Abstract
Context: Liver disease is a serious ailment and the scenario is worsened by the lack of precise therapeutic regimens. Currently available therapies for liver ailments are not apposite and systemic toxicity inhibits their long term use. Medicinal plants have been traditionally used for treating liver diseases since centuries as the toxicity factor appears to be on the lower side.
Objective: Several phytochemials have been identified which have significant hepatoprotective activity with minimal systemic adverse effects which could limit their long term use. The scenario calls for extensive investigations which can lead to development of lead molecules for hepatoprotective molecules of future. This review deals with the biological activity, mode of action and toxicity and forthcoming application of some of these leads.
Methods: These generally have strong antioxidative potential and cause induction of antioxidant enzymes like superoxide dismutase, reduced glutathione and catalase. Additional mechanisms of hepatoprotection include stimulation of heme oxygenase-1 activity, inhibition of nitric oxide production, hepatocyte apoptosis and nuclear factor-κB activation.
Results and conclusion: Out of the several leads obtained from plant sources as potential hepatoprotective agents, silymarin, andrographolide, neoandrographolide, curcumin, picroside, kutkoside, phyllanthin, hypophyllanthin, and glycyrrhizin have been established as potent hepatoprotective agents. The hepatoprotective potential of several herbal medicines has been clinically evaluated. Significant efficacy has been seen with silymarin, glycyrrhizin and Liv-52 in treatment of hepatitis, alcoholic liver disease and liver cirrhosis.
Introduction
Chronic liver diseases represent a major health burden worldwide, with liver cirrhosis being the ninth leading cause of death in Western countries (CitationKim et al., 2002). Chronic viral hepatitis B and C, alcoholic liver disease, non-alcoholic fatty liver disease, and hepatocellular carcinoma are the major entities and many problems remain unresolved. Treatment options for common liver diseases such as cirrhosis, fatty liver, and chronic hepatitis are often limited in efficacy, carry the risk of adverse effects and are often too costly, especially for the developing world. The effectiveness of treatments such as interferon, colchicine, penicillamine and corticosteroids are inconsistent and the incidence of side-effects profound. Physicians and patients are in need of effective therapeutic agents with a low incidence of side-effects. Herbal medicines potentially constitute such a group. In recent years many researchers have examined the effects of plants used traditionally by indigenous healers to support liver function and treat diseases of the liver (CitationSchuppan et al., 1999). In most cases, research has confirmed traditional experience and wisdom by discovering the mechanisms and modes of action of these plants as well as reaffirming the therapeutic effectiveness of certain plants or plant extracts in clinical studies (CitationStrader et al., 2002).
Therefore, treating liver diseases with plant-derived compounds which are accessible and do not require arduous synthetic steps seems highly attractive (CitationFogden & Neuberger, 2003). In spite of the advances in conventional medicine in the last decades, research professionals are paying increasing attention to phytomedicine. Several recent surveys from Europe and the United States have demonstrated a sharp rise in the use of phytomedicine within a few years, and up to 65% of patients with liver disease take herbal preparations (CitationDe Smet, 2002). Many factors contribute to herbal medicine’s appeal, including the claim that herbal medicine may both treat and prevent diseases. This adds to a deep belief that these treatments are safe because they are “natural” and, therefore, harmless alternative to conventional medicine. In addition, herbal products are often exempt from rigorous regulations, such as in the US, and prescriptions are usually not required for these inexpensive products. The following review describes the current scientific evidence regarding herbal drugs and the liver, especially in regard to their presumed beneficial effect.
Hepatotoxicity
Liver is one of the largest organs in the human body that performs numerous interrelated vital functions. Some of the commonly known disorders include viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, autoimmune liver disease, metabolic liver disease, drug induced liver injury, toxin-induced liver injury, etc. As a consequence of chronic liver disease, patient may develop portal hypertension and liver cirrhosis. Liver toxicity mainly occurs due to alcohol, viral and induced by drugs.
Alcoholic liver disease, including acute alcoholic hepatitis and alcoholic cirrhosis, is a major cause of morbidity and mortality in the Western world. In alcoholic liver disease, oxidative stress is caused by pro-oxidant formation, inadequate intake of antioxidants, antioxidant depletion, and alcohol-mediated inhibition of glutathione synthesis. Acetaldehyde is the most important metabolite of ethanol leading to liver damage. Alcohol-induced liver diseases are mediated by cytokines, which are secreted by liver and other parts of the body. In the liver, persistent cytokine secretion results in chronic inflammation leading to the conditions such as hepatitis, fibrosis, and cirrhosis. Cytokines, tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) regulate apoptosis, which is in part responsible for alcohol-induced destruction of liver tissue. Fibrogenesis within the liver takes place due to the activation of collagen-producing stellate cells which is mediated through expression of interleukins (ILs), such as IL-1, IL-6, IL-8 ultimately causing precipitation of collagen deposition (CitationTome & Lucey, 2004). Viral hepatitis is responsible for both acute and chronic liver diseases. Hepatitis A is caused by hepatitis A virus, a picornavirus transmitted by the fecal-oral route often associated with ingestion of contaminated food. Hepatitis B is caused by hepatitis B virus, a hepadnavirus that can cause both acute and chronic hepatitis. Identified methods of transmission include blood, tattoos, sexual, or via mother to child by breast feeding. Hepatitis C may lead to chronic form of hepatitis culminating to cirrhosis. Hepatitis A is rarely life threatening, while B and C are quite serious and may be fatal. Several cytokines, including interferon-γ (IFN-γ) and TNF-α, are implicated in the pathogenesis of hepatitis. Chronic hepatitis B and C, mostly in the cirrhotic stage, are responsible for the great majority of cases of hepatocellular carcinoma worldwide (CitationMichielsen et al., 2005).
Phytoconstituents with hepatoprotective potentials
Compounds with different structure but with the same therapeutic activity isolated from different plant species act as active moieties for the treatment of various diseases. Several phytomolecules including flavonoids, alkaloids, glycosides and saponins obtained from various plant sources have been reported as potent hepatoprotective agents (CitationFlora et al., 1996). Plant tissues contain a wide variety of compounds with antioxidant activity. Phenolic compounds (flavonoids and phenolic acids), nitrogen compounds (alkaloids, chlorophyll derivatives, amino acids and amines), carotenoids, lignans and terpenes were reported to possess antioxidative activity in suppressing the initiation or propagation of the chain reactions (CitationHall & Cuppett, 1997). Flavonoids and phenolic compounds are the main antioxidative compounds of herbal drugs. The phenolic compounds exhibit considerable free radical scavenging activities, through their reactivity as hydrogen or electron-donating agents, and metal ion chelating properties (CitationRice-Evans et al., 1996). Flavonoids are polyphenolic compounds that occur ubiquitously in foods of plant origin. Over 4000 different flavonoids have been described. They may have beneficial health effects because of their antioxidant properties and their inhibitory role in various stages of tumor development (CitationLevy et al., 2004). Some potent herbs with established hepatoprotective activity are described in the following section and in , where as some investigational lead molecules with significant hepatoprotective effects are mentioned in .
Table 1. Herbs which are being investigated for use as hepatoprotective agents.
Table 2. List of lead molecules with hepatoprotective activity.
Silymarin
Silymarin obtained from Silybum marianum (L.) Gaertn. (Asteraceae/Compositae) commonly known as “milk thistle,” is one of the oldest and thoroughly researched plants in the treatment of liver diseases (CitationLuper, 1998; CitationMayer et al., 2005). It is the gold standard of hepatoprotective agents and has been widely used as comparative standard for test drugs in preclinical studies. It is a polyphenolic flavonoid that has anti-inflammatory, cytoprotective and anticarcinogenic effects. The pharmacological profile of silymarin has been well defined and hepatoprotective properties of silymarin have been investigated both in vitro and in vivo (CitationPradhan & Girish, 2006). Experimental studies have demonstrated antioxidant and free radical scavenging properties. Silymarin also has significant antifibrotic activity. The active constituents of the plant are obtained from the dried seeds, where it is present in higher concentrations and consist of four flavonolignans which are collectively known as silymarin. Silymarin is a complex mixture of four flavonolignan isomers (CitationFlora et al., 1998), namely silybin, isosilybin, silydianin and silychristin with an empirical formula C25H22O10. The structural similarity of silymarin to steroid hormones is believed to be responsible for its protein synthesis facilitatory actions (CitationSaller et al., 2001). Among the isomers silybin is the major and most active component and represents about 60–70%, followed by silychristin (20%), silydianin (10%), and isosilybin (5%). Most of its hepatoprotective properties are attributed to silybin ().
Hepatoprotective activity against ethanol, acetaminophen, Amanita phalloides (Vaill. ex Fr.) Link and carbon tetrachloride (CCl4) induced liver injury has been demonstrated (CitationMuriel et al., 1992). It also produced hepatoprotective effects in acute viral hepatitis and alcohol related liver cirrhosis. The cytoprotective effects of silymarin are mainly attributable to its antioxidant and free radical scavenging properties (CitationMiller, 1996). Silymarin also interacts directly with cell membrane components to prevent any aberration in the content of lipid fraction responsible for maintaining normal fluidity (CitationMuriel & Mourelle, 1990; CitationWiseman, 1996). Anti-inflammatory actions of silymarin are due to inhibition of 5-lipoxygenase pathway which results in impaired leukotriene synthesis (CitationDehmlow et al., 1996). Liver fibrosis can result in remodeling of liver architecture leading to hepatic insufficiency, portal hypertension and hepatic encephalopathy. The conversion of hepatic stellate cells (HSCs) into myofibroblasts is considered as the central event in fibrogenesis. Silymarin inhibits nuclear factor-κB (NF-κB) and retards HSC activation. Silymarin blocked TNF-induced activation of NF-κB in a dose-and time-dependent manner, mediated through inhibition of phosphorylation and degradation of Iota κB α, an inhibitor of NF-κB. Silymarin blocked the translocation of p65 to the nucleus without affecting its ability to bind to the DNA. NF-κB dependent gene transcription was also suppressed by silymarin. Silymarin also inhibited the TNF-induced activation of mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase and abrogated TNF-induced cytotoxicity and caspase activation. Silymarin suppressed the TNF-induced production of reactive oxygen intermediates and lipid peroxidation (LPO) (CitationManna et al., 1999). It also inhibits protein kinases and other kinases involved in signal transduction and may interact with intracellular signaling pathways (CitationSaliou et al., 1998; CitationJohnson et al., 2002). Stimulation of protein synthesis by silymarin has important therapeutic implications in the repair of damaged hepatocytes and restoration of normal functions of liver (CitationSonnenbichler & Zetl, 1986). Silymarin has inhibitory effect on nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) gene expression in macrophages. Silymarin dose-dependently suppressed the lipopolysaccharide (LPS) induced production of NO in isolated mouse peritoneal macrophages and RAW 264.7 (murine macrophage-like cell line). Silymarin inhibits NO production and iNOS gene expression by inhibiting NF-κB activation (CitationKang et al., 2002).
Glycyrrhizin
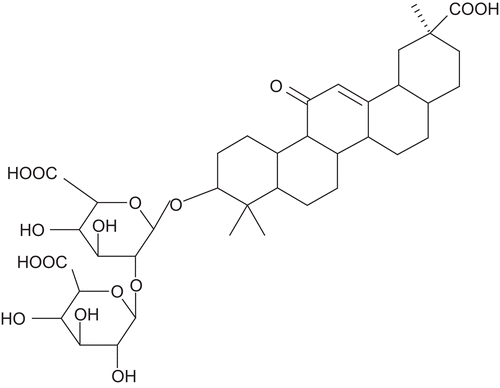
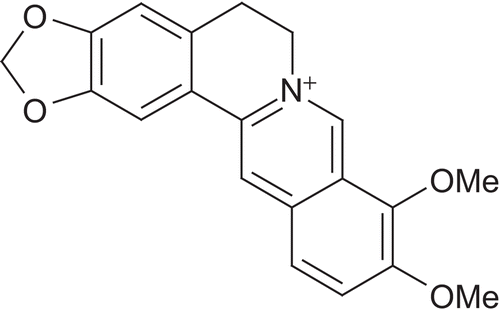
Glycyrrhizin is the major active constituent liquorice root [Glycyrrhiza glabra L., (Leguminacae)] and has been used in traditional medicine to alleviate bronchitis, gastritis and jaundice. It is a mixture of calcium and potassium salts of glycyrrhizinic acid. The major constituents are glycyrrhetinic acid (GA), flavonoids, hydroxycoumarins, and β-sitosterol, the latter with probable glucocorticoid and mineralocorticoid properties. Standardization of liquorice is done based on glycyrrhizin content. Enzymatic hydrolysis of glycyrrhizin using glucuronidase yields GA as an aglycone. Chemically, glycyrrhizin is a triterpenoid saponin named as (3-β, 20-β) -20- carboxy-11-oxo-30-norolean-12-en-3-yl 2- O-β- d-glucopyranuronosyl-α- d-glucopyranosiduronic acid ().
Glycyrrhizin possesses anti-inflammatory and antioxidant activities. Glycyrrhizin inhibits CD4+T cell and TNF-mediated cytotoxicity (CitationYoshikawa et al., 1997). Glycyrrhizin has a membrane stabilizing effect (CitationShiki et al., 1992) and also stimulates endogenous production of interferons (CitationAbe et al., 1994). In cell culture experiments, glycyrrhizin modifies glycosylation and blocks sialylation of hepatitis B surface antigen (HBsAg), leading to its retention in the Golgi apparatus (CitationTakahara et al., 1994). Glycyrrhizin counteracts several forms of experimental hepatic injury and inhibits the activity of 11-β-hydroxysteroid dehydrogenase, PGE2 production by macrophages, and has antioxidant properties through induction of glutathione S-transferase (GST) and catalase. Antifibrotic activity of glycyrrhizin could be attributed to its inhibitory activity on NF-κB (CitationWang et al., 1998).
The beneficial effect of glycyrrhizin in CCl4-induced liver injury has been evaluated. The serum activities of alanine transaminase (ALT) and aspartate aminotransferase (AST) and the hepatic level of malondialdehyde (MDA) were significantly higher after the CCl4 treatment, while the concentration of reduced glutathione (GSH) was lower. These changes were attenuated by glycyrrhizin. CCl4 increased the level of circulating TNF-α markedly, which was reduced by glycyrrhizin. The levels of hepatic iNOS, cyclooxygenase-2 (COX), and heme oxygenase-1 (HO-1) protein expression were markedly higher after the CCl4 treatment. The mRNA expression of HO-1 was augmented by the glycyrrhizin treatment, while glycyrrhizin attenuated the increase in TNF-α, iNOS, and COX-2 mRNA expressions. These results suggest that glycyrrhizin alleviates CCl4-induced liver injury, and this protection is likely due to the induction of HO-1 and the downregulation of proinflammatory mediators (CitationLee et al., 2007).
The protective effects of 18β-GA, the aglycone of glycyrrhizin derived from liquorice, on CCl4-induced hepatotoxicity and the possible mechanisms involved in this protection were investigated in mice. Pretreatment with GA prior to the administration of CCl4 significantly prevented an increase in ALT, AST activity and hepatic LPO in a dose-dependent manner. In addition, pretreatment with GA also significantly prevented the depletion of GSH content in the livers of CCl4 intoxicated mice. Protective effects of GA against the CCl4-induced hepatotoxicity may be due to its ability to block the bioactivation of CCl4, primarily by inhibiting the expression and activity of P450 2E1 (CitationJeong et al., 2002). In Japan, a standardised extract containing glycyrrhizin, cysteine and glycine is an established treatment for chronic hepatitis as Stronger Neo-minophagen C (SNMC) (CitationHidaka et al., 2007). SNMC can effectively protect liver against fulminant hepatic failure induced by galactosamine (D-GalN) and LPS. The levels of plasma TNF-α, NO, IL-6, and the degree of hepatic tissue injury were decreased in the SNMC-treated groups. SNMC prevented hepatocyte apoptosis evident from decreased expression of Cyt-C and caspase-3. SNMC stabilizes mitochondria membrane to suppress the release of Cyt-C and sequent activation of caspase-3 (CitationYang et al., 2007).
Andrographolide
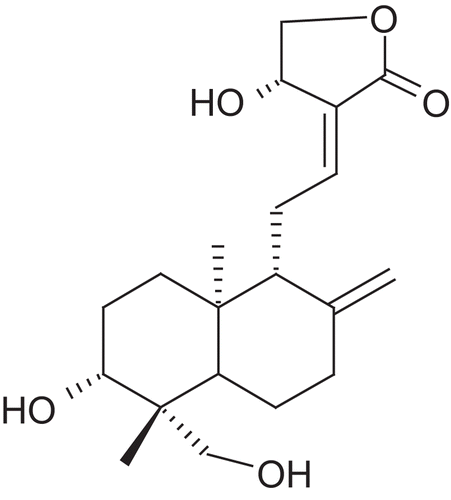
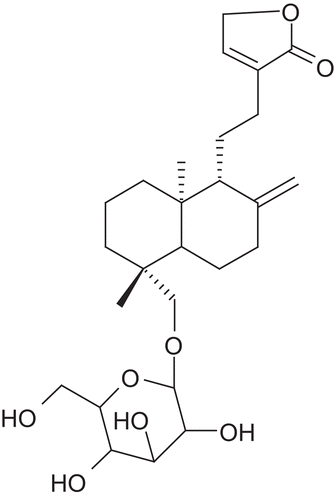
Andrographis paniculata Nees. (Ap) (Acanthaceae) is a herbaceous plant native to India and Sri Lanka. Andrographis is called “king of bitters” due to its extremely bitter taste. Mostly the leaves and roots are used for medicinal purposes. The principal constituents are 14-deoxy-11-dehydroandrographolide, 14-deoxy-11-oxoandrographolide, andrographolide, andrographine, neoandrographolide, panicoline, paniculide-A, paniculide-B and paniculide-C. Chemically, andrographolide is a labdane diterpene lactone named as, 3-[2-{decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl}ethylidene]dihydro-4-hydroxy, 2(3H)-furanone ( and ).
Andrographolide, chief constituent extracted from the leaves of the plant, is a bitter water-soluble lactone and is responsible for the hepatoprotective activity of A. paniculata (CitationVisen et al., 1993). Andrographolide, showed a significant dose-dependent protective activity against acetaminophen induced toxicity on isolated rat hepatocytes. It significantly increased the percentage viability of the hepatocytes as tested by Trypan blue exclusion and oxygen uptake tests. It completely antagonized the toxic effects of acetaminophen on certain enzymes (AST, ALT and ALP) in serum as well as in isolated hepatic cells. Antihepatotoxic activity of andrographolide (100 mg/kg, i.p.) was evaluated using CCl4 intoxicated rats. Biochemical parameters like serum AST, ALT, ALP, serum bilirubin and hepatic triglycerides were estimated to assess the liver function (Handa & Sharma, Citation1990a). Hepatoprotective effect of andrographolide was studied on acute hepatitis induced in rats by single dose of galactosamine (800 mg/kg, i.p.). The results confirmed the in vivo hepatoprotective effect of andrographolide against galactosamine induced hepatotoxicity in rats (CitationHanda and Sharma, 1990b). The protective activity of andrographolide against ethanol-induced hepatotoxicity in mice has been established (CitationSingha et al., 2007).
The diterpenes andrographolide, andrographiside and neoandrographolide were investigated for their protective effects on hepatotoxicity induced in mice by tert-butylhydroperoxide (tBHP) intoxication. Pretreatment of mice with the diterpenes produced significant hepatoprotection in the toxin treated groups. Reduction in MDA formation and GSH depletion was observed in the animals treated with diterpenes, which suggests that antihepatotoxic activity of the diterpenes could be due to antioxidant mechanism (CitationKapil et al., 1993). Neoandrographolide has been reported to possess free radicals scavenging activity by donating the allylic hydrogen of the unsaturated lactone ring (CitationKamdem et al., 2002). Andrographolide and neoandrographolide were found to suppress NO production in LPS activated macrophages (CitationBatkhuu et al., 2002). Also it has been found that neoandrographolide inhibits the activation of p38 MAPKs instead of JNK, ERK1/2, or NF-κB. These results indicated that the anti-inflammatory properties of neoandrographolide might result from the inhibition of iNOS and COX-2 expression through inhibiting p38 MAPKs activation (CitationLiu et al., 2007).
Picrorhiza kurroa
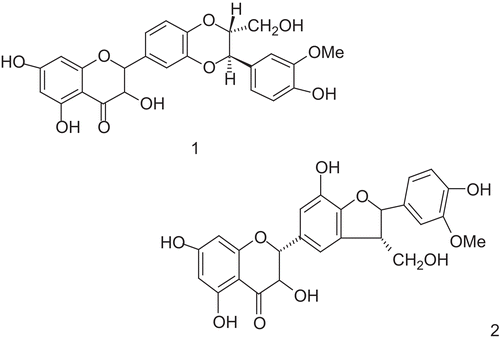
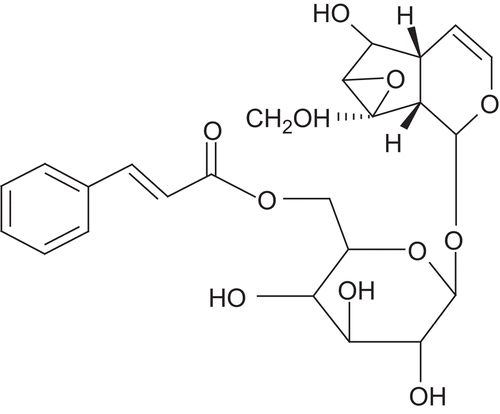
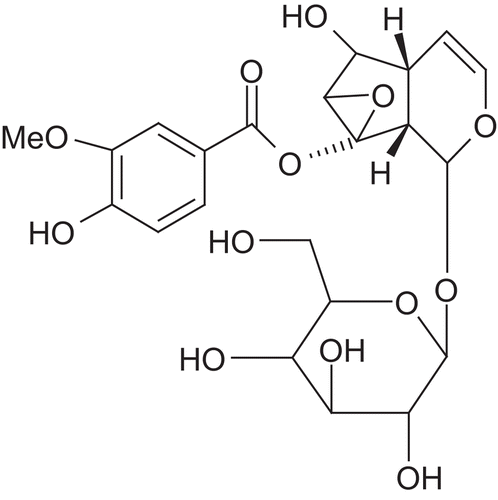
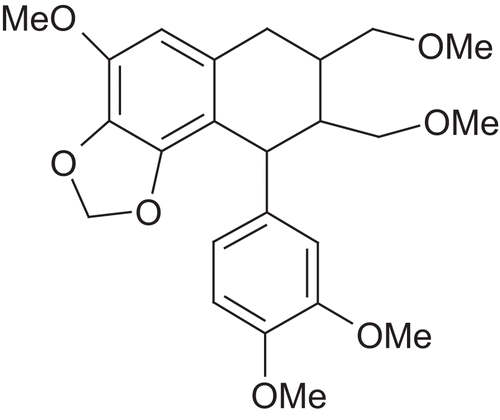
Picrorhiza kurroa Royle Benth. (Scrophulariaceae) is a well-known herb in the Ayurvedic system of medicine and has traditionally been used to treat disorders of the liver and upper respiratory tract, reduce fevers, and to treat dyspepsia, chronic diarrhea, and scorpion sting. It is a small perennial herb from the Scrophulariaceae family, found in the Himalayan region. The active constituents are obtained from the root and rhizomes. Kutkin is the active principle of Picrorhiza kurroa and is comprised of kutkoside and the iridoid glycoside picrosides I, II, and III. Other identified active constituents are apocynin, drosin and nine cucurbitacin glycosides. Picroliv is a standardized iridoid glycoside mixture isolated from the roots and rhizomes of Picrorhiza kurroa. It contains at least 60% of a 1:1.5 mixture of picroside I and kutkoside; the remainder (40%) is a mixture of iridoid as well as cucurbitacin glycosides and some still unidentified substances (CitationVerma et al., 2009). Chemically, these are iridoid glycosides with a common unit known as “catalpol.” Picroside I is established as 6’-O-cinnamoylcatalpol, while kutkoside is 10-vanilloylcatalpol ( and ).
Picroliv has been found to possess potent hepatoprotective activity against different hepatotoxins (CitationDwivedi et al., 1993). Hepatoprotective activity of picroliv has been established against thioacetamide (TAA) (CitationDwivedi et al., 1991), CCl4 (CitationDwivedi et al., 1990) and alcohol (CitationRastogi et al., 1996). CCl4 treatment resulted in elevation of serum ALT and AST and reduction of liver GSH, G6PD, catalase and membrane-bound Na+/K+ ATPase which was reversed by administration of Picroliv. Thus, Picroliv offers significant protection against liver damage by CCl4 by acting as free radical scavenger and inhibitor of LPO of liver plasma membrane (CitationSantra et al., 1998).
Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family, Curcuma longa L. (Zingiberaceae). Apart from culinary use, turmeric has been used in traditional medicine for the treatment of jaundice and other disorders of liver, parasitic infections, ulcers, inflammation of joints, various skin diseases, etc. Curcuminoids are a mixture of several structurally close phenolic compounds present in the rhizomes of turmeric. Three curcuminoids of major occurrence are curcumin (60–80%), demethoxycurcumin (10–20%), and bisdemethoxycurcumin (5–10%). Chemically, curcumin is a diferuloylmethane having a diferulic acid moiety fused with another carbon atom or methylene moiety. Thus, it has a methylene-1, 3-diketo group showing keto-enol tautomerism due to stabilization by hydrogen bonding. Curcumin exists mainly in keto-enol form rather than in a diketo form ().
Curcumin attenuates liver injury induced by acetaminophen, CCl4, ethanol, TAA, iron overdose and cholestasis (Girish et al., Citation2009a; CitationBruck et al., 2007). Curcuminoids suppressed the increase of lactate dehydrogenase (LDH), ALT, and AST levels caused by d-galactosamine treatment (CitationMiyakoshi et al., 2004). Aflatoxin B (1) is a potent hepatotoxic and hepatocarcinogenic mycotoxin. LPO and oxidative DNA damage are the principal manifestations of aflatoxin B1 induced toxicity that could be counteracted by antioxidants. Aflatoxin B (1) administration significantly increased serum ALT, AST and γ-GT levels, LPO and reduced activities of GSH, SOD, catalase and GSH-Px. Curcumin showed a significant hepatoprotective activity by lowering the levels of serum marker enzymes, LPO and elevating the levels of GSH, SOD, catalase and GSH-Px (CitationEl-Agamy, 2010; CitationNayak & Sashidhar, 2010).
A study was carried out to demonstrate the potential protective effect of curcumin pretreatment against ethanol-induced hepatocytes oxidative damage, with emphasis on HO-1 induction. HO-1 is an important antioxidant enzyme (AOE) that plays a pivotal role in cytoprotection against noxious stimuli of both endogenous and exogenous origin. Ethanol exposure resulted in a sustained MDA elevation, GSH depletion and evident release of cellular LDH and AST, which was significantly ameliorated by curcumin pretreatment. Induction of HO-1 by curcumin was dose-dependent. It was concluded that hepatoprotective activity of curcumin against ethanol involves HO-1 induction (CitationBao et al., 2010). It has been reported to induce expression of the AOEs in various cell lines. In one study, it was found that oral administration of curcumin at 200 mg/kg dose for four consecutive days not only protected against dimethylnitrosamine (DMN)-induced hepatic injury, but also resulted in more than threefold induction of HO-1 protein expression as well as activity in rat liver. Inhibition of HO-1 activity by zinc protoporphyrin-IX abrogated the hepatoprotective effect of curcumin against DMN toxicity. NF-E2-related factor 2 (Nrf2) plays a role in the cellular protection against oxidative stress through antioxidant response element (ARE)-directed induction of several phase-2 detoxifying and AOEs including HO-1. Curcumin administration resulted in enhanced nuclear translocation and ARE-binding of Nrf2. These findings suggest that ARE-driven induction of HO-1 expression by curcumin is involved in protection against DMN-induced hepatotoxicity (CitationFarombi et al., 2008).
One study used liver slice culture model to demonstrate hepatoprotective activity of curcumin in vitro. Ethanol was used as hepatotoxin and the cytotoxicity of ethanol was estimated by quantization of the release of LDH. Ethanol induced increased release of LDH from the liver cells and twice the amount of LPO as compared to the cells from untreated liver tissue and this was significantly reduced in presence of curcumin (5 μM). The activity of AOEs namely SOD, catalase and peroxidase was measured and found that in ethanol treated cells activity of all three enzymes was elevated. However, when curcumin was added along with ethanol their levels were kept low. The fact that release of LDH is significantly reduced along with LPO and the activity of AOEs is kept low indicates that curcumin by its antioxidant activity reduced the oxidative stress induced by ethanol and protected the liver cells in vitro (CitationNaik et al., 2004). Curcumin is capable of preventing NF-κB activation and therefore to prevent the secretion of proinflammatory cytokines (CitationReyes-Gordillo et al., 2007). CCl4 administration in rats resulted in increased TNF-α, IL-1β and IL-6 production, while curcumin remarkably suppressed these mediators of inflammation in liver damage. Administration of CCl4-induced the translocation of NF-κB to the nucleus, which was blocked by curcumin treatment (CitationRivera-Espinoza & Muriel, 2009). Thus, curcumin prevents acute liver damage by acting as an antioxidant and by inhibiting NF-κB activation and thus reducing production of proinflammatory cytokines.
Phyllanthin
Phyllanthin is a lignan obtained from Phyllanthus niruri L. (Euphorbiaceae). It is a well-known Ayurvedic plant used in folk remedy for jaundice and other liver disorders. The main plant constituents include alkaloids, astragalin, brevifolin, ellagitannins, amariin, repandusinic acid, phyllanthusiin D gallocatechins, geraniin, hypophyllanthin, lignans, lintetralins, lupeols, nirurin, phyllanthin, phyllanthine and phyllanthenol. Phyllanthin and hypophyllanthin have been established as the hepatoprotective agents (CitationHarish & Shivanandappa, 2006). Chemically, both phyllanthin and hypophyllanthin are lignans ( and ). Whole plant, fresh leaves and fruits are used to treat various ailments, particularly hepatitis. This herb has shown potent antioxidant activity and hepatoprotective activity (CitationHarish & Shivanandappa, 2006). Methanol and aqueous extract of leaves and fruits of P. niruri showed inhibition of membrane LPO, scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and inhibition of reactive oxygen species (ROS) in vitro. Antioxidant activities of the extracts were also demonstrable in vivo by the inhibition of CCl4-induced formation of lipid peroxides in liver of rats by pretreatment with the extracts. CCl4-induced hepatotoxicity in rats was prevented by pretreatment with the extracts, demonstrating the hepatoprotective action of P. niruri. Treatment with protein isolates of P. niruri significantly increase SOD and catalase which were reduced due to CCl4 administration (CitationBhattacharjee & Sil, 2007).
In a study, the hepatoprotective potential of aqueous extract of the herb P. niruri on nimesulide induced hepatotoxicity by antioxidant activity was evaluated in mice. Nimesulide administration (8 mg/kg) for 7 days caused significant depletion of the levels of SOD, CAT and GSH, along with the increased levels of LPO. Intraperitoneal administration of the extract at a dose of 50 mg/kg body weight for 7 days, prior to nimesulide treatment, significantly increased levels of SOD, CAT, GSH and decreased MDA levels suggesting beneficial effect of the aqueous extract of P. niruri could be due to its antioxidant property (CitationChatterjee & Sil, 2006). A study investigated the hepatoprotective and antioxidative property of phyllanthin using CCl4-induced toxicity in human hepatoma HepG2 cell line. Free radical scavenging activity of phyllanthin was examined using DPPH assay. CCl4 treatment caused a significant decrease in cell viability, initiated LPO and caused leakage of ALT and LDH with a significant decrease in GSH levels. It was observed that phyllanthin effectively alleviated the changes induced by CCl4 in a concentration-dependent manner (CitationKrithika et al., 2009). The protective effect of phyllanthin on ethanol-induced rat liver cell injury has been investigated. Primary cultures of rat hepatocytes were pretreated with phyllanthin (1, 2, 3 and 4 μg/ml) for 24 h. After 24 h pretreatment, cells were treated with ethanol (80 μl/ml) for 2 h. Ethanol decreased %MTT, increased the release of ALT and AST with the increase in the production of intracellular ROS and LPO. Phyllanthin demonstrated its role in protection by antagonizing the above effect induced by ethanol. Phyllanthin also restored the antioxidant capability of rat hepatocytes including level of total glutathione, and activities of SOD and glutathione reductase (GR) which were reduced by ethanol. These results suggest the hepatoprotective effect of phyllanthin against ethanol-induced hepatotoxicity is through its antioxidant activity (CitationChirdchupunseree & Pramyothin, 2010). The antioxidant activity of some of the principal constituents of Phyllanthus namely Amariin, 1-galloyl-2,3-dehydrohexahydroxydiphenyl-glucose, repandusinic acid, geraniin, corilagin, phyllanthusiin D, rutin and quercetin 3-O-glucoside were examined for their ability to scavenge free radicals in a range of systems including DPPH, ABTS, ferric reducing antioxidant power and pulse radiolysis. In addition, their ability to protect rat liver mitochondria against oxidative damage was determined by measuring the ROO• radical induced damage to proteins and lipids and •OH radical induced damage to plasmid DNA. The compounds showed significant antioxidant activities with differing efficacy depending on the assays employed. The ellagitannins Amariin, repandusinic acid and phyllanthusiin D showed higher antioxidant activity (CitationLondhe et al., 2008).
Berberine
Berberine is an alkaloid obtained from Berberis aristata L. (Berberidaceae). It is an edible plant employed in the South Asian Traditional Medicine, particularly its fruits being used as a tonic remedy for liver and heart. Berberis aristata is rich in alkaloids like berberine, oxyberberine, berbamine, aromoline, karachine, and oxycanthine. The isoquinoline alkaloid, berberine, is the major active constituent (). Berberine has experimentally established hepatoprotective activity (CitationJanbaza & Gilanib, 2000). Pretreatment of animals with berberine (4 mg/kg; orally twice daily for 2 days) prevented acetaminophen induced rise in serum levels of ALP, AST and ALT, suggestive of hepatoprotection. Pretreatment of animals with a single oral dose of berberine (4 mg/kg) induced prolongation of the pentobarbital (60 mg/kg, i.p.) induced sleeping time as well as increased strychnine (0.3 mg/kg; i.p.)-induced toxicity, suggestive of inhibitory effect on microsomal drug metabolizing enzymes, cytochrome P450s (CYPs). The effects of berberine on ion channels of isolated rat hepatocytes have been studied. Berberine 1–300 μmol/l reduced delayed outward potassium currents and Ca++ release-activated Ca++ currents in a concentration-dependent manner. Thus it was concluded that berberine has inhibitory effects on potassium and calcium currents in isolated rat hepatocytes, which may be involved in hepatoprotection (CitationWang et al., 2004).
One study investigated the effect of berberine on the early phase of hepatocarcinogenesis stimulated by diethylnitrosamine (DENA, 150 mg/kg, 4 weeks) plus phenobarbital (PB, 75 mg/kg, 7 days) in rats. The expressions of proliferating cell nuclear antigen (PCNA) and iNOS were evaluated by immunohistochemistry. Results showed that expressions of PCNA and iNOS were induced by DENA + PB in liver tissues. Oral administration of berberine (50 mg/kg) inhibited the hepatocyte proliferation and iNOS expression, decreased cytochrome P450 content and inhibited activities of CYP2E1 and CYP1A2 in DENA + PB treated rats in vivo. Also, berberine (10, 50 and 100 μM) inhibited activities of CYP2E1 and CYP1A2 in microsomes isolated from DENA + PB treated rats in vitro, suggesting that anti-hepatocarcinogenetic potential of berberine might be due to inhibiting oxidative metabolic activities of CYP 2E1 and CYP1A2, and decreasing NO production in rats (CitationZhao et al., 2008).
Embelin
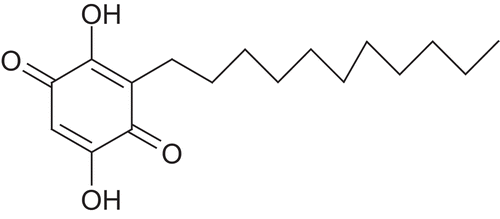
Embelia ribes Burm.f. (Myrsinaceae) is a large scandent shrub with slender branches and elliptic-lanceolate and gland dotted leaves. The plant contains embelin, christembine, quercitol and resins. It is a widely used herb for its anthelmintic, analgesic, antifertility, hepatoprotective and antitumour properties. Embelin is the major active constituent and is responsible for the antioxidant and hepatoprotective activity (CitationMukherjee et al., 2007). Chemically embelin is 2,5-dihydroxy-3-undecyl-1,4-benzoquinone ().
Free radical scavenging activity and LPO studies have been conducted using embelin to elucidate the hepatoprotective activity of embelin. After the induction of liver damage by CCl4 intoxication to rats, the concentration of LPO was significantly higher in liver and serum, along with concomitant decrease in the levels of antioxidants and CYP450 enzyme in liver as compared to vehicle controls. The activities of marker enzymes-AST, ALT, ALP, γ-glutamyl transpeptidase, LDH, total bilirubin and total protein levels were altered significantly in the serum of CCl4 treated rats. Treatment with embelin (oral, 25 mg/kg), significantly reduced hepatopathy, and results were comparable to silymarin. The study concluded that embelin acts as a natural antioxidant against hepatotoxicity induced in rats (CitationSingh et al., 2009). The free radical scavenging reactions and antioxidant activity of embelin have been studied. It has been found to scavenge DPPH radical and inhibit hydroxyl radical induced deoxyribose degradation. It has been also found to inhibit LPO and restore impaired Mn-SOD in rat liver mitochondria. Further, kinetics and mechanism of the reactions of embelin with hydroxyl, one-electron oxidizing, organo-haloperoxyl and thiyl radicals have been studied using nanosecond pulse radiolysis technique. Its redox potential has been also evaluated with cyclic voltammetry. These studies suggest that embelin can act as a competitive antioxidant in physiological conditions (CitationJoshi et al., 2007).
X-Linked inhibitor of apoptosis protein (XIAP) is a promising molecular target for the design of new anticancer drugs aiming at promoting apoptosis in cancer cells (CitationChen et al., 2006). Embelin has been recognized as a fairly potent, nonpeptidic, cell-permeable, small-molecule inhibitor of XIAP. The effects of embelin (50 mg/kg/day) against N-nitrosodiethylamine (DENA)-initiated and PB-promoted hepatocarcinogenesis were studied in Wistar rats. It was able to prevent the induction of hepatic hyper plastic nodules, body weight loss, increase in the levels of hepatic diagnostic markers, and hypoproteinemia induced by DENA + PB treatment indicating possible chemopreventive effects of embelin against DENA + PB-induced hepatocarcinogenesis in Wistar rats (CitationSreepriya & Bali, 2005). In a further study of effects of administration of embelin on LPO, hepatic glutathione antioxidant defense were examined during DENA + PB promoted hepatocarcinogenesis in rats. DENA + PB-induced hepatic damage was manifested by a significant drop in the hepatic glutathione antioxidant defense, increased LPO and histological alterations like dysplasia, and atypical cells with abnormal chromatin pattern. Treatment with embelin (50 mg/kg) prevented the drop in hepatic glutathione antioxidant defense, decreased LPO, and minimized the histological alterations induced by DENA + PB. Results indicate beneficial effects of embelin against oxidative tissue damage during chemically induced hepatocarinogenesis in rats (CitationSreepriya & Bali, 2006).
Resveratrol
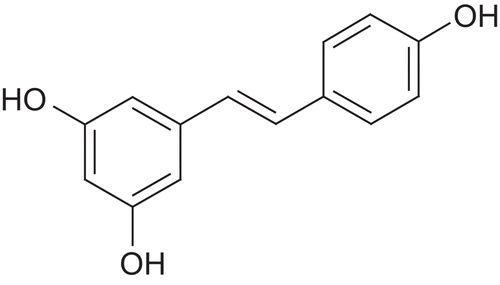
Resveratrol is a naturally occurring polyphenol which shows pleiotropic health beneficial effects, including antioxidant, anti-inflammatory, anti-aging, cardioprotective and neuroprotective activities. Resveratrol is a phytoalexin which is produced when plants are under attack from bacteria or fungi. Resveratrol is present in many plants and fruits, including red grapes, eucalyptus, spruce, blueberries, mulberries, peanuts and giant knotweed. Also red wine is an abundant source of it. Chemically it is trans-3,5,4′-trihydroxystilbene ().
It is a very potent antioxidant and this property has implications in anticarcinogenic, antidiabetic, neuprotective and cardioprotective benefits. In order to investigate protective antioxidant action of resveratrol, primary rat hepatocyte cultures were exposed to 300 µM tBHP and various concentrations of resveratrol (25, 50, 75 μM). Necrosis was evaluated by LDH liberation to the medium. Resveratrol inhibited necrosis induced by tBHP. It also was effective in eliminating ROS. Results show that resveratrol protects primary rat hepatocytes in culture from oxidative stress induced cell death (CitationRubiolo and Vega, 2008). In order to elucidate the molecular mechanism of resveratrol in protecting liver cells from oxidative stress induced damage the activation of Nrf2 transcription factor that regulates the expression of antioxidant and phase II detoxifying enzymes was studied. Resveratrol pretreatment effectively protected hepatocytes in culture exposed to oxidative stress, increasing the activities of catalase, SOD, GPx, NADPH quinone oxidoreductase and GST. Resveratrol increases the level of Nrf2 and induces its translocation to the nucleus. Also, it increases the concentration of the mRNA for Nrf2. These results suggest that resveratrol could enhance the antioxidant status of hepatic cells and could be a useful drug for the protection of liver cells from oxidative stress induced damage (CitationRubiolo et al., 2008). In one study, the effect of subacute pretreatment with this natural compound on LPS-induced hepatotoxicity was investigated. Resveratrol counteracted LPS-induced lipoperoxidation and depletion of AOE activities as SOD and catalase. The polyphenol also abrogated LPS-induced liver and plasma NO elevation and attenuated endotoxemia-induced hepatic tissue injury. Resveratrol treatment abolished LPS-induced iron sequestration from plasma to liver. The results suggest that mode of action of resveratrol responsible for alleviating LPS-induced hepatotoxicity involves differential iron compartmentalization via iron shuttling proteins (CitationSebai et al., 2010).
A study was conducted to evaluate the preventive effect of resveratrol on ethanol-induced oxidative stress in rat liver. Rats were treated daily with 35% ethanol solution for 6 weeks. Chronic ethanol administration leads to hepatotoxicity as monitored by the increase in the level of hepatic marker enzymes and the appearance of fatty change, necrosis, fibrosis and inflammation in liver sections. Ethanol also enhanced the formation of MDA in the liver and decreased the activities of hepatic SOD, GPx and catalase. Dietary supplementation with resveratrol during ethanol treatment inhibited hepatic LPO and ameliorated SOD, GPx and CAT activities in the liver, indicating that resveratrol could have a beneficial effect in inhibiting the oxidative damage induced by chronic ethanol administration (CitationKasdallah-Grissa et al., 2007). The effects of resveratrol pretreatment on LPS (0.5 μg/kg) induced hepatitis in d-galactosamine (800 mg/kg) sensitized rats has been investigated. Effect of resveratrol on iNOS and inducible HO-1 were studied. Resveratrol administration ameliorates liver damage induced by LPS plus d-galactosamine. Effects of resveratrol include reduction in NO, downregulation of NOS-2 and modulation of HO-1 which led to overall improvement in hepatotoxic markers and morphology after the hepatic insult (CitationFarghali et al., 2009).
One study investigated the bioavailability of resveratrol from dietary sources and its effect on CCl4-induced liver LPO. Ten rats were intragastrically administered for 14 days with a grape-stalk extract determining a daily dosage of resveratrol (3 mg/kg). After 1 week, the induction of liver LPO by CCl4 was carried out. Serum and liver samples, at different time intervals, were collected to evaluate resveratrol content. Liver MDA as marker of oxidative stress was measured. Significant accumulation of resveratrol in liver was found after 14 days. The increase of MDA liver concentration due to CCl4 injection after 1 week was reduced by 63% by treatment with resveratrol. Thus it was concluded that consumption of resveratrol from a dietary source resulted in a time-dose-dependent liver accumulation, which was able to counteract CCl4-induced liver LPO thus demonstrating the hepatoprotective property of resveratrol (CitationVitaglione et al., 2009). Resveratrol inhibits NF-kB, which regulates the transcription of several genes including cytokines such as the profibrogenic TGF-β. To elucidate the antifibrogenic mechanism of resveratrol, the activation of NF-kB and the production of TGF-β were measured in CCl4-induced cirrhosis in the rats. CCl4 increased levels of NF-κB and TGF-β along with increased fibrosis. Resveratrol administration decreased levels of NF-κB and TGF-β and fibrosis. Results indicate that resveratrol possesses a strong antifibrogenic effect which is associated with its ability to reduce NF-κB activation and TGF-β (CitationChávez et al., 2008). Resveratrol (10 mg/kg) was found to inhibit pyrogallol (40 mg/kg) induced hepatotoxicity. Resveratrol reduced pyrogallol-mediated increase in ALT, AST, bilirubin, LPO and catalytic activity of CYP2E1 and CYP1A2. Pyrogallol-mediated decrease in GST, GPx and GSH content was significantly attenuated in resveratrol treated animals (CitationUpadhyay et al., 2008).
Acteoside
Acteoside is a phenylethanoid glycoside obtained from Cistanche tubulosa (Schrenk) Wight (Orobanchaceae) and Syringa vulgaris L. (Oleaceae) (). One study investigated the protective effects of acteoside on the CCl4-induced hepatotoxicity as well as the possible mechanisms involved in this protection. Pretreatment with acteoside prior to the administration of CCl4 significantly prevented the increased serum enzymatic activities of ALT and AST and hepatic MDA in a dose-dependent manner. Glutathione content in the liver of CCl4 intoxicated mice was increased. The effects of acteoside on cytochrome P450 2E1, the major isozyme involved in CCl4 bioactivation were also investigated. Treatment of the mice with acteoside resulted in a significant decrease in the P450 2E1-dependent p-nitrophenol and aniline hydroxylation in a dose-dependent manner. The level of CYP2El protein was reduced. Acteoside exhibited antioxidant effects on FeCl2-ascorbate induced LPO in a mouse liver homogenate, and on superoxide radical scavenging activity. These results suggest that the protective effects of acteoside against the CCl4-induced hepatotoxicity possibly involve mechanisms related to its ability to block the P450-mediated CCl4 bioactivation and free radical scavenging effects (CitationLee et al., 2004). Acetoside inhibited d-galactosamine induced death of hepatocytes. Acetoside has been found to reduce TNF-α induced cytotoxicity in L929 cells, thus indicating cytoprotective activities (CitationMorikawa et al., 2010).
Acetoside exhibited stronger free radical scavenging activities than α-tocopherol on DPPH radical and xanthine/xanthine oxidase generated superoxide anion radical (CitationXiong et al., 1996). In a study, acteoside, 2’-acetylacteoside and isoacteoside significantly suppressed NADPH/CCl4-induced LPO in rat liver microsomes. Addition of them to primary cultured rat hepatocytes efficiently prevented cell damage induced by exposure to CCl4 or d-galactosamine (CitationXiong et al., 1998). The hepatoprotective effect of acteoside was evaluated in BCG plus LPS-induced immunological liver injury (ILI) in mice. Acteoside (50, 150, or 300 mg/kg) was administered for 12 days. The liver index, liver homogenate levels of AST and ALT, hepatic NO, MDA content, SOD activity, production of TNF-γ and IL-2, 4, 10, as well as histopathological changes of the liver were evaluated following the 12-day treatment. Moreover, the modulation influence of acteoside on the expression of Bcl-2 (hepatocyte apoptosis inhibitor) and Bcl-2 associated X protein (Bax, hepatocyte apoptosis promoter) in the mice liver with immunological hepatic injury was also studied. Acteoside (50, 150, or 300 mg/kg) effectively reduced the BCG/LPS-induced elevated liver index, liver homogenate AST and ALT levels, hepatic NO and MDA contents, restored hepatic SOD activity and reduced the degree of liver injury. The expression of Bax was decreased (vs. BCG + LPS group), while the expression of Bcl-2 increased (vs. BCG + LPS group). These results suggest that acteoside has a protective and therapeutic effect on ILI mice, which might be associated with its antioxidant properties, immunoregulatory function and regulation of hepatic apoptosis (CitationZhao et al., 2009).
The effect of acteoside on hepatic apoptosis and the subsequent liver failure induced by d-GalN and LPS was assessed. A co-administration of d-GalN (700 mg/kg) and LPS (35 μg/kg) in mice evoked typical hepatic apoptosis characterized by DNA fragmentation and apoptotic body formation, resulting in fulminant hepatitis. Pre-administration of acteoside at 10 or 50 mg/kg subcutaneously at 12 and 1 h prior to d-GalN/LPS intoxication significantly inhibited hepatic apoptosis, hepatitis and lethality. TNF-α secreted from LPS-stimulated macrophages is an important mediator of apoptosis. Acteoside prevented in vitro TNF-α (100 ng/ml) induced cell death in d-GalN (0.5 mM)-sensitized hepatocytes at the concentrations of 50, 100 and 200 μM. These results indicated that d-GalN/LPS-induced hepatic apoptosis can be blocked by acetoside suggesting its antiapoptotic activity (CitationXiong et al., 1999).
Sauchinone
Saururus chinensis (Lour.) Bail. (Saururaceae) has been used in Chinese folk medicine for treatment of various diseases, such as edema, jaundice, gonorrhea, antipyretic, diuretic, and anti-inflammatory agents. The diastereomeric lignan sauchinone which is the active constituent of Saururus chinensis has cytoprotective and antioxidant activities (). The hepatoprotective and antifibrotic effects of Saururus chinensis extract in CCl4-induced liver fibrosis rats has been evaluated (CitationWang et al., 2009). Saururus chinensis extract effectively reduced the elevated levels of serum ALT, AST, and hepatic MDA contents, enhance the reduced hepatic SOD activity in CCl4 treated rats. The histopathological analysis suggested that Saururus extract alleviated the degree of liver fibrosis induced by CCl4. One study investigated the potency of sauchinone as a hepatic HO-1 inducer and its protective effects in HepG2 cells (CitationSung et al., 2000). Treatment of the cells with sauchinone induced HO-1 expression and increased HO activity in a concentration and time-dependent manner. This expression conferred cytoprotection against oxidative injury induced by tBHP. HO-1 expression by sauchinone also suppressed tBHP induced ROS generation in HepG2 cells. Moreover, sauchinone promoted the nuclear accumulation of Nrf2, and increased the activity of ARE. Furthermore, treatment of the cells with a p38 MAPK inhibitor (SB203580) reduced sauchine-induced HO-1 expression and its protective effects (CitationJeong et al., 2010). These results suggest that sauchinone increases the cellular resistance of HepG2 cells to tBHP induced oxidative injury, presumably through the p38 MAPK pathway-Nrf2/ARE-dependent HO-1 expression. At a concentration of 50 μM, sauchinone significantly reduced the release into the culture medium of GPT from CCl4 damaged cultures of rat hepatocytes. Sauchinone protects primary cultured rat hepatocytes exposed to CCl4 from significant drops in the levels of glutathione, SOD and GPx. Sauchinone also seemed to ameliorate LPO as demonstrated by a reduction in the production of MDA. These results suggest that sauchinone may exert hepatoprotective activity through antioxidant activity (CitationSung & Kim, 2000).
Asiatic acid
Asiatic acid (AA) is one of the triterpenoid components of Terminalia catappa L. (Combretaceae) which has antioxidative, anti-inflammatory and hepatoprotective activity. The effect of the chloroform extract of T. catappa on CCl4-induced acute liver damage and d-GalN induced hepatocyte injury has been evaluated. The effects of AA on mitochondria and free radicals were investigated to determine the mechanism underlying the action of Terminalia on hepatotoxicity. Hepatotoxicity induced by CCl4 was reversed with pretreatment with 50 and 100 mg/kg extract of T. catappa (CitationTang et al., 2006). The increased ALT and AST levels in the medium of primary cultured hepatocytes induced by d-GalN were blocked by pretreatment with 0.05, 0.1, 0.5 g/l T. catappa extract. In addition, Ca2+ induced mitochondrial swelling was inhibited by AA. AA at concentrations ranging from 50 to 500 μM, showed dose-dependent superoxide anion and hydroxyl radical scavenging activity (CitationGao et al., 2004). Also the disruption of mitochondrial membrane potential, intramitochondrial Ca2+ overload and suppression of mitochondrial Ca2+-ATPase activity in the liver of CCl4 insulted mice were effectively prevented by pretreatment with T. catappa extract. Thus it could be concluded that the hepatoprotective activity of T. catappa extract is related to protection of liver mitochondria and the scavenging action on free radicals. It was also found to markedly suppress the CCl4-induced over-transcription of IL-6 gene. As a result, the expression of IL-6 protein was blocked by it in CCl4 stimulated mice. Mitochondrial protection of AA against acute liver injury induced by LPS and d-GalN in mice has been studied. It was found that pretreatment with 25, 50 or 100 mg/kg AA significantly blocked the LPS + d-GalN-induced increase in both serum ALT and AST levels. Different doses of AA decreased the synthesis of voltage-dependent anion channels (VDACs), and blocked the translocation of cytochrome c from mitochondria to cytosol. Also pre-incubation with 25, 50 and 100 μg/ml AA inhibited the Ca2+ induced mitochondrial permeability transition (MPT), including mitochondrial swelling, membrane potential dissipation and releasing of matrix Ca2+ in liver mitochondria separated from normal mice, indicating the direct role of AA on mitochondria. Results suggest that AA could protect liver from damage and the mechanism might be related to upregulating mitochondrial VDACs and inhibiting the process of MPT (CitationGao et al., 2006).
The protective effects of AA and the relative mechanism in the d-GalN + LPS induced hepatotoxicity in hepatocytes and kupffer cells co-cultured system have been explored. The cultures were pretreated with AA for 12 h, followed by d-GalN + LPS exposure for 12 h. AA reduced AST and LDH generation and increased cell viability in a concentration-dependent manner. The effects of AA in leukotriene C4 synthase (LTC4S) expression and cellular redox status including ROS and GSH content were detected. The results showed that D-GalN + LPS induced the increase of ROS followed by extracellular signal-regulated kinase 1/2 (ERK 1/2) and NF-κB activation. Treatment with ERK 1/2 specific inhibitor 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene (U0126) abolished the ERK1/2 protein phosphorylation and blunted LTC4S expression. ROS signaling pathway inhibitor pyrrolidine dithiocarbamate (PDTC) inhibited ROS generation and NF-κB activation, which in turn blocked LTC4S expression and attenuated the injury. Thus AA protects against d-GalN + LPS-induced hepatotoxicity partly via redox-regulated LTC4S expression pathway (CitationMa et al., 2009). Two AA derivatives, 3β,23-dihydroxyurs-2-oxo-12-ene-28-oic acid (AS-10) and 3β,23-dihydroxyurs-12-ene-28-oic acid (AS-14) exhibited significant protective activity against CCl4-induced hepatotoxicity in primary cultures of rat hepatocytes. AS-10 and AS-14 preserved the level of glutathione and the activities of AOEs such as GR, GPx, SOD and catalase. In addition, these compounds ameliorated LPO, as demonstrated by a reduction in the production of MDA. Results suggest that both AS-10 and AS-14 exerted their hepatoprotective activities against CCl4-induced injury by preserving the cellular antioxidative defense system (CitationLee et al., 2009).
Solanum nigrum (Solanaceae)
Solanum nigrum L. has been widely used as hepatoprotective and anti-inflammation agent. The principal constituents are solamargine-A, solamargine-B, solasodine and solasodine. Its hepatoprotective and antioxidant activity has been established experimentally (CitationLin et al., 2008) in CCl4-induced chronic hepatotoxicity in rats. Solanum nigrum extract was orally administered which reversed hepatotoxicity caused by CCl4. This hepatoprotective effect could be contributed to its antioxidant effects as the reduced hepatic content and activities of GSH, SOD and GST were restored by Solanum extract. The effects of Solanum extract on TAA induced liver fibrosis (0.2 g/kg, i.p., 3 times/week for 12 weeks) in mice have been investigated. Treatment with Solanum extract reduced the hepatic hydroxyproline levels of TAA-treated mice and TAA induced TGF-β1 mRNA levels in the liver (CitationHsieh et al., 2008). One 150 kDa glycoprotein isolated from S. nigrum produced significant increases in the activities of AOEs. The activities of SOD, catalase and GPx were increased after treatment with the glycoprotein (CitationLee et al., 2005). In one study CitationLin et al., 2007 demonstrated the cytotoxic effect of Solanum extracts on HepG2 cells. High doses of Solanum extract (2 and 5 mg/ml) induced apoptotic cell death in HepG2 cells, as evidenced by increases in the expressions of p-JNK and Bax, mitochodrial release of cytochrome c, and caspase activation. On the other hand, cells treated with low concentrations of Solanum (50–1000 μg/ml) induced autophagocytic death evidenced by increased levels of autophagic vacuoles and LC3-I and LC3-II proteins, which are specific markers of autophagy. This study suggests that Solanum could be important in treatment of liver cancers (CitationLin et al., 2007).
Current clinical status of some hepatoprotective medicines
Many herbal preparations which demonstrated significant hepatoprotective activity in preclinical studies have been successfully evaluated in clinical studies on patients suffering from dysfunctional hepatic system. To determine the effect of silymarin on the outcome of patients with cirrhosis, a double blind, prospective, randomized study was performed in 170 patients with cirrhosis. 87 patients received 140 mg silymarin three times daily whereas 83 received placebo. All patients received the treatment for 2 years. The 4-year survival rate was 58% in silymarin-treated patients and 39% in the placebo group indicating effectiveness of silymarin treatment (CitationFerenci et al., 1989). In a 6-month double blind clinical trial the effects of silymarin therapy on liver function tests, serum procollagen III peptide level and liver histology was studied in 36 patients with chronic alcoholic liver disease. During silymarin treatment serum bilirubin, ALT and AST values normalized, while procollagen III peptide level decreased. The histological alterations showed an improvement in the silymarin group, while remained unchanged in the placebo group. These results indicated that silymarin improves liver functions in alcoholic patients (CitationFehér et al., 1989). In a different randomized, placebo-controlled trial efficacy of silymarin treatment in patients with acute clinical hepatitis was evaluated. The intervention consisted of daily administration of 140 mg of silymarin. Patients randomized to the silymarin group had quicker resolution of symptoms related to biliary retention, viz., dark urine, jaundice and scleral icterus. There was a reduction in indirect bilirubin among those assigned to silymarin (CitationEl-Kamary et al., 2009). Efforts have been made to study the effect of silymarin treatment in patients with chronic hepatitis C. In one study patients aged 21–65 years old with a diagnosis of chronic hepatitis C who were not using antiviral therapy were asked to participate. Patients were randomized to treatment with silymarin 160 mg orally three times a week for 4 weeks or to no-treatment. The percent change for ALT, AST and viral load were compared between the treated and control group demonstrating a statistically significance difference for ALT and AST, but not for viral load. These results suggest that silymarin may have a protective effect in the inflammatory response to HCV (CitationTorres et al., 2004).
SNMC is a drug with glycyrrhizin as the principal ingredient. The therapeutic effects of administration of SNMC three times a week for 12 weeks in patients with chronic viral hepatitis were evaluated in a randomized clinical trial in which SNMC administration suppressed ALT levels in a dose-dependent manner (CitationMiyake et al., 2002). The efficacy and safety of SNMC were evaluated on 194 patients with chronic hepatitis B in nine hospitals in China. All patients received oral SNMC tablets. SNMC was efficacious in normalizing ALT levels (CitationZhang and Wang, 2002). In a multicenter double blind study, ALT levels decreased in the patients who received 40 ml/day of SNMC for 4 weeks. Furthermore, SNMC 100 ml/day for 8 weeks improved liver histology in 40 patients with chronic hepatitis, in correlation with improved ALT levels in serum. Liver cirrhosis occurred less frequently in 178 patients on long-term SNMC than in 100 controls. Finally, hepatocellular carcinoma (HCC) developed less frequently in the 84 patients on long-term SNMC than in the 109 controls (CitationKumada, 2002). Long term administration of SNMC in the treatment of chronic hepatitis C was effective in preventing liver carcinogenesis (CitationArase et al., 1997).
To validate the claims of Liv-52 being a potent hepatoprotective several clinical studies have been conducted. The efficacy of Liv-52 was determined on liver cirrhotic patients, a randomized, double blind, placebo-controlled study was conducted in 36 cirrhotic patients in Tehran Hepatic Center. The efficacy of Liv-52 on liver cirrhosis outcomes was compared with the placebo for 6 months. The results demonstrated that the patients treated with Liv-52 for 6 months had significantly better child-pugh score, decreased ascites, decreased serum ALT and AST, whereas no such effect was observed in placebo administered patients. Results of this study conclude that Liv-52 possess hepatoprotective effect in cirrhotic patients (CitationHuseini et al., 2005). In one retrospective study, effect of Liv-52 on 19 patients with alcoholic liver damage was investigated. It was found that administration of Liv-52 improved the subjective condition and clinical parameters in patients with liver damage. No undesirable side-effects were detected even after one year of treatment (CitationKaláb & Krechler, 1997).
The effectiveness of Phyllanthus amarus in treatment of hepatitis has been demonstrated in a randomized study where 35 patients with chronic viral hepatitis B were treated with P. amarus derivative for 3 months in the treatment group while another 25 patients were treated with recombinant human IFNα-1b for 3 months as control. Effective rate in the treatment group was 83.3%. The normalization rates of ALT and bilirubin in the treatment group were significantly higher than that in the control. The negative conversion rates of HBeAg and HBV-DNA in the treatment group were 42.3 and 47.8%, showing no significant difference from the control. It is indicated that Phyllanthus amarus derivative has remarkable effect for chronic viral hepatitis B in recovery of liver function and inhibition of the replication of HBV (CitationXin-Hua et al., 2001). There are several herbal preparations which are used as hepatoprotective drugs all over the world, some of which are listed in .
Table 3. List of herbal preparations available as hepatoprotective drugs.
Conclusion
Herbal medicines derived from plant extracts are being increasingly utilized to treat a wide variety of clinical diseases, with large efforts being made to elucidate their modes of action. Large sections of patients with liver disease use botanicals. Future efforts will have to implement extensive methodological improvements to separate the real therapeutic value of these agents from unsubstantiated hopes associated with them. The active molecules must be isolated and tested through well designed experiments and finally in randomized, placebo-controlled studies to enable rational clinical use of the agents. There are many such isolated lead molecules which if rigorously investigated could become hepatoprotective drugs of the future (). Sound and controlled clinical studies with herbals and especially their active ingredients in chronic liver diseases are important, with regard to discover novel antifibrotic and anti-inflammatory activities. There is a huge demand for both experimental and clinical research to validate the potential of herbal drugs and rigorous scientific testing along the principles of evidence-based medicine will help herbal medicine to become a very justifiable scientific treatment regime.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Abe Y, Ueda T, Kato T, Kohli Y. (1994). [Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C]. Nippon Rinsho, 52, 1817–1822.
- Ahmed B, Khan S, Masood MH, Siddique AH. (2008). Anti-hepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. J Asian Nat Prod Res, 10, 223–231.
- Ajith TA, Janardhanan KK. (2002). Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J Ethnopharmacol, 81, 387–391.
- Akindele AJ, Ezenwanebe KO, Anunobi CC, Adeyemi OO. (2010). Hepatoprotective and in vivo antioxidant effects of Byrsocarpus coccineus Schum. and Thonn. (Connaraceae). J Ethnopharmacol, 129, 46–52.
- Alqasoumi S. (2010). Carbon tetrachloride-induced hepatotoxicity: Protective effect of ‘Rocket’ Eruca sativa L. in rats. Am J Chin Med, 38, 75–88.
- Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. (1997). The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer, 79, 1494–1500.
- Bao W, Li K, Rong S, Yao P, Hao L, Ying C, Zhang X, Nussler A, Liu L. (2010). Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol, 128, 549–553.
- Batkhuu J, Hattori K, Takano F, Fushiya S, Oshiman K, Fujimiya Y. (2002). Suppression of NO production in activated macrophages in vitro and ex vivo by neoandrographolide isolated from Andrographis paniculata. Biol Pharm Bull, 25, 1169–1174.
- Bhakta T, Banerjee S, Mandal SC, Maity TK, Saha BP, Pal M. (2001). Hepatoprotective activity of Cassia fistula leaf extract. Phytomedicine, 8, 220–224.
- Bhandarkar MR, Khan A. (2004). Antihepatotoxic effect of Nymphaea stellata Willd., against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol, 91, 61–64.
- Bhattacharjee R, Sil PC. (2007). Protein isolate from the herb, Phyllanthus niruri L. (Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Food Chem Toxicol, 45, 817–826.
- Bhattacharyya D, Pandit S, Jana U, Sen S, Sur TK. (2005). Hepatoprotective activity of Adhatoda vasica aqueous leaf extract on d-galactosamine-induced liver damage in rats. Fitoterapia, 76, 223–225.
- Bhattacharyya D, Pandit S, Mukherjee R, Das N, Sur TK. (2003). Hepatoprotective effect of Himoliv, a polyherbal formulation in rats. Indian J Physiol Pharmacol, 47, 435–440.
- Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. (2002). Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci, 27, 139–146.
- Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H, Genina O, Helpern Z, Pines M. (2007). Prevention of liver cirrhosis in rats by curcumin. Liver Int, 27, 373–383.
- Chatterjee M, Sil PC. (2006). Hepatoprotective effect of aqueous extract of Phyllanthus niruri on nimesulide-induced oxidative stress in vivo. Indian J Biochem Biophys, 43, 299–305.
- Chattopadhyay RR, Sarkar SK, Ganguly S, Banerjee RN, Basu TK, Mukherjee A. (1992). Hepatoprotective activity of Azadirachta indica leaves on paracetamol induced hepatic damage in rats. Indian J Exp Biol, 30, 738–740.
- Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. (2008). Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol, 28, 35–43.
- Chen J, Nikolovska-Coleska Z, Wang G, Qiu S, Wang S. (2006). Design, synthesis, and characterization of new embelin derivatives as potent inhibitors of X-linked inhibitor of apoptosis protein. Bioorg Med Chem Lett, 16, 5805–5808.
- Chirdchupunseree H, Pramyothin P. (2010). Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. J Ethnopharmacol, 128, 172–176.
- Dange SV, Shah KU, Bulakh PM, Joshi DR. (1992). Efficacy of stimuliv, an indigenous compound formulation, against hepatotoxicity of antitubercular drugs–a double blind study. Indian J Chest Dis Allied Sci, 34, 175–183.
- De Smet PA. (2002). Herbal remedies. N Engl J Med, 347, 2046–2056.
- Dehmlow C, Erhard J, de Groot H. (1996). Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology, 23, 749–754.
- Devi KP, Sreepriya M, Balakrishna K, Devaki T. (2004). Protective effect of Premna tomentosa (L. Verbenaceae) extract on membrane-bound phosphatases and inorganic cations transport in acetaminophen-induced hepatotoxicity rats. J Ethnopharmacol, 93, 371–375.
- Dhanabal SP, Syamala G, Satish Kumar MN, Suresh B. (2006). Hepatoprotective activity of the Indian medicinal plant Polygala arvensis on d-galactosamine-induced hepatic injury in rats. Fitoterapia, 77, 472–474.
- Dwivedi Y, Rastogi R, Chander R, Sharma SK, Kapoor NK, Garg NK, Dhawan BN. (1990). Hepatoprotective activity of picroliv against carbon tetrachloride-induced liver damage in rats. Indian J Med Res, 92, 195–200.
- Dwivedi Y, Rastogi R, Garg NK, Dhawan BN. (1993). Perfusion with picroliv reverses biochemical changes induced in livers of rats toxicated with galactosamine or thioacetamide. Planta Med, 59, 418–420.
- Dwivedi Y, Rastogi R, Sharma SK, Garg NK, Dhawan BN. (1991). Picroliv affords protection against thioacetamide-induced hepatic damage in rats. Planta Med, 57, 25–28.
- El-Agamy DS. (2010). Comparative effects of curcumin and resveratrol on aflatoxin B(1)-induced liver injury in rats. Arch Toxicol, 84, 389–396.
- El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A, El-Kassas M, Esmat G, Strickland GT. (2009). A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine, 16, 391–400.
- Farghali H, Cerný D, Kameníková L, Martínek J, Horínek A, Kmonícková E, Zídek Z. (2009). Resveratrol attenuates lipopolysaccharide-induced hepatitis in d-galactosamine sensitized rats: Role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide, 21, 216–225.
- Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. (2008). Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol, 46, 1279–1287.
- Fehér J, Deák G, Müzes G, Láng I, Niederland V, Nékám K, Kárteszi M. (1989). [Liver-protective action of silymarin therapy in chronic alcoholic liver diseases]. Orv Hetil, 130, 2723–2727.
- Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. (1989). Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol, 9, 105–113.
- Flora K, Hahn M, Rosen H, Benner K. (1998). Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol, 93, 139–143.
- Flora KD, Rosen HR, Benner KG. (1996). The use of naturopathic remedies for chronic liver disease. Am J Gastroenterol, 91, 2654–2655.
- Fogden E, Neuberger J. (2003). Alternative medicines and the liver. Liver Int, 23, 213–220.
- Gao J, Chen J, Tang X, Pan L, Fang F, Xu L, Zhao X, Xu Q. (2006). Mechanism underlying mitochondrial protection of asiatic acid against hepatotoxicity in mice. J Pharm Pharmacol, 58, 227–233.
- Gao J, Dou H, Tang XH, Xu LZ, Fan YM, Zhao XN. (2004). Inhibitory effect of TCCE on CCl4-induced overexpression of IL-6 in acute liver injury. Acta Biochim Biophys Sin (Shanghai), 36, 767–772.
- Gao J, Tang X, Dou H, Fan Y, Zhao X, Xu Q. (2004). Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. J Pharm Pharmacol, 56, 1449–1455.
- Girish C, Koner BC, Jayanthi S, Ramachandra Rao K, Rajesh B, Pradhan SC. (2009a). Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol, 23, 735–745.
- Girish C, Koner BC, Jayanthi S, Rao KR, Rajesh B, Pradhan SC. (2009b). Hepatoprotective activity of six polyherbal formulations in paracetamol induced liver toxicity in mice. Indian J Med Res, 129, 569–578.
- Gopal N, Sengottuvelu S. (2008). Hepatoprotective activity of Clerodendrum inerme against CCl4 induced hepatic injury in rats. Fitoterapia, 79, 24–26.
- Gupta NK, Dixit VK. (2009a). Evaluation of hepatoprotective activity of Cleome viscosa Linn. extract. Indian J Pharmacol, 41, 36–40.
- Gupta NK, Dixit VK. (2009b). Hepatoprotective activity of Cleome viscosa Linn. extract against thioacetamide-induced hepatotoxicity in rats. Nat Prod Res, 23, 1289–1297.
- Gupta YK, Sharma M, Chaudhary G, Katiyar CK. (2004). Hepatoprotective effect of New Livfit, a polyherbal formulation, is mediated through its free radical scavenging activity. Phytother Res, 18, 362–364.
- Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK, Kim CH. (2005). Protective effect of Lycium chinense fruit on carbon tetrachloride-induced hepatotoxicity. J Ethnopharmacol, 96, 529–535.
- Hall CA, Cuppett SL. (1997). Structure-activities of natural antioxidants, in Antioxidant Methodology In Vivo and in Vitro Concepts, Ed by Auroma OI and Cuppett SL, AOCS Press, Champaign, IL, pp. 141–172.
- Handa SS, Sharma A. (1990a). Hepatoprotective activity of andrographolide against galactosamine & paracetamol intoxication in rats. Indian J Med Res, 92, 284–292.
- Handa SS, Sharma A. (1990b). Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J Med Res, 92, 276–283.
- Harish, R., Shivanandappa, T. (2006). Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem, 95, 180–185.
- Hidaka I, Hino K, Korenaga M, Gondo T, Nishina S, Ando M, Okuda M, Sakaida I. (2007). Stronger Neo-Minophagen C, a glycyrrhizin-containing preparation, protects liver against carbon tetrachloride-induced oxidative stress in transgenic mice expressing the hepatitis C virus polyprotein. Liver Int, 27, 845–853.
- Hsieh CC, Fang HL, Lina WC. (2008). Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. J Ethnopharmacol, 119, 117–121.
- Huseini HF, Alavian SM, Heshmat R, Heydari MR, Abolmaali K. (2005). The efficacy of Liv-52 on liver cirrhotic patients: A randomized, double-blind, placebo-controlled first approach. Phytomedicine, 12, 619–624.
- Hwang YP, Choi CY, Chung YC, Jeon SS, Jeong HG. (2007). Protective effects of puerarin on carbon tetrachloride-induced hepatotoxicity. Arch Pharm Res, 30, 1309–1317.
- Jain A, Soni M, Deb L, Jain A, Rout SP, Gupta VB, Krishna KL. (2008). Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol, 115, 61–66.
- Janbaza KH, Gilanib AH. (2000). Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia, 71, 25–33.
- Jeong GS, Lee DS, Li B, Byun E, Kwon DY, Park H, Kim YC. (2010). Protective effect of sauchinone by upregulating heme oxygenase-1 via the P38 MAPK and Nrf2/ARE pathways in HepG2 cells. Planta Med, 76, 41–47.
- Jeong HG, You HJ, Park SJ, Moon AR, Chung YC, Kang SK, Chun HK. (2002). Hepatoprotective effects of 18beta-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharmacol Res, 46, 221–227.
- Johnson VJ, Osuchowski MF, He Q, Sharma RP. (2002). Physiological responses to a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: II. Alterations in thymic differentiation correlate with changes in c-myc gene expression. Planta Med, 68, 961–965.
- Joshi R, Kamat JP, Mukherjee T. (2007). Free radical scavenging reactions and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. Chem Biol Interact, 167, 125–134.
- Kaláb M, Krechler T. (1997). [The effect of the heptoprotective agent LIV 52 on liver damage]. Cas Lek Cesk, 136, 758–760.
- Kamdem RE, Sang S, Ho CT. (2002). Mechanism of the superoxide scavenging activity of neoandrographolide - a natural product from Andrographis paniculata Nees. J Agric Food Chem, 50, 4662–4665.
- Kang JS, Jeon YJ, Kim HM, Han SH, Yang KH. (2002). Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther, 302, 138–144.
- Kapil A, Koul IB, Banerjee SK, Gupta BD. (1993). Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem Pharmacol, 46, 182–185.
- Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. (2007). Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci, 80, 1033–1039.
- Kim HY, Kim JK, Choi JH, Jung JY, Oh WY, Kim DC, Lee HS, Kim YS, Kang SS, Lee SH, Lee SM. (2010). Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J Pharmacol Sci, 112, 105–112.
- Kim WR, Brown RS Jr, Terrault NA, El-Serag H. (2002). Burden of liver disease in the United States: Summary of a workshop. Hepatology, 36, 227–242.
- Krithika R, Mohankumar R, Verma RJ, Shrivastav PS, Mohamad IL, Gunasekaran P, Narasimhan S. (2009). Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chem Biol Interact, 181, 351–358.
- Kumada H. (2002). Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology, 62 Suppl 1, 94–100.
- Lee CH, Park SW, Kim YS, Kang SS, Kim JA, Lee SH, Lee SM. (2007). Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull, 30, 1898–1904.
- Lee CP, Shih PH, Hsu CL, Yen GC. (2007). Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol, 45, 888–895.
- Lee KJ, Woo ER, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG. (2004). Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci, 74, 1051–1064.
- Lee MK, Kim SH, Yang H, Lim DY, Ryu JH, Lee ES, Jew SS, Park HG, Sung SH, Kim YC. (2009). Asiatic acid derivatives protect primary cultures of rat hepatocytes against carbon tetrachloride-induced injury via the cellular antioxidant system. Nat Prod Commun, 4, 765–768.
- Lee SJ, Ko JH, Lim K, Lim KT. (2005). 150 kDa glycoprotein isolated from Solanum nigrum Linne enhances activities of detoxicant enzymes and lowers plasmic cholesterol in mouse. Pharmacol Res, 51, 399–408.
- Levy C, Seeff LD, Lindor KD. (2004). Use of herbal supplements for chronic liver disease. Clin Gastroenterol Hepatol, 2, 947–956.
- Lin HM, Tseng HC, Wang CJ, Chyau CC, Liao KK, Peng PL, Chou FP. (2007). Induction of autophagy and apoptosis by the extract of Solanum nigrum Linn in HepG2 cells. J Agric Food Chem, 55, 3620–3628.
- Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. (2008). Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem Biol Interact, 171, 283–293.
- Liu J, Wang ZT, Ji LL, Ge BX. (2007). Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol Cell Biochem, 298, 49–57.
- Londhe JS, Devasagayam TP, Foo LY, Ghaskadbi SS. (2008). Antioxidant activity of some polyphenol constituents of the medicinal plant Phyllanthus amarus Linn. Redox Rep, 13, 199–207.
- Luper S. (1998). A review of plants used in the treatment of liver disease: part 1. Altern Med Rev, 3, 410–421.
- Ma K, Zhang Y, Zhu D, Lou Y. (2009). Protective effects of asiatic acid against d-galactosamine/lipopolysaccharide-induced hepatotoxicity in hepatocytes and kupffer cells co-cultured system via redox-regulated leukotriene C4 synthase expression pathway. Eur J Pharmacol, 603, 98–107.
- Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. (1999). Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol, 163, 6800–6809.
- Mayer KE, Myers RP, Lee SS. (2005). Silymarin treatment of viral hepatitis: A systematic review. J Viral Hepat, 12, 559–567.
- Michielsen PP, Francque SM, van Dongen JL. (2005). Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol, 3, 27.
- Miller AL. (1996). Antioxidant flavonoids: Structure, function and clinical usage. Altern Med Rev, 1, 103–111.
- Mitra SK, Seshadri SJ, Venkataranganna MV, Gopumadhavan S, Udupa UV, Sarma DN. (2000). Effect of HD-03–a herbal formulation in galactosamine-induced hepatopathy in rats. Indian J Physiol Pharmacol, 44, 82–86.
- Mitra SK, Venkataranganna MV, Gopumadhavan S, Anturlikar SD, Seshadri SJ, Venkatesha Udupa U. (2001). The protective effect of HD-03 in CCl4-induced hepatic encephalopathy in rats. Phytother Res, 15, 493–496.
- Mitra SK, Venkataranganna MV, Sundaram R, Gopumadhavan S. (1998). Protective effect of HD-03, a herbal formulation, against various hepatotoxic agents in rats. J Ethnopharmacol, 63, 181–186.
- Miyake K, Tango T, Ota Y, Mitamura K, Yoshiba M, Kako M, Hayashi S, Ikeda Y, Hayashida N, Iwabuchi S, Sato Y, Tomi T, Funaki N, Hashimoto N, Umeda T, Miyazaki J, Tanaka K, Endo Y, Suzuki H. (2002). Efficacy of Stronger Neo-Minophagen C compared between two doses administered three times a week on patients with chronic viral hepatitis. J Gastroenterol Hepatol, 17, 1198–1204.
- Miyakoshi M, Yamaguchi Y, Takagaki R, Mizutani K, Kambara T, Ikeda T, Zaman MS, Kakihara H, Takenaka A, Igarashi K. (2004). Hepatoprotective effect of sesquiterpenes in turmeric. Biofactors, 21, 167–170.
- Morikawa T, Pan Y, Ninomiya K, Imura K, Matsuda H, Yoshikawa M, Yuan D, Muraoka O. (2010). Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg Med Chem, 18, 1882–1890.
- Mukherjee T, Joshi R, Kamat JP. (2007). Free radical scavenging reactions and antioxidant activity of embelin: Biochemical and pulse radiolytic studies. Chem Biol Interact, 167, 125–134.
- Mulla WA, Salunkhe VR, Bhise SB. (2009). Hepatoprotective activity of hydroalcoholic extract of leaves of Alocasia indica (Linn.). Indian J Exp Biol, 47, 816–821.
- Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. (1992). Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol, 12, 439–442.
- Muriel P, Mourelle M. (1990). Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol, 10, 275–279.
- Naik RS, Mujumdar AM, Ghaskadbi S. (2004). Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro. J Ethnopharmacol, 95, 31–37.
- Nayak S, Sashidhar RB. (2010). Metabolic intervention of aflatoxin B1 toxicity by curcumin. J Ethnopharmacol, 127, 641–644.
- Oliveira FA, Chaves MH, Almeida FR, Lima RC Jr, Silva RM, Maia JL, Brito GA, Santos FA, Rao VS. (2005). Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol, 98, 103–108.
- Orhan DD, Orhan N, Ergun E, Ergun F. (2007). Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. J Ethnopharmacol, 112, 145–151.
- Parabia, MH, Adhvaryu MR, Reddy N. (2007). Effects of four Indian medicinal herbs on Isoniazid, Rifampicin and Pyrazinamide induced hepatic injury and immunosuppression in guinea pigs. World J Gastroenterol, 13, 3199–205.
- Pradhan SC, Girish C. (2006). Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res, 124, 491–504.
- Raja S, Ahamed KF, Kumar V, Mukherjee K, Bandyopadhyay A, Mukherjee PK. (2007). Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J Ethnopharmacol, 109, 41–47.
- Rajesh MG, Latha MS. (2004). Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol, 91, 99–104.
- Ram VJ. (2001). Herbal preparations as a source of hepatoprotective agents. Drug News Perspect, 14, 353–363.
- Rao GM, Rao CV, Pushpangadan P, Shirwaikar A. (2006). Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol, 103, 484–490.
- Rastogi R, Saksena S, Garg NK, Kapoor NK, Agarwal DP, Dhawan BN. (1996). Picroliv protects against alcohol-induced chronic hepatotoxicity in rats. Planta Med, 62, 283–285.
- Rawat AK, Mehrotra S, Tripathi SC, Shome U. (1997). Hepatoprotective activity of Boerhaavia diffusa L. roots–a popular Indian ethnomedicine. J Ethnopharmacol, 56, 61–66.
- Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. (2007). Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta, 1770, 989–996.
- Rice-Evans CA, Miller NJ, Paganga G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20, 933–956.
- Rivera-Espinoza Y, Muriel P. (2009). Pharmacological actions of curcumin in liver diseases or damage. Liver Int, 29, 1457–1466.
- Rubiolo JA, Mithieux G, Vega FV. (2008). Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol, 591, 66–72.
- Rubiolo JA, Vega FV. (2008). Resveratrol protects primary rat hepatocytes against necrosis induced by reactive oxygen species. Biomed Pharmacother, 62, 606–612.
- Saliou C, Rihn B, Cillard J, Okamoto T, Packer L. (1998). Selective inhibition of NF-kappaB activation by the flavonoid hepatoprotector silymarin in HepG2. Evidence for different activating pathways. FEBS Lett, 440, 8–12.
- Saller R, Meier R, Brignoli R. (2001). The use of silymarin in the treatment of liver diseases. Drugs, 61, 2035–2063.
- Saller R, Melzer J, Reichling J, Brignoli R, Meier R. (2007). An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed, 14, 70–80.
- Sanmugapriya E, Venkataraman S. (2006). Studies on hepatoprotective and antioxidant actions of Strychnos potatorum Linn. seeds on CCl4-induced acute hepatic injury in experimental rats. J Ethnopharmacol, 105, 154–160.
- Santra A, Das S, Maity A, Rao SB, Mazumder DN. (1998). Prevention of carbon tetrachloride-induced hepatic injury in mice by Picrorhiza kurrooa. Indian J Gastroenterol, 17, 6–9.
- Sathesh Kumar S, Ravi Kumar B, Krishna Mohan G. (2009). Hepatoprotective effect of Trichosanthes cucumerina var cucumerina L. on carbon tetrachloride induced liver damage in rats. J Ethnopharmacol, 123, 347–350.
- Schuppan D, Jia JD, Brinkhaus B, Hahn EG. (1999). Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology, 30, 1099–1104.
- Sebai H, Sani M, Yacoubi MT, Aouani E, Ghanem-Boughanmi N, Ben-Attia M. (2010). Resveratrol, a red wine polyphenol, attenuates lipopolysaccharide-induced oxidative stress in rat liver. Ecotoxicol Environ Saf, 73, 1078–1083.
- Sharma M, Tripathi P, Singh VP, Tripathi YB. (1995). Hepatoprotective and toxicological evaluation of hepatomed, an ayurvedic drug. Indian J Exp Biol, 33, 34–37.
- Shiki Y, Shirai K, Saito Y, Yoshida S, Mori Y, Wakashin M. (1992). Effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J Gastroenterol Hepatol, 7, 12–16.
- Singh B, Saxena AK, Chandan BK, Agarwal SG, Anand KK. (2001). In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian J Physiol Pharmacol, 45, 435–441.
- Singh D, Singh R, Singh P, Gupta RS. (2009). Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin Pharmacol Toxicol 105, 243–248.
- Singha PK, Roy S, Dey S. (2007). Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in mice. J Ethnopharmacol, 111, 13–21.
- Sonnenbichler J, Zetl I. (1986). Biochemical effects of the flavonolignane silibinin on RNA, protein and DNA synthesis in rat livers. Prog Clin Biol Res, 213, 319–331.
- Sreepriya M, Bali G. (2005). Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia, 76, 549–555.
- Sreepriya M, Bali G. (2006). Effects of administration of embelin and curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol Cell Biochem, 284, 49–55.
- Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. (2002). Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol, 97, 2391–2397.
- Suja SR, Latha PG, Pushpangadan P, Rajasekharan S. (2004). Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J Ethnopharmacol, 92, 61–66.
- Sun ZL, Wang Y, Guo ML, Li YX. (2010). Two new hepaprotective saponins from Semen celosiae. Fitoterapia, 81, 375–380.
- Sung SH, Kim YC. (2000). Hepatoprotective diastereomeric lignans from Saururus chinensis herbs. J Nat Prod, 63, 1019–1021.
- Sung SH, Lee EJ, Cho JH, Kim HS, Kim YC. (2000). Sauchinone, a lignan from Saururus chinensis, attenuates CCl4-induced toxicity in primary cultures of rat hepatocytes. Biol Pharm Bull, 23, 666–668.
- Takahara T, Watanabe A, Shiraki K. (1994). Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. J Hepatol, 21, 601–609.
- Tang X, Gao J, Wang Y, Fan YM, Xu LZ, Zhao XN, Xu Q, Qian ZM. (2006). Effective protection of Terminalia catappa L. leaves from damage induced by carbon tetrachloride in liver mitochondria. J Nutr Biochem, 17, 177–182.
- Tome S, Lucey MR. (2004). Review article: Current management of alcoholic liver disease. Aliment Pharmacol Ther, 19, 707–714.
- Torres M, Rodríguez-Serrano F, Rosario DJ, Rodríguez-Perez F, Toro DH. (2004). Does Silybum marianum play a role in the treatment of chronic hepatitis C? P R Health Sci J, 23, 69–74.
- Upadhyay G, Singh AK, Kumar A, Prakash O, Singh MP. (2008). Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur J Pharmacol, 596, 146–152.
- Verma PC, Basu V, Gupta V, Saxena G, Rahman LU. (2009). Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa Royle ex benth. (kutki). Curr Pharm Biotechnol, 10, 641–649.
- Vidyashankar S, K Mitra S, Nandakumar KS. (2010). Liv.52 protects HepG2 cells from oxidative damage induced by tert-butyl hydroperoxide. Mol Cell Biochem, 333, 41–48.
- Vidyashankar S, Patki PS. (2010). Liv.52 attenuate copper induced toxicity by inhibiting glutathione depletion and increased antioxidant enzyme activity in HepG2 cells. Food Chem Toxicol, 48, 1863–1868.
- Visen PKS, Shukia B, Patnaik GK, Dhawan BN. (1993). Andrographolide protects rat hepatocytes against paracetamol-induced damage. J Ethnopharmacol, 40, 131–136.
- Vitaglione P, Ottanelli B, Milani S, Morisco F, Caporaso N, Fogliano V. (2009). Dietary trans-resveratrol bioavailability and effect on CCl4-induced liver lipid peroxidation. J Gastroenterol Hepatol, 24, 618–622.
- Wang F, Zhou HY, Zhao G, Fu LY, Cheng L, Chen JG, Yao WX. (2004). Inhibitory effects of berberine on ion channels of rat hepatocytes. World J Gastroenterol, 10, 2842–2845.
- Wang JY, Guo JS, Li H, Liu SL, Zern MA. (1998). Inhibitory effect of glycyrrhizin on NF-kappaB binding activity in CCl4- plus ethanol-induced liver cirrhosis in rats. Liver, 18, 180–185.
- Wang L, Cheng D, Wang H, Di L, Zhou X, Xu T, Yang X, Liu Y. (2009). The hepatoprotective and antifibrotic effects of Saururus chinensis against carbon tetrachloride induced hepatic fibrosis in rats. J Ethnopharmacol, 126, 487–491.
- Wang T, Sun NL, Zhang WD, Li HL, Lu GC, Yuan BJ, Jiang H, She JH, Zhang C. (2008). Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J Ethnopharmacol, 117, 300–308.
- Wiseman H. (1996). Dietary influences on membrane function: Importance in protection against oxidative damage and disease. J Nutr Biochem, 7, 2–5.
- Xin-Hua W, Chang-Qing L, Xing-Bo G, Lin-Chun F. (2001). A comparative study of Phyllanthus amarus compound and interferon in the treatment of chronic viral hepatitis B. Southeast Asian J Trop Med Public Health, 32, 140–142.
- Xiong Q, Hase K, Tezuka Y, Namba T, Kadota S. (1999). Acteoside inhibits apoptosis in d-galactosamine and lipopolysaccharide-induced liver injury. Life Sci, 65, 421–430.
- Xiong Q, Hase K, Tezuka Y, Tani T, Namba T, Kadota S. (1998). Hepatoprotective activity of phenylethanoids from Cistanche deserticola. Planta Med, 64, 120–125.
- Xiong Q, Kadota S, Tani T, Namba T. (1996). Antioxidative effects of phenylethanoids from Cistanche deserticola. Biol Pharm Bull, 19, 1580–1585.
- Xue Q, Sun ZL, Guo ML, Wang Y, Zhang G, Wang XK. (2010). Two new compounds from Semen celosiae and their protective effects against CCl(4)-induced hepatotoxicity. Nat Prod Res, 1–8.
- Yang BS, Ma YJ, Wang Y, Chen LY, Bi MR, Yan BZ, Bai L, Zhou H, Wang FX. (2007). Protective effect and mechanism of stronger neo-minophagen C against fulminant hepatic failure. World J Gastroenterol, 13, 462–466.
- Yanpallewar SU, Sen S, Tapas S, Kumar M, Raju SS, Acharya SB. (2003). Effect of Azadirachta indica on paracetamol-induced hepatic damage in albino rats. Phytomedicine, 10, 391–396.
- Yoshikawa M, Matsui Y, Kawamoto H, Umemoto N, Oku K, Koizumi M, Yamao J, Kuriyama S, Nakano H, Hozumi N, Ishizaka S, Fukui H. (1997). Effects of glycyrrhizin on immune-mediated cytotoxicity. J Gastroenterol Hepatol, 12, 243–248.
- Zeashan H, Amresh G, Singh S, Rao CV. (2008). Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem Toxicol, 46, 3417–3421.
- Zhang L, Li HZ, Gong X, Luo FL, Wang B, Hu N, Wang CD, Zhang Z, Wan JY. (2010). Protective effects of Asiaticoside on acute liver injury induced by lipopolysaccharide/d-galactosamine in mice. Phytomedicine, 17, 811–819.
- Zhang L, Wang B. (2002). Randomized clinical trial with two doses (100 and 40 ml) of Stronger Neo-Minophagen C in Chinese patients with chronic hepatitis B. Hepatol Res, 24, 220.
- Zhao J, Liu T, Ma L, Yan M, Zhao Y, Gu Z, Huang Y. (2009). Protective effect of acteoside on immunological liver injury induced by Bacillus Calmette-Guerin plus lipopolysaccharide. Planta Med, 75, 1463–1469.
- Zhao X, Zhang JJ, Wang X, Bu XY, Lou YQ, Zhang GL. (2008). Effect of berberine on hepatocyte proliferation, inducible nitric oxide synthase expression, cytochrome P450 2E1 and 1A2 activities in diethylnitrosamine- and phenobarbital-treated rats. Biomed Pharmacother, 62, 567–572.