Abstract
Context: Saffron extract can inhibit the metabolic disorders induced by stress but the mechanism of action of saffron extract in the central nervous system is not clear.
Objective: The present research investigated the effects of saffron water extract and its constituent, safranal on the behavioral and metabolic signs induced by electroshock stress in male Wistar rats (W: 250–300 g).
Materials and methods: Dried saffron material and maceration method was used for extraction. Animals received intra-amygdala (1, 5, and 10 µg/rat) or intraperitoneal (1, 5, and 10 mg/kg) administration of the extract, safranal (Fluka, Germany), or saline 5 or 30 min before stress induction, respectively.
Results: The result showed that stress elevated the corticosterone plasma (115 nmol/L) concentration in the control and intra-amygdala (1, 5, and 10 µg/rat)-treated groups but not in groups that received extract or safranal (55 nmol/L) intraperitoneally (1, 5, and 10 mg/kg). Moreover, anorexia was reduced only in groups that received the extract (1, 5, and 10 mg/kg) or safranal (1, 5, and 10 mg/kg) intraperitoneally (50 sec). Stress increased sniffing, rearing, locomotion, and coping time, which were decreased by intraperitoneal (1, 5, and 10 mg/kg) but not by intra-amygdala (1, 5, and 10 µg/rat) administration of saffron extract and safranal.
Discussion and conclusion: The results revealed that saffron water extract and safranal had an important impact on the reduction of both metabolic and behavioral signs of stress in male Wistar rats. Moreover, the involvement of the amygdala in this observation can be ruled out.
Introduction
Stressful events are considered as one of the most environmental factors that can alter organism’s activity. Stress can interact with several body functions by activation of the nervous as well as the endocrine systems (CitationMcEwen, 2006, Citation2007, Citation2008, Citation2009). Studies concerned with the effects of stress reveal that stress hormones that release during stress are among the important mediators of stress influence (CitationLupien et al., 2009). It is now clear that chronic stress can influence the function and morphology of the cells in different parts of body including adrenal medulla (CitationMcEwen, 2007), as well as several parts of the nervous system (CitationHunter et al., 2007; CitationJohnson et al., 2009). However, protection of the body against stress can also reduce damage induced by stress. In this regard, studies have indicated that glucocorticoid hormones including cortisol and corticosterone are the main mediators for stress-induced tissue damage and other unwanted effects of stress (CitationMcEwen, 2008). Accordingly, if we can inhibit the release of glucocorticoid hormones during chronic stress, then we can inhibit the unwanted effects of stress as well. It is now clear that the synthesis of glucocorticoid hormones in the adrenal cortex is under the influence of µ, κ, and σ opioids and N-methyl-d-aspartate (NMDA) glutamatergic receptors (CitationIyengar et al., 1990). So, inhibition of these receptors may lead to a reduction of corticosterone and cortisol release from the adrenal gland during stress.
Crocus sativus L. (Iridaceae), commonly known as saffron, has been used as a food ingredient for several centuries. The plant was used as an aphrodisiac, antispasmodic, and expectorant in folk medicine (CitationAbdullaev, 1993; CitationAbdullaev & Espinosa-Aguirre, 2004). In modern medicine, antimicrobial (CitationAung et al., 2007) and anticancer (CitationAung et al., 2007; CitationDas et al., 2009; CitationDhar et al., 2009) effects have been described for its extract. It is now clear that saffron water or alcohol extracts may have anti-anxiety (CitationPitsikas et al., 2008; CitationHosseinzadeh & Noraei, 2009) and antidepressant effects (CitationAkhondzadeh et al., 2004; CitationMoshiri et al., 2006; CitationAkhondzadeh Basti et al., 2007). Moreover, the extract can improve the signs of Alzheimer’s disease (CitationAkhondzadeh et al., 2010).
However, despite of comprehensive studies regarding the effects of saffron extract on neuronal function such as memory improvement (CitationAbe & Saito, 2000; CitationPitsikas & Sakellaridis, 2006), it is not clear if the saffron water extract can inhibit side effects of stress or not. These experiments were conducted to determine the effects of saffron water extract on metabolic and behavioral effects of stress.
Material and methods
Animals
Male Wistar rats (W: 235 ± 15 g; Pasture Institute, Tehran, Iran) were used in the present experiments (n = 6–9/each experiment). The animals were kept four per cage with 12/12 h dark and light cycle and free ad libitum access to food and water except during the experiments. During the experiments, animals were kept one per cage for food and water consumption recording. All experiments were conducted in accordance with standard ethical guidelines and approved by Baqyiatallah University of Medical Sciences Committee on the Use and Care of Animals (86/14, March 12, 2008).
Plant material
The saffron used in this study was donated by Talakaran-E-Mazraeh Agricultural Co. (Torbat Heydarieh, Khorasan, Iran). The plant was authenticated by Mohammad Kamalinejad (Department of Pharmacognosy, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran) and a voucher specimen coded P-408 has been deposited at the herbarium of Department of Pharmacognosy, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The flower stigma was used for extraction. The stigma’s extract was prepared as follows: 25 g of dried and milled stigmas were extracted with 250 mL distilled water by maceration procedure. The extract was dried by evaporation in temperature 35°C. The yield of extraction was 6 g of freeze-dried powder for 25 g of the dry stigma. The extract was dissolved in normal saline and was immediately administered intraperitoneally (i.p.) to the animals expressed as milligrams of extract per kilogram body weight. The method of Hooseinzadeh and Noraei (2009) was used for safranal in saffron extract with modifications. Safranal percentage of the extract was calculated as 15.5% and the extract dose used in these experiments was standardized according to its safranal content. Safranal (Fluka Chemical Co., Germany) was also dissolved in normal saline and used immediately after preparation.
Stress induction procedure
Stress was applied to the animals using an apparatus explained in detail by CitationRosales et al. (2002), with modifications. In brief, the communication box (Borj-e-Sanat Co., Tehran, Iran) consists of nine distinct compartment (16 × 16 cm), made by plexiglas with eight holes (2-mm diameter) in their contact sides allowing receiving visual, olfactory, and auditory cues. The compartment floor was equipped by stainless steel rods (4-mm diameter) placed 1.3 cm apart. The rods were attached to the generator (Borj-e-Sanat Co., Tehran, Iran) that was controlled by a PC and produced an electrical current of 0.1 mA to generate an electric foot shock for 10 sec.
Experimental design
Electro foot shock was applied during light period (10:00–11:00). In brief, each animal was transferred to the experimental room 1 h before the experiments were begun for environmental adaptation. Then the stressed animals were placed in the compartments individually and 30 min later an electro foot shock was applied. After electro foot shock termination, the animals were left in the compartment for additional 30 min and then they removed to their home cages. Controls were just placed in the compartment for 60 min without any foot shock. Each animal was randomly assigned stressed or control groups. After stress period termination, animals were kept in their home cages four per cage for additional 14 days. On 21st day, the animals were transferred to the stress box without any foot shock application and their response to the stress-experienced environment was recorded by a video camera. Several dopamine-related behaviors including sniffing, rearing, and locomotion were considered to be analyzed off-line. In addition, metabolic factors including animal’s weight, food, and water intake, the anorexic time (the time elapsed by the animals to start eating chow when they returned to their cages), and plasma corticosterone level were also recorded.
Blood sampling
Blood samples were taken from retroorbital sinus (0.5 mL of the blood in 0.5 mL sodium citrate 1%) between 12:00 and 12:30. The samples were centrifuged in 2500 rpm for 5 min in 4°C and the supernatant serum was collected for corticosterone detection. Corticosterone concentration was determined by ELISA kit (Rat Corticosterone ELISA kit; EIA-4164; DRG Instruments GmbH, Germany) in 450 nm.
Dopamine-related behaviors evaluation
The behavior of each animal was digitally recorded by a video camera for a period of 10 min. The records were later analyzed off-line by a person who was not familiar to the experiments. Dopamine-related behaviors, including sniffing, rearing, and locomotion, were distinguished (CitationFedele et al., 1998).
Surgical procedures
Rats were anesthetized with ketamine hydrochloride (70 mg/kg, i.p.) + xylazine (10 mg/kg, i.p.) and one or two stainless steel cannulas (23-gauge) were placed stereotaxically (Stoelting instruments, Wood Dale, IL) into the basolateral amygdala (BLA). Stainless steel, 23-gauge guide cannulas were implanted bilaterally 0.5 mm above the intended site of injection according to the atlas of CitationPaxinos and Watson (1987). Stereotaxic coordinates for the BLA were incisor bar (−3.3 mm), −2.56 mm anterior to the bregma, ±4.1 mm lateral to the sagittal suture, and 7 mm down from top of the skull. The lengths of the cannulas were considered in the way that the tips of the cannulas were placed 0.5 mm above the amygdala. Cannulas were secured to jewelers’ screws with dental acrylic. After completing the surgery, a dummy inner cannula was inserted into the guide cannula and left in place until injections were made. The length of the dummy cannula matched whit the guide cannula. Animals were allowed 7 days to recover from surgery and anesthesia. For drug infusion, the animals were gently restrained by hand; the stylets were removed from the guide cannulas and replaced by 29-gauge injection needles (0.5 mm below the tip of the guide cannula). The solutions were slowly administered in a total volume of 1 µL/rat (0.5 µL in each side) over a period of 60 sec. Injection needles were left in place for an additional 60 sec to facilitate diffusion of the drugs.
Histology
After the completion of testing, all animals were anesthetized and received a transcardiac perfusion with 0.9% normal saline followed by 10% buffered formalin. The brains were removed, blocked, and cut coronally in 40 µm sections through the cannula placements. The tissues were stained with cresyl violet and were examined by light microscopy by an unfamiliar observer to the behavioral data. Only the animals with correct cannula placements were included in the data analysis ().
Statistical analysis
Data are shown as mean ± SEM. In order to test the hypothesis, one-way analysis of variance (one-way ANOVA) and Tukey’s post hoc test were used. Differences P < 0.05 were considered statistically significant.
Results
Plasma corticosterone level after stress application in the rats treated with saffron water extract and safranal
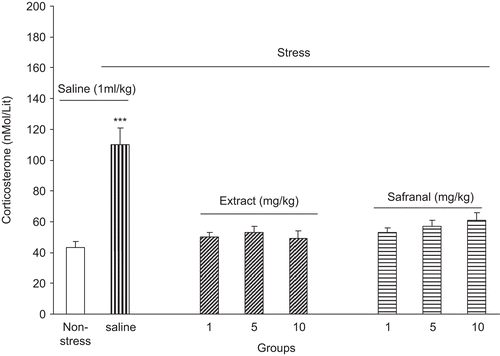
The effects of saffron water extract and safranal on stress-induced blood corticosterone level elevation is shown in . As it is shown in the figure, stress can increase plasma corticosterone level in the control group. However, intraperitoneal administration of saffron water extract or safranal could abolish stress-induced corticosterone plasma level elevation [F(6, 49) = 22.03, P < 0.0001] ().
Figure 2. Plasma corticosterone level increment after foot shock stress in rats received intraperitoneal saffron extract or safranal. Plasma corticosterone level was increased in the saline-treated group but in the extract and safranal-treated groups plasma corticosterone level did not change. Data shown as mean ± SEM, for 8/9 rats. ***p < 0.001 different from non-stressed group.
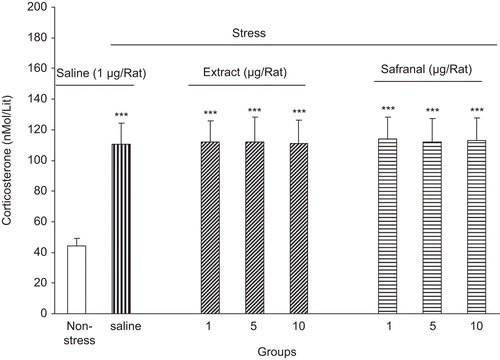
On the other hand, intra-amygdala administration of both saffron extract and safranal did not inhibit the effects of stress [F(6, 49) = 1.09, P > 0.05] ().
Figure 3. Plasma corticosterone level increment after foot shock stress in rats received intra-amygdala saffron extract or safranal. Plasma corticosterone level was increased in the saline as well as extract and safranal-treated groups. Data shown as mean ± SEM, for 6/8 rats. ***p < 0.001 different from non-stressed group.
Effects of intraperitoneal and intra-amygdala saffron water extract and safranal on the stress-induced anorexia
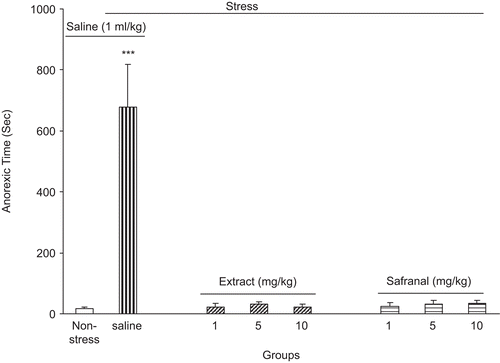
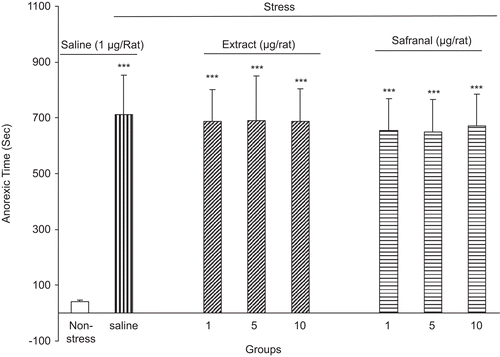
After each session when the animals were returned to their home cages, the time elapsed for initiation of food consumption was recorded. Our data indicated that the control group needed more time for initiation of food intake [F(6, 49) = 19.23, P < 0.0001] ().
Figure 4. The anorexic time (the time elapsed for initiation of food consumption) after foot shock stress in rats received intraperitoneal saffron extract or safranal. The anorexic time was increased in the saline-treated group but in the extract and safranal-treated groups, the anorexic time did not change. Data shown as mean ± SEM for 8/9 rats. ***p < 0.001 different from non-stressed group.
Anorexic time did not differ in the groups that received intra-amygdala administration of saffron extract and safranal [F(6, 49) = 0.35, P > 0.05] ().
Figure 5. The anorexic time (the time elapsed for initiation of food consumption) after foot shock stress in rats received intra-amygdala saffron extract or safranal. The anorexic time was increased in the saline as well as extract and safranal-treated groups. Data shown as mean ± SEM for 6/8 rats. ***p < 0.001 different from non-stressed group.
Effects of intraperitoneal and intra-amygdala saffron water extract and safranal on the stress-induced change in dopamine-related behaviors
shows that dopamine-related behaviors were also changed in the stressed group. Intraperitoneal injections of the saffron water extract completely inhibit these behaviors. For sniffing: [F(6, 49) = 12.76, P < 0.0001], for rearing: [F(6, 49) = 18.3, P < 0.0001], for locomotion: [F(6, 49) = 12.15, P < 0.001], for coping time: [F(6, 49) = 12.31, P < 0.0001] ().
Table 1. Effects of intraperitoneal administration of saffron water extract on dopamine-related behaviors modified by foot shock stress in rats.
However, intra-amygdala administration of the extract did not change the sniffing, rearing, locomotion, and coping time in the animals. For sniffing: [F(6, 49) = 0.09, P > 0.05], for rearing: [F(6, 49) = 1.1, P > 0.05], for locomotion: [F(6, 49) = 1.05, P > 0.05], for coping time: [F(6, 49) = 1.011, P > 0.05] ().
Table 2. Effects of intra-amygdala administration of saffron water extract on dopamine-related behaviors modified by foot shock stress in rats.
Similarly, intraperitoneal injections of safranal inhibit sniffing, rearing, locomotion, and coping time in the animals after exposure to stress. For sniffing: [F(6, 49) = 12.76, P < 0.0001], for rearing: [F(6, 49) = 18.3, P < 0.0001], for locomotion: [F(6, 49) = 12.35, P < 0.0001], for coping time: [F(6, 49) = 12.31, P < 0.0001] ().
Table 3. Effects of intraperitoneal administration of safranal on dopamine-related behaviors modified by foot shock stress in rats.
However, intra-amygdala administration of safranal did not change the sniffing, rearing, locomotion and coping time in the animals. For sniffing: [F(6, 49) = 0.09, P > 0.05], for rearing: [F(6, 49) = 1.1, P > 0.05], for locomotion: [F(6, 49) = 1.05, P > 0.05], for coping time: [F(6, 49) = 1.011, P > 0.05] ().
Table 4. Effects of intra-amygdala administration of safranal on dopamine-related behaviors modified by foot shock stress in rats.
Discussion
Our present data revealed an important role for saffron water extract and safranal—as an important constituent of saffron extract—on amelioration of both metabolic and behavioral signs of stress.
The present data are in agreement with previous studies, which have shown that stress can induce corticosterone release from the adrenal gland in the rodents (CitationMcEwen, 2006, Citation2007). Studies have shown that corticosterone plasma level increases in response to the stressful events in rodents and HPA axis is responsible for hormone increment (CitationMcEwen, 2008, Citation2009). The same mechanism can be considered for response observed in our experiments as well. In addition, our data show that stress can induce anorexia in the animals as it is shown by increasing the time for beginning of food intake after termination of stress, for example, anorexic time. In accordance with our finding, other investigators also insist that stress can induce anorexia both in human and animals (CitationMorley et al., 1981; CitationAdam & Epel, 2007). However, it is generally accepted that stress can induce a response that originates from hypothalamus, passes through pituitary gland, and terminates in adrenal gland, an axis that is called HPA axis (CitationMcEwen, 2008). The corticotropin-releasing factor (CRF) released from hypothalamus has been considered as the main responsible element for anorexia during stress (CitationKoob, 2008; CitationMcEwen, 2009), which may be the responsible element in our experiments as well. In this direction, our data also show that food intake and weight gain decrease in the stressed animals. The observed signs can be attributed to the anorexia induced by stress and the same mechanism(s) may also be involved in this issue. Moreover, the present data showed that dopamine-related behaviors including sniffing, rearing, locomotion, and coping time were also changed in stressed animals. Dopamine-related behaviors are among the important signs of stress initiated from several parts of the central nervous system (CNS) including amygdala, nucleus accumbens, ventral tegmental area, and prefrontal cortex, which collectively called the brain stress system (CitationKoob, 2008; CitationRadley et al., 2008; CitationMcEwen, 2009; CitationRoozendaal et al., 2009). The exact mechanism(s) that underlies these behaviors is not fully understood but several studies indicated that alteration in brain glutamate neurotransmission may be the cause of changes observed (CitationHunter et al., 2007; CitationMcEwen, 2008). These alterations are thought to be mediated by corticosterone level increment after stress (CitationLupien et al., 2009; CitationMcEwen, 2009; CitationYuen et al., 2009).
Our data showed that intraperitoneal pretreatment with saffron water extract and safranal can inhibit all of the signs observed in the stressed animals. It is an interesting finding showing that saffron extract may be a useful tool for reduction of both metabolic and behavioral stress side effects. The effects of saffron extract on several functions of CNS including learning and memory improvement (CitationPitsikas & Sakellaridis, 2006; CitationPitsikas et al., 2007), reduction of anxiety signs (CitationPitsikas et al., 2008; CitationHosseinzadeh & Noraei, 2009), improvement of depression (CitationRios et al., 1996; CitationSchmidt et al., 2007), and Alzheimer’s disease (CitationAkhondzadeh et al., 2010) have been indicated in previous studies in human and animal models. In addition, data exist showed that saffron extract can inhibit sigma opioid and NMDA glutamate receptors in vivo (CitationLechtenberg et al., 2008). Moreover, it is now clear that the extract can reduce DNA damage and histone H1 function (CitationLai and Roy, 2004), which may indicate its anticancer properties. Inhibition of stress-induced corticosterone release in the animals receiving different doses of saffron extract and/or safranal indicates that the extract or one of its constituents (probably safranal?) can interact with a part (or all parts?) of the HPA axis and inhibit its function, which reduces corticosterone release from adrenal gland as a result. In this way, CitationLechtenberg and colleagues (2008) showed that saffron extract can inhibit NMDA glutamate and sigma opioid receptors in the rat. In addition, some investigators have shown that regulation of opioid receptors plays a key role in regulation of corticosterone release from adrenal gland (CitationIyengar et al., 1990). It is likely that the extract may inhibit corticosterone release by such mechanism. On the other hand, the inhibition of stress-induced anorexia indicates that the effect of extract is more likely to be a central than just is a peripheral interaction. Studies indicated that activation of HPA axis during stress leads to a CRF release, which in fact reduces appetite to food in the animals and human (CitationKoob, 2008). Inhibition of stress-induced anorexia by the extract means that activity of HPA axis is reduced under extract influence and probably reduction of CRF release as a result. However, the exact mechanism that is responsible for this inhibition is not clear and needs more experiments. In this regard, animals weight gain and food intake were improved in the extract and safranal-treated animals, which further explained the role of these agents on anorexia induced by stress. The effects observed from safranal may indicate that safranal is an active component of the saffron extract in reduction of metabolic and behavioral side effects observed in the present study.
In the next part of experiments, we observed that dopamine-related behaviors that were among the functional indicators for mesolimbic dopamine system were altered in the animals pretreated with saffron extract and safranal. This data further indicated that the effects of saffron extract and safranal may at least partially mediate in the CNS. Dopamine-related behaviors have been postulated to be changed during stress (CitationFedele et al., 1998). These changes are the manifestations of the effects of CRF, polyopiomelanocortin (POMC), and corticosterone released during stress from the hypothalamus, pituitary, and adrenal gland, respectively (CitationKoob, 2008). As proposed above, the extract can interact with glutamate and opioid receptors in the brain and one can conclude that the interaction of the extract with these receptors may be the cause of reduction of stress-induced dopamine-related behaviors changes in our experiments. However, more experiments are needed for further clear-cut clarification of the effect of the extract on stress.
Finally, our study showed that intra-amygdala injections of the extract and/or safranal cannot inhibit the metabolic or behavioral side effects of the stress in the rats. These findings may indicate that amygdala is not the site of action of the extract and safranal in reduction of stress side effects. Previous studies have shown that amygdala plays a central role in mediation of brain response to stress (CitationMcEwen, 2009). However, despite of extensive investigations indicating the role of different parts of amygdala in integration of response to stress, our results showed that intra-amygdaloid complex injections of safranal and water extract of saffron could not inhibit the alterations in dopamine-related behaviors including rearing, sniffing, coping, and locomotion induced by stress. So, one can conclude that the amygdala did not share in the extract and safranal effects on stress amelioration; we proposed that the other possible sites within the CNS (such as hippocampus) must be considered for investigation in this regard in future. It must be noted that intra-amygdala injections of the saffron extract and/or safranal might led to some behavioral and/or biochemical changes; however, we did not observe any behavioral changes after intra-amygdala injections of the saffron extract and/or safranal but we suggested that biochemical changes must also be checked in future experiments.
In conclusion, it is clear from the present study that the water extract of saffron and safranal can inhibit both the metabolic and behavioral effects of the foot shock stress in the rats when injected intraperitoneally but not intra-amygdala. The effects observed may be due to interaction of the extract and safranal with possibly NMDA and opioid receptors located both in the CNS and peripheral.
Acknowledgement
This study was supported by a grant from Applied Neuroscience Research Center, Baqyiatallah (a.s.) University of Medical Sciences and Neuroscience Research Center, Shahid Beheshti University of Medical Sciences.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Abdullaev FI. (1993). Biological effects of saffron. Biofactors, 4, 83–86.
- Abdullaev FI, Espinosa-Aguirre JJ. (2004). Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev, 28, 426–432.
- Abe K, Saito H. (2000). Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res, 14, 149–152.
- Adam TC, Epel ES. (2007). Stress, eating and the reward system. Physiol Behav, 91, 449–458.
- Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. (2004). Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement Altern Med, 4, 12.
- Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, Rezazadeh SA, Yousefi A, Zare F, Moradi A, Vossoughi A. (2010). A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology (Berl), 207, 637–643.
- Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S. (2007). Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry, 31, 439–442.
- Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama CY, Yuan CS. (2007). Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol, 29, 175–180.
- Das I, Das S, Saha T. (2009). Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: A histopathological study. Acta Histochem, 112, 317–327.
- Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, Van Veldhuizen P, Campbell DR, Banerjee SK. (2009). Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther, 8, 315–323.
- Fedele E, Varnier G, Ansaldo MA, Raiteri M. (1998). Nicotine administration stimulates the in vivo N-methyl-d-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol, 125, 1042–1048.
- Hosseinzadeh H, Noraei NB. (2009). Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res, 23, 768–774.
- Hunter RG, Bellani R, Bloss E, Costa A, Romeo RD, McEwen BS. (2007). Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res, 1152, 234–240.
- Iyengar S, Mick S, Dilworth V, Michel J, Rao TS, Farah JM, Wood PL. (1990). Sigma receptors modulate the hypothalamic–pituitary–adrenal (HPA) axis centrally: Evidence for a functional interaction with NMDA receptors, in vivo. Neuropharmacology, 29, 299–303.
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT. (2009). Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience, 163, 34–39.
- Koob GF. (2008). Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism. Proc Natl Acad Sci USA, 105, 8809–8810.
- Lai PK, Roy J. (2004). Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem, 11, 1451–1460.
- Lechtenberg M, Schepmann D, Niehues M, Hellenbrand N, Wünsch B, Hensel A. (2008). Quality and functionality of saffron: quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and sigma1 (sigma-1) receptors. Planta Med, 74, 764–772.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci, 10, 434–445.
- McEwen BS. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol, 583, 174–185.
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev, 87, 873–904.
- McEwen BS. (2006). Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci, 8, 367–381.
- McEwen BS. (2009). The brain is the central organ of stress and adaptation. Neuroimage, 47, 911–913.
- Morley JE, Levine AS, Rowland NE. (1981). Stress induced eating. Life Sci, 32, 2169–2182.
- Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S. (2006). Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine, 13, 607–611.
- Paxinos G, Watson D. (1987). The Rat Brain in Stereotaxic Coordinates, 2nd Edition, Academic Press, New York.
- Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, Sakellaridis N. (2008). Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine, 15, 1135–1139.
- Pitsikas N, Sakellaridis N. (2006). Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res, 173, 112–115.
- Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. (2007). Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory. Behav Brain Res, 183, 141–146.
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. (2008). Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol, 507, 1141–1150.
- Rios JL, Recio MC, Manez S. (1996). An update review of saffron and its active constituent. Phytother Res, 10, 189–193.
- Roozendaal B, McEwen BS, Chattarji S. (2009). Stress, memory and the amygdala. Nat Rev Neurosci, 10, 423–433.
- Rosales VP, Ikeda K, Hizaki K, Naruo T, Nozoe S, Ito G. (2002). Emotional stress and brux-like activity of the masseter muscle in rats. Eur J Orthod, 24, 107–117.
- Schmidt M, Betti G, Hensel A. (2007). Saffron in phytotherapy: Pharmacology and clinical uses. Wien Med Wochenschr, 157, 315–319.
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA, 106, 14075–14079.