Abstract
Context: Tanacetum parthenium Schultz Bip. (Asteraceae) is an aromatic perennial plant, widely distributed in the northern hemisphere. This species traditionally has been used in insecticides, cosmetics, balsams, dyes, medicines and preservatives.
Material and methods: The essential oil of T. parthenium was obtained by hydrodistillation in three developmental stages and analyzed by gas chromatography-mass spectrometry. The antibacterial activity of the oils was investigated against four Gram-positive and four Gram-negative bacteria. The oil was tested for cytotoxicity against THP-1 cells using the Trypan blue assay.
Results: Twenty-nine components were identified in the essential oil; the highest amount was extracted at the flowering stage. The main component, in the flowering stage, was camphor (18.94%) and other major components were bornyl acetate (18.35%), camphene (13.74%), bornyl isovalerate (3.15%), borneol (10.93%), juniper camphor (6.23%) and β-eudesmol (2.65%). Minimum inhibitory concentration of essential oil was evaluated from 4 µL mL−1 against Staphylococcus subtilis to 38 µL mL−1 against Entrobacter aerogenes. Toxicity assay showed that the oil has no significant toxicity at 5–15% v/v concentrations on THP-1 cells.
Discussion and conclusion: This study demonstrates the occurrence of camphor/bornyl acetate chemotype of T. parthenium in western regions of Iran. The finding showed also the studied oils have relatively good antibacterial activity without significant toxicity, thus have great potentiality to be used as natural health product.
Introduction
Asteraceae is the largest plant family; its many genera and species, worldwide distribution, and the fact that it comprises many useful plants have made it the subject of many studies (CitationFunk et al., 2009). Tanacetum is one of the most important genera. Tanacetum species are aromatic perennial plants, widely distributed in the northern hemisphere (CitationSalamci et al., 2007). They have also been cultured in gardens and used in salads, omelets, and cakes (CitationGrieve, 1984). Some members of this genus have traditionally been used in insecticides, cosmetics, balsams, dyes, medicines, and preservatives (CitationGrieve, 1984).
According to recent studies, essential oils and extracts of some members of the genus Tanacetum exhibit anti-inflammatory (CitationBrown et al., 1997), antibacterial (CitationHolopainen & Kauppinen, 1989; CitationHethelyi et al., 1991; CitationNeszmelyi et al., 1992; CitationNori-Shargh et al., 1999; CitationAkpulat et al., 2005; CitationSalamci et al., 2007), and antifungal effects (CitationHethelyi et al., 1991; CitationNeszmelyi et al., 1992). The chemical composition of essential oils of various Tanacetum species was reported by some earlier researchers (CitationNori-Shargh et al., 1999; CitationBaser et al., 2001; CitationGoren et al., 2001; CitationAkpulat et al., 2005; CitationSalamci et al., 2007). Camphor and chrysanthenyl acetate were determined as the main components of the essential oil of T. parthenium Schultz Bip. originated from England and Netherlands, whereas camphene, p-cymene and (E)-chrysanthenol were found together with the previous compounds in other studies (CitationHendriks et al., 1996; CitationChristensen et al., 1999; CitationSalamci et al., 2007). Several studies have also shown that there are high quantities of sesquiterpenes, lactones, parthenolides, and flavonoids in T. parthenium, which exhibit strong antibacterial activity (CitationSmith & Burford, 1992; CitationAwang, 1998; CitationJain & Kulkarni, 1999; CitationWilliams et al., 1999; CitationLong et al., 2003; CitationSalamci et al., 2007).
The above mentioned studies display the different oil chemotypes, which strongly correlate with a different geographical origin, the plant material, the vegetative period, and the method used for isolating the essential oils. In this research work, we studied the chemical composition of the hydrodistilled oils of T. parthenium of Iranian origin that were collected in the three growth stages, pre-flowering, flowering and post-flowering to compare the results with the previous reports. We also tested the antibacterial activity of prepared essential oils from the species against some bacterial strains.
Material and methods
Plant materials
Aerial parts of Tanacetum parthenium were collected from naturally growing area in Hamedan (west of Iran) in August 2007 at the three developmental stages including pre-flowering, flowering and post-flowering, and dried in the shade. Herbarium vouchers of the species are deposited in the local herbarium in the Department of Biology, Faculty of Science, Bu-Ali Sina University, Iran. The plant materials were authenticated by Prof. Shahin Zarre who is a botanist in Tehran University. The location information is as following:
Tanacetum parthenium Schultz Bip.
Iran, Hamedan, Road of Hamedan to Imamzadeh Mohsen, Soulan village, 2500 m (), Chehregani & Hajisadeghian, 10. 8. 2007, n = x = 9, 2n = 2x = 18.
Isolation procedure
The dried plant samples (500 g) were subjected to direct hydrodistillation (plant material in boiling water) using a Clevenger type apparatus for 4 h. The oils were dried over anhydrous Na2SO4. Hydrodistillation of T. parthenium yielded 0.85, 1.02 and 0.75% (v/w) of essential oils in the pre-flowering, flowering and post-flowering stages, respectively. The yields were based on dry mass of the plant samples. The procedure was repeated three times and data were presented as the mean of the three samples.
Identification of components
Essential oil components were identified by gas chromatography-mass spectrometry via peak matching and by utilizing their retention indices on an Innowax FSC column. n-Alkanes (C9-C20) were used as reference points in the calculation of retention indices (RI) (CitationCurvers et al., 1985). Computer matching against commercial libraries (Wiley and Mass Finder Ver. 2.1) (CitationMcLafferty & Staffer, 1989; CitationJoulain et al., 2001), “Baser Library of Essential Oil Constituents” which was built from genuine compounds and components of known oils, and the reported MS literature library data (CitationJenning & Shibamoto, 1980; CitationJoulain & Koenig, 1998; E.S.O., 2000) were utilized in the final characterization of oil components. The experiments were repeated three times and the data represented as the mean of three samples.
Test organisms for antibacterial assay
The standard strains of the following microorganisms were used as test organisms. Enterobacter aerugenes (PTCC 10009), Serratia marcescens (PTCC 1330), Proteus vulgaris (Lio), Citrobacter amalonaticus (Lio), Bacillus cereus (ATCC 7064), Basilus megaterium (PTCC 1672), Staphylococcous subrogation (Lio), Staphylococcous aureus (ATCC 6633). Some microorganisms were obtained from Persian Type Culture Collection, Tehran, Iran and others locally isolated (Lio). The organisms were sub-cultured in nutrient broth and nutrient agar (Oxiod Ltd.) for using in experiments, while diagnostic sensitivity test agar (DST) (Oxoid Ltd.) was used in antibiotic sensitivity testing.
Sensitivity testing
For bioassays, suspension of ~1.5 × 108 cells per mL in sterile normal saline was prepared as described by CitationForbes et al. (1990). The sensitivity testing was determined using agar-gel diffusion method (CitationRussell and Furr, 1977; CitationChehregani et al., 2010). In each disk 30 µL essential oil were loaded. The minimum inhibitory concentration (MIC) of essential oils was also determined using a twofold dilutions method (CitationNCCLS, 2008). The isolated bacterial strains were first grown in nutrient broth for 18 h before use. The inoculum suspensions were standardized and then tested against the effect of the essential oils at amount of 30 µL for each disk in DST medium. The plates were later incubated at 37 ± 0.5°C for 24 h after which they were observed for zones of inhibition. The effects were compared with the standard antibiotic chloramphenicol at a concentration of 1 mg/mL (CitationKhan & Omosto, 2003). The MICs of essential oils were also determined by tube dilution techniques in Mueller–Hinton broth (Merck) according to CitationNCCLS (2008). The experiments were repeated at least three times for each organism and the data were presented as the mean ± SE of 3–5 samples.
Cytotoxicity assay
A monocyte cell line THP-1, derived from acute monocyte leukemia, was obtained from cell line bank of Pasture Institute, Tehran, Iran. Cells were cultured in RPMI-1640 culture medium (Sigma-Bio Sciences, St. Louis, MO) supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 U/mL). Cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2. After incubation for 10 min at room temperature, the effect of the different concentrations (5, 10 and 15% v/v) of essential oils on THP-1 cells were evaluated by 0.2% (final concentration) Trypan blue dye exclusion analysis. Briefly, the cell number was determined by counting the viable cells in a hemocytometer. The percentage of viable cells from each well after incubation with essential oil was obtained by applying the following equation: % viable cells = (VC/TC) × 100, where VC = viable cells counted and TC = total cells counted (stained plus unstained cells). The experiments were repeated at least three times for each organism and the data were presented as the mean ± SE of 3–5 samples.
Statistical analysis
The differences between the control and experimental groups were analyzed statistically by the Kruskal–Wallis and Mann–Whitney tests (SPSS version 10.0; SPSS Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
Results
The essential oil yields (v/w, on dried mass basis) of Tanacetun parthenium were 0.85, 1.02 and 0.75% in the pre-flowering, flowering and post-flowering stages respectively. The results of the analysis of essential oils are present in . Results showed that totally 29 compounds were identified in the oils of T. parthenium in all above mentioned stages; nevertheless results indicated that there are some differences between the stages. In the stage of pre-flowering, camphor (16.75%) and bornyl acetate (14.31%) were in higher percentage than the other compounds. The presence of camphene (11.12%), juniper camphor (4.71%) and bornyl isovalerate (2.62%) is also important for the oil profile. Our results showed that the amounts of the most compounds were increased in the flowering stages. Camphor (18.94%) and bornyl acetate (18.35%) are the major compounds of essential oil in this stage. The amounts of some chemicals were decreased in the flowering time in comparison to the pre-flowering stage. They are including to α-thujene, β-pinene, broneol, cis-p-menth-2-en-1-ol, terpinen-4-ol, ascaridole and 6,10,14-trimethy l-2-pentadecanone. In the stage of post-flowering, the amounts of most compounds were decreased in the essential oils. However, comphore (12.65%) and bornyl acetate (11.48%) are the prominent chemicals in this stage, too.
Table 1. Chemical composition of aerial parts of T. parthenium in three developmental stages. Amounts were expressed as percentage*.
Antibacterial activities of the oils were studied against 8 bacterial strains (). The oils inhibited the growth of bacterial strains producing a zone diameter of inhibition from 12.0 to 29.0 mm, depending on susceptibility of the tested bacteria. For the oil of flowering stage, the inhibition zones against some bacterial strains (Citrobacter amalonaficus, Bacillus megaterium, Bacillus cereus) were even greater than those of chloramphenicol, and showed a wide inhibition zones at a very low concentration.
Table 2. Antibacterial activity of the essential oil of T. parthenium that was expressed as diameter of inhibition zone (mm) and minimum inhibitory concentration (MIC).
Since the comparison of the size of inhibition zones merely is not trustworthy, the MIC of the plant oils was also determined according to the method of CitationNCCLS (2008). Results indicated that the MIC of plant oils against the tested organisms varied between 4 against Staphylococcus subltilis and 38 Enterobacter aerogenes µL mL−1. The standard chloramphenicol had MIC values varying between 1 and 8 µg mL−1. The results indicated that standard antibiotic chloramphenicol had stranger activity than plant oil against some bacterial strains. Antibacterial effect of the oils is also notable. The lowest MIC (4 µL mL−1) was detected for T. parthenium against Staphylococcus subltilis and for other bacterial strains were as 8 (St. aureus), 16 (Bacilus cereus), 20 (B. megaterium and Proteus vulgaris), and 25 (Citrobacter amalonaficus and Serratia marcescens) ().
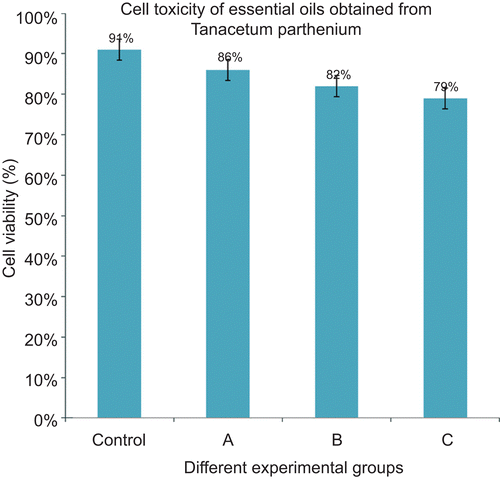
The cytotoxic effect of the studied essential oil was studied by Trypan blue dye exclusion technique (CitationCosta et al., 1999) and results are shown in . The results showed that cell viability was not affected by different concentration of essential oil (5, 10 and 15%) when compared to the control groups. Cell viability was evaluated 91%, in the control group, after 24 h incubation of THP-1 cells with Trypan blue dye. The lowest cell viability (79%) was evaluated for the group that treated with 15% v/v essential oil. Statistical analysis indicated that the reduction of cell viability in the oil-treated groups is not significant (P ≤ 0.05).
Figure 2. Effect of 24-h incubation of essential oil obtained from T. parthenium, at flowering stage, on THP-1 cells by Trypan blue dye exclusion assay expressed as percentage of viable cells in control and test groups. Results indicated that the oil has not significant toxic effect (P ≤ 0.05). 1, Control; (A) the group treated with 5%; (B) the group treated with 10%; (C) the group treated with 15% essential oil. Each datum represented the means ± SE of five samples.
Discussion
The essential oil yields (v/w, on dried mass basis) of Tanacetun parthenium were 0.85, 1.02 and 0.75% in the pre-flowering, flowering and post-flowering stages, respectively; close to those reported for T. argyrophyllum C. Koch (0.96–1.03%) and T. parthenium (0.30–0.83%) (CitationHendriks et al., 1996; CitationGoren et al., 2001). Twenty-nine compounds were identified in the oil of Tanacetum parthenium, at all the above mentioned stages. In the stage of pre-flowering, camphor was determined to be present at a high percentage (16.75%) but it was increased in flowering stage (18.94%) and then was decreased in post-flowering stage (12.65%). Similar data was reported for Artemisia vulgar Linn. (Asteraceae) that showed to have 15.7–23% camphor at pre-flowering stage and 38.7% at flowering stage. It seems that camphor is an essential factor for flowering process in plants (CitationHaider et al., 2003). But there is only a different report that showed camphor is decreased at flowering stage in compared to pre-flowering stage in Salvia officinalis L. (Labiateae) (CitationStancheva et al., 2010).
A similar pattern was observed for some other compounds. For example bornyl acetate and camphene was determined as 14.31 and 11.12%, in pre-flowering stage; they were increased up to 18.3 and 13.74% in flowering stage, and then decreased to 11.8 and 9.50% in post-flowering stage. The higher percentages of bornyl acetate and camphene in the flowering and post-flowering than pre-flowering stage was reported in the oil of Salvia bracteata Banks et Sol. (Labiateae) (CitationAmiri, 2007a) that is accordance with our data. Instead, the amounts of some chemicals were decreased in the flowering time in comparison with pre-flowering stage. They are α-thujene, β-pinene, broneol, cis-p-menth-2-en-1-ol, terpinen-4-ol, ascaridole and 6,10,14-trimethyl-2-pentadecanone that is accordance with the finding of some prior reports (CitationAmiri, 2007a). Unfortunately, the reason for these differences was not clear in the previous literature.
Results of this research work showed that trans-chrysanthenyl acetate was not detected in the oil of T. partheium. However, this component together with camphor has been considered as the characteristic constituents of essential oil in T. parthenium (CitationSmith & Burford, 1992; CitationHendriks et al., 1996). This difference should be due to different geographical location of the studied species. Our data were also differed from those of T. parthenium oil from Danish origin, which contained trans-chrysanthenol (7.2%), trans-chrysanthenyl acetate (15.7%) and more amounts of camphor (26.7%) (CitationChristensen et al., 1999). Camphor (11–16%) also characterizes the essential oils of T. armenum and T. haradjami, whereas it ranged between 0.06 and 73.02% (CitationBaser et al., 2001; CitationKeskitalo et al., 2001) in 20 genotypes of T. vulgare collected from different geographical locations in Finland. The oil obtained from the dried leaves of T. argyrophyllum is reported to contain a high percentage of 1,8-cineole (CitationGoren et al., 2001) but its amounts were few in the our studied plants (0.38–0.58%). Some reports indicated that the genus Tanacetum, rich in cis-thujone (T. praetheritum subsp. massicyticum) and trans-thujone (T. vulgare) (CitationGoren et al., 2001; CitationKeskitalo et al., 2001) but they was absent in the oil profile of the our studied plants. Considerable amounts of cis-thujone (about 12%) have also been found in the essential oils of T. argenteum subsp. canum var. canum and T. balsamita (CitationGoren et al., 2001; CitationBaser et al., 2001). In conclusion, this study demonstrates the occurrence of camphor/bornyl acetate chemotype of T. parthenium in western region of Iran. This is also the first report of oil composition of T. parthenium in different developmental stages.
Antibacterial activity of the oils was studied against eight bacterial strains and results indicated that the oils obtained from the all stages, were able to inhibit the growth of studied bacterial strains. The oil obtained from flowering stage, had the highest inhibition zones. For example, the inhibition zones against Citrobacter amalonaficus, Bacillus megaterium, and Bacillus cereus were even greater than standard chloramphenicol. The MIC of the oils, against the tested organisms, varied between 4 and 38 µL mL−1. The results indicated that although the activity of standard antibiotic chloramphenicol is stranger than to plant oil, against some bacterial strains but antibacterial activity of the oils is also considerable. The lowest MIC (4 µL mL−1) was detected for the oil against Staphylococcus subltilis. Results were also indicated that the oil of flowering stage was more effective than the oils of two other stages. It seems that the reason of is the presence a higher percentage of camphor and bornyl acetate (18.94 and 18.35%) at the flowering stage. Antibacterial effect of camphor was reported previously (CitationAmiri, 2007b). Although there are several reports on the analysis of essential oils from Tanacetum species in the literature, but there are few reports about antibacterial effects of Tanacetum oils against only a limited number of bacteria (CitationKalodera et al., 1997; CitationNori-Shargh et al., 1999; CitationEl-Shazly et al., 2002; CitationAmiri, 2007b). Oxygenated monoterpenes such as camphor, camphene and borneol, which were detected in the oils of T. parthenium, have been also demonstrated to exhibit antibacterial activity (CitationAmiri, 2007b). Antibacterial effect of essential oils containing 1,8-cineole, camphor, borneol and terpinene-4-ol was reported, previously (CitationKordali et al., 2005). Therefore, the broad spectrum antibacterial activity of T. parthenium oils may be attributed to these major components.
The Trypan blue dye exclusion technique was chosen for cytotoxicity assay because it is easy to perform and allows for distinguishing non-viable cells from viable ones by microscopic analysis. Although accurate procedures for determination of cell viability are reported in literature (CitationCosta et al., 1999), the analysis by Trypan blue assay reveals the disruption of cell membrane integrity. Trypan blue staining of non-viable cells is a common procedure used in cell culture research, and it relies on the premise that vital cells will not allow the stain to penetrate through cell membranes (CitationFreshney, 2000). Results of the cytotoxicity assay showed that the toxicity of the studied essential oil is not significant at concentrations of 5–15% (v/v).
Conclusion
This study demonstrates the occurrence of camphor/bornyl acetate chemotype of T. parthenium in western region of Iran. We can also conclude that the essential oil of T. parthenium have relatively good antibacterial activities without significant toxicity, at the applied concentration, and thus have great potentiality to be used as a resource for natural health products. The antibacterial activity of the oil that obtained from flowering stage is greater than others due to greater amounts of camphor and bornyl acetate.
Declaration of interest
This research was supported by a grant provided by the Research Council of Bu-Ali Sina University.
References
- Akpulat AH, Tepe B, Sokmen A, Daferera D, Polissiou M. (2005). Composition of the essential oils of Tanacetum argyrophyllum (C. Koch) Tvzel. var. argyrophyllum and Tanacetum parthenium (L.) Schultz Bip. (Asteraceae) from Turkey. Biochem System Ecol, 33, 511–516.
- Amiri H. (2007a). Quantative and qualative changes of essential oil of Salvia bracteata Bank et Sol. in different growth stages. Daru, 15, 79–82.
- Amiri H. (2007b). Chemical composition, antibacterial and antioxidant activity of essential oil of Tanacetum polycephalum Schutz. Bip. Int J Bot, 3, 321–324.
- Awang DV. (1998). Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz Bip., syn. Chrysanthemum parthenium (L.) (Bernh.). Integr Med, 5, 11–13.
- Baser KHC, Demirci B, Tabanca N, Ozek T, Goren N. (2001). Composition of the essential oils of Tanacetum armenum (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch. & Mey) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. Fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flav Frag J 16, 195–200.
- Brown AMG, Edwards CM, Davey MR, Power JB, Lowe KC. (1997). Effects of extracts of Tanacetum species on human polymorphonuclear leucocyte activity in vitro. Phytother Res 11, 479–484.
- Chehregani A, Mohsenzadeh F, Mirazi N, Hajisadeghian S, Baghali Z. (2010). Chemical composition and antibacterial activity of essential oils of Tripleurospermum disciforme in three developmental stages. Pharm Biol, 48, 1280–1284.
- Christensen LP, Jakobsen HB, Paulsen E, Hodal L, Andersen KE. (1999). Airborne Compositae dermatitis: Monoterpenes and no parthenolide are released from flowering Tanacetum parthenium (feverfew) plants. Arch Dermatol Res, 291, 425–431.
- Costa AO, Assis MC, Marques EA, Plotkowski MC. (1999). Comparative analysis of three methods to assess viability of mammalian cells in culture. Biol Cell, 23, 65–72.
- Curvers J, Rijks J, Cramers C, Knauss K, Larson P. (1985). Temperature programmed retention indices: Calculation from isothermal data. Part 1: Theory. High Res Chromatogr, 8, 607–610.
- E.S.O. (2000). The Complete Database of Essential Oils. Boelens Aroma Chemical Information Service, The Netherlands.
- El-Shazly A, Dorai G, Wink M. (2002). Composition and antimicrobial activity of essential oil and hexane-ether extract of Tanacetum santolinoides (dc.) Feinbr. and Fertig. Z Naturforsch, C, J Biosci, 57, 620–623.
- Funk VA, Susanna A, Stuessy T, Bayer R. (2009). Systematic, Evolution and Biogeography of the Compositae. Vienna, Austria: International Association for Plant Taxonomy.
- Forbes AA, Sahm DF, Weissfeld AS, Trevino EA. (1990). Method for testing antimicrobial effectiveness. In: Baron, EJ, Peterson LR and Finegold SM, ed. Bailey Scott’s Diagnostic Microbiology. Missouri: Mosby Co., 171–194.
- Freshney RI. (2000). Culture of Animal Cells: A Manual of Basic Technique. New York, USA: Wiley-Liss.
- Goren N, Demirci B, Baser KHC. (2001). Composition of the essential oils of Tanacetum spp. from Turkey. Flav Frag J, 16, 191–194.
- Grieve M. (1984). A modern herbal. In: Leyel CF, ed. Tansy. Middlesex: Penguin Books Ltd, 789–790.
- Haider F, Dwivedi PD, Naqvi AA, Bagchi GD. (2003). Essential oil composition of Artemisia vulgaris harvested at different growth periods under Indo-genetic plain conditions. J Essent Oil Res, 15, 376–380.
- Hendriks H, Bos R, Woerdenbag J. (1996). The essential oil of Tanacetum parthenium (L.) Schultz-Bip. Flav Frag J, 11, 367–371.
- Hethelyi E, Tetenyi P, Danos B, Koczka I. (1991). Phytochemical and antimicrobial studies on the essential oils of the Tanacetum vulgare clones by gas chromatography/mass spectrometry. Herba Hungarica, 30, 82–90.
- Holopainen M, Kauppinen V. (1989). Antimicrobial activity of different chemotypes of Finnish tansy. Planta Med, 55, 103–108.
- Jain NK, Kulkarni SK. (1999). Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol, 68, 251–259.
- Jenning WG, Shibamoto T. (1980). Quantitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary GC. New York, USA: Academic Press.
- Joulain D, Koenig WA. (1998). The Atlas of Spectra Data of Sesquiterpene Hydrocarbons. Hamburg, Germany: E.B.Verlag.
- Joulain D, Koenig WA, Hochmuth DH. (2001). Terpenoids and Related Constituents of Essential Oils: Library of Mass Finder 2.1. Hamburg, Germany: E.B.Verlag.
- Kalodera Z, Pepeljnjak S, Blazevic N, Petrak T. (1997). Chemical composition and antimicrobial activity of Tanacetum parthenium essential oil. Pharmazie, 52, 885–886.
- Keskitalo M, Pehu E, Simon JE. (2001). Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem Syst Ecol, 29, 267–285.
- Khan MR, Omosto AD. (2003). Antimicrobial activity of extractives of Sarcocephalus coadunatus. Fitoterapia 74: 4494–4497.
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. (2005). Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem, 53, 1408–1416.
- Long C, Sauleau P, David B, Lavaud C, Cassabois V, Ausseil F, Massiot G. (2003). Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry, 64, 567–569.
- McLafferty FW, Staffer DB. (1989). The Wiley/NBS Registry of Mass Spectral Data. New York, USA: John Wiley and Sons.
- NCCLS. (2008). Performance standards for antimicrobial susceptibility testing; Ninth informational supplement. NCCLS document M100-S9. National Committee for Clinical Laboratory Standards, Wayne, PA.
- Neszmelyi A, Milne GWA, Hethelyi E. (1992). Composition of the essential oil of clone 409 of Tanacetum vulgar and 2D NMR investigation of trans-chrysanthenyl acetate. J Essent Oil Res 4: 243–250.
- Nori-Shargh D, Norouzi-Arasi H, Mirza M, Jaimand K, Mohammadi S. (1999). Chemical composition of the essential oil of Tanacetum polycephalum (Schultz Bip. ssp. heterophyllum). Flav Frag J, 14, 105–106.
- Russell AD, Furr JR. (1977). The antibacterial activity of a new chloroxylenol preparation containing ethylenediamine tetraacetic acid. J Appl Bacteriol, 43, 253–260.
- Salamci E, Kordali S, Kotan R, Cakir A, Kaya Y. (2007). Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem System Ecol, 35, 569–581.
- Smith RM, Burford MD. (1992). Supercritical fluid extraction and gas chromatographic determination of the sesquiterpene lactone parthenolide in the medicinal herb feverfew (Tanacetum parthenium). J Chromatogr, 67, 255–261.
- Stancheva I, Geneva M, Georgiev G, Todorova M, Evstatieva L. (2010). Essential oil variation of Salvia officinalis leaves during vegetation after treatment with foliar fertilizer and thidiazuro. Comm Soil Sci Plant Analysis, 41, 417–423.
- Williams CA, Harborne JB, Geiger H, Hoult JR. (1999). The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry, 51, 417–423.