-reperfusion animal model in vivo. Oxygen free radicals are a key factor involved in myocardial ischemia-reperfusion injury. Renin–angiotensin-aldosterone system and endothelin not only increase infarct size but also lead to microvascular obstruction (CitationNiccoli et al., 2009). Our study also indicated that ginsenoside Rb3 can alleviate the changes of endothelin and angiotensin II induced by ischemia and reperfusion. To our knowledge, this is the first ever study conducted to report the cardioprotective effect of ginsenoside Rb3 on the basis of myocardial infarct size and histopathological changes in myocardial ischemia-reperfusion rats.
Myocardial cell contains marker enzymes such as CK-MB and LDH. Ischemia-reperfusion injury makes cell membrane become permeable or rupture, which results in the leakage of these enzymes into blood. Hence, the CK-MB and LDH activities in serum reflect the degree of myocardial injury. Our results showed myocardial ischemia-reperfusion caused significant elevation of CK-MB and LDH activities. However, administration with ginsenoside Rb3 at dose of 5, 10 or 20 mg/kg significantly lowered the ischemia and reperfusion-induced elevation of CK-MB and LDH activities. Left ventricles from myocardial ischemia-reperfusion rats showed widespread myocardial structure disorder and subendocardial necrosis. Administration with ginsenoside Rb3 significantly alleviated the ischemia and reperfusion-induced myocardial injury. These results further confirmed the cardioprotective property of ginsenoside Rb3.
Reintroduction of abundant oxygen at the onset of reperfusion evokes within the first few minutes of reflow a burst of potent free radicals. The release of free radicals in the early phase of reperfusion, in combination with the ischemia and reperfusion-induced decrease in antioxidant activity, renders the myocardium extremely vulnerable (CitationMoens et al., 2005). Overproduction of reactive oxygen species can result in lipid peroxidative process. MDA is a major lipid peroxidant end product. SOD constitutes the first defense line against oxidative injury, decomposing superoxide anion and hydrogen dioxide before their interaction to form the more harmful hydroxyl radical (CitationJi et al., 1988). In this study, MDA content was higher while SOD activity was lower in myocardial ischemia-reperfusion group than that in control group. Administration with ginsenoside Rb3 at dose of 5, 10 or 20 mg/kg not only reduced MDA content but also increased SOD activity. The findings indicated that the cardioprotective property of ginsenoside Rb3 was associated with its antioxidant activity, at least, in part.
Recently, there are both a number of studies reported that the renin-angiotensin system was involved in the pathogenesis of ischemia and reperfusion-induced myocardial injury (CitationNeves et al., 1997; Citationde Gusmão et al., 2005). Angiotensin II, the key product of the renin–angiotensin system, increases intracellular calcium levels of myocytes and smooth muscle cells, leading to impairment of diastolic function, and coronary vasoconstriction. Endothelin including ET-1, ET-2 and ET-3 is the most potent vasoconstrictors. ET-1 is abundant isoform in cardiovascular system. ET-1 is released from cardiomyocytes and vascular cells on account of stimulus such as hypoxia or myocardial ischemia-reperfusion. Ischemic insult has been found to be one of the contributing factors for 2–6-fold increase in ET-1 release (CitationOzdemir et al., 2006). This increased biosynthesis of ET during ischemia is considered as a major candidate for the microcirculatory dysfunction. In accordance with previous studies, we also observed an increase in levels of ET-1 and angiotensin II. However, administration of ginsenoside Rb3 significantly blocks the changes of ET-1 and angiotensin II induced by myocardial ischemia-reperfusion. Therefore, the cardioprotection exerted by ginsenoside Rb3 might be implicated in the microcirculatory improvement.
Conclusion
Our data showed that ginsenoside Rb3 is effective against myocardial ischemia-reperfusion injury. The mechanisms mediating these protective effects of ginsenoside Rb3 may be through regulating oxidative stress and attenuating ischemia and reperfusion-induced microcirculatory disturbance. This study raises the intriguing possibility of the potential value of ginsenoside Rb3 in protecting myocardial ischemic heart disease.
Declaration of interest
The authors report no conflicts of interest.
References
- Attele AS, Wu JA, Yuan CS. (1999). Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol, 58, 1685–1693.
- Braunwald E, Kloner RA. (1985). Myocardial reperfusion: A double-edged sword? J Clin Invest, 76, 1713–1719.
- de Gusmão FM, Becker C, Carvalho MH, Barros LF. (2005). Angiotensin II inhibition during myocardial ischemia-reperfusion in dogs: Effects on leukocyte infiltration, nitric oxide synthase isoenzymes activity and left ventricular ejection fraction. Int J Cardiol, 100, 363–370.
- Haiyun L, Yijia L, Honggang L, Honghai W. (2004). Protective effect of total flavones from Elsholtzia blanda (TFEB) on myocardial ischemia induced by coronary occlusion in canines. J Ethnopharmacol, 94, 101–107.
- Ji LL, Stratman FW, Lardy HA. (1988). Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch Biochem Biophys, 263, 150–160.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- McCord JM, Fridovich I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem, 244, 6049–6055.
- Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. (2005). Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol, 100, 179–190.
- Neves LA, Almeida AP, Khosla MC, Campagnole-Santos MJ, Santos RA. (1997). Effect of angiotensin-(1-7) on reperfusion arrhythmias in isolated rat hearts. Braz J Med Biol Res, 30, 801–809.
- Niccoli G, Burzotta F, Galiuto L, Crea F. (2009). Myocardial no-reflow in humans. J Am Coll Cardiol, 54, 281–292.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Ozdemir R, Parlakpinar H, Polat A, Colak C, Ermis N, Acet A. (2006). Selective endothelin a (ETA) receptor antagonist (BQ-123) reduces both myocardial infarct size and oxidant injury. Toxicology, 219, 142–149.
- Wang T, Yu XF, Qu SC, Xu HL, Sui DY. (2010). Ginsenoside Rb3 inhibits angiotensin II-induced vascular smooth muscle cells proliferation. Basic Clin Pharmacol Toxicol, 107, 685–689.
- Wang Z, Li M, Wu WK, Tan HM, Geng DF. (2008). Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther, 22, 443–452.
- Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan CS. (2006). Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol, 532, 201–207.
- Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen CJ. (2004). Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit, 10, RA187–RA192.