Abstract
Objective: Different species of Artemisia (Asteraceae) have shown to exhibit antitumor activity. The aim of this study was to identify the antiproliferative effect of some Artemisia species from Iran on cultured human cancer cells.
Materials and methods: Methanol, ethyl acetate, dichloromethane and n-hexane extracts from aerial parts of seven species of Artemisia were prepared and their antiproliferative effects on four cancer (AGS, HeLa, HT-29 and MCF-7) and normal cell line (L929) were determined. Different concentrations of extracts were added to cultured cells and incubated for 72 h. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was employed to assess the cell viability.
Results: Different extracts exert various growth inhibitory effects. In case of AGS cells, dichloromethane and methanol extracts of A. ciniformis Krasch. & Popov ex Poljak. (IC50 values: 35 and 60 µg/ml, respectively) showed the highest growth inhibitory effects. HeLa cells were more sensitive to both A. diffusa Krasch. ex Poljak. dichloromethane (IC50 value: 71 µg/ml) and A. ciniformis ethylacetate (IC50 value: 73 µg/ml) extracts. Dichloromethane extracts of A. diffusa, A. santolina Schrenk and A. ciniformis (IC50 values: 42, 91 and 94 µg/ml, respectively) exhibited more inhibition on HT-29 cells in comparison to other extracts. MCF-7 cells were best inhibited by A. ciniformis dichloromethane (IC50 value: 29 µg/ml) and A. vulgaris L. ethyl acetate (IC50 value: 57 µg/ml) extracts.
Discussion and conclusion: This study shows the antiproliferative effects of Artemisia extracts on malignant cell lines. Artemisia could be also considered as a promising chemotherapeutic agent in cancer treatment.
Introduction
Prevention of cancer is one of the most important public health and medicinal issues in this century. In past two decades, many natural products have been studied for their chemopreventive effects. Many plants with different pharmacological properties are known to contain a variety of chemical compounds that may have the potential for the prevention or treatment of malignancies (CitationRao et al., 2002; CitationZhang et al., 2002). Plants have many phytochemicals with various bioactivities, including antioxidant, anti-inflammatory and antitumoral activities. It has been shown that many extracts from natural products have positive effects against cancer, compared with chemotherapy or recent hormonal treatments (CitationPezzuto, 1997). Many plants have also been examined to identify new and effective anticancer compounds (CitationKim et al., 1998; Pietta et al., 1998; CitationSwamy & Tan, 2000). Biological methods have been extensively employed to evaluate antitumoral activity of natural products in the past three decades.
The genus Artemisia L. is one of the largest and most widely distributed of the Astraceae (Compositae). This genus is a large taxon, numbering over 400 species distributed mainly in the temperate zone of Europe, Asia and North America. These species are perennial, biennial and annual herbs or small shrubs. Leaves are alternate, capitulate small, usually race mouse, paniculate or capitate, inflorescence, rarely solitary. Involucral bracts in few rows. Receptacle flat to hemispherical, without scales and sometimes hirsute. Florets all tubular, achiness obvoid, pappus absent or sometimes a small scarious ring (CitationTutin & Persson, 1976; CitationPodlech, 1986; CitationPolyako, 1995; CitationHeywood & Humphries, 1997; CitationMucciaralli & Maffel, 2002). The genus in Iran has 30 species, two of which are endemic to the country (CitationPodlech, 1986; CitationGhahreman & Attar, 1999; CitationEmami & Aghazari, 2008). Some chemical components of the genus include monoterpenes, sesquiterpenes, sesquiterpene lactones, flavonoides, coumarins, sterols, polyacetylenes (CitationTan et al., 1998; CitationMucciaralli & Maffel, 2002). Different species of Artemisia have a wide range of biological effects including antimalarial (CitationTan et al., 1998), cytotoxic (CitationZheng, 1994), antibacterial, antifungal (CitationTan et al., 1998), anti-inflammatory (CitationEmami et al., 2010) and antioxidant (CitationCakir et al., 2005) characteristics.
This study was designed to determine the antiproliferative effects of methanol, ethylacetate, dichloromethane and n-hexane extracts from some Artemisia species of Iran on human cancer cells lines.
Materials and methods
Plant material
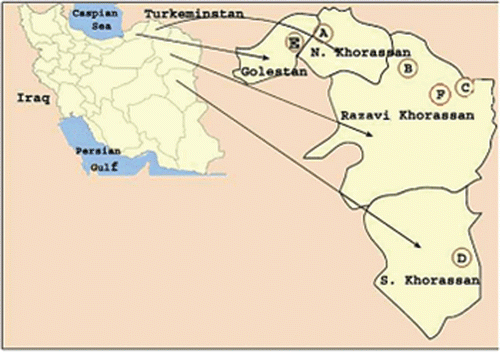
Seven species of Artemisia were collected from different regions of Iran ( and ). Dr. Mozaffarian, Research Institute of Forest and Rangelands, Ministry of Jahad- Keshavarzi, Iran, identified these species. Voucher specimens have been deposited in the Herbarium of School of Pharmacy, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. Related information on these plants and their names in Farsi is summarized in (CitationMozaffarian, 2003).
Table 1. Characteristics of tested Artemisia species.
Preparation of the plant extracts
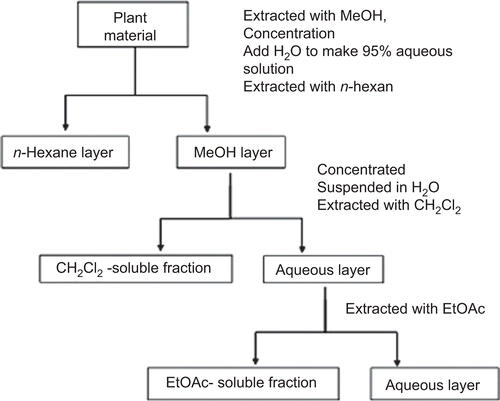
Plant aerial parts of each species were shade-dried at room temperature (25–30°C) and later ground into a fine powder using a household blender and sieved with a 2 mm-diameter mesh. Pulverized materials (100 g) were extracted using a percolator with methanol and were then concentrated at 50°C under reduced pressure to dry. This material was then extracted three times with an equal volume of n-hexane to give an extract containing nonpolar compounds. Then, the solution was successively partitioned between dichloromethane (CH2Cl2), ethyl acetate (EtOAc; CitationOtsuka, 2006). These isolated extracts were dried. A partitioning scheme of the methanol extract of each Artemisia species is presented in .
Cell cultures
Four different cell lines, AGS (human gastric adenocarcinoma cell line, NCBI#: C131), HeLa (human cervix carcinoma cell line, NCBI#: C115), HT-29 (human colon adenocarcinoma cell line, NCBI#: C466) and MCF-7 (human breast carcinoma cell line, NCBI#: C135) and L929 (mouse fibroblast-like cell line NCBI#: C161), were obtained from National Cell Bank of Iran (Tehran, Iran). Cell lines were grown as a monolayer culture in Dulbecco modified Eagle’s medium (DMEM) from Gibco Laboratories (Detroit, USA) and supplemented with 10% fetal bovine serum (Gibco Laboratories, Detroit, USA), 1% penicillin/streptomycin (100 IU/ml and 100 µg/ml, respectively) from Gibco Laboratories (Detroit, USA) in a 5% CO2-humidified atmosphere at 37°C. For the experiments, cells were removed from the flasks using a 0.25% trypsin-EDTA solution.
In vitro proliferation assay
Growth inhibition of normal and tumor cells by obtained extracts was measured using a rapid 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma Chemical Co., St. Louis, MO) colorimetric assay (CitationMosmann, 1983) and compared with untreated controls. Briefly, cells (1 × 104 cells per each well) were seeded into 96-well microculture plates and allowed to adhere for 24 h before treatment. Then, each tumor cell line was exposed to extracts at 20, 40, 60, 80, 100, 250, 500, 750, 1000, 1500 and 2000 µg/ml concentrations for 72 h in triplicate. The first column of each microplate was assumed as negative control (containing no extracts). As obtained Artemisia extracts could interfere with MTT salts, the medium was replaced with fresh culture medium to add the MTT salts. Then, to assay the cell survival, 25 µl of MTT solution (5 mg/ml in phosphate buffered solution) was added to each well and the plates were subsequently incubated for 3 h at 37°C. Then, the produced formazan crystals were dissolved using dimethyl sulfoxide (DMSO). The optical density (OD) was read on a plate reader at 570 nm. The inhibitory rate of cell proliferation was calculated according to the following formula:
The growth inhibitory activity of extracts against a variety of cancer cell lines expressed as IC50 values (the extract concentration reducing by 50% the absorbance in treated cells with respect to untreated cells) were determined from dose-response curves.
All treatments were done by setting up three independent experiments on separate days, each time with three or more wells for each concentration in 96-well plates.
Results
In this study, growth inhibitory activity of methanol, ethyl acetate, dichloromethane and n-hexane extracts from seven Artemisia species were screened against a panel of human cancer cell lines representing different histological types including AGS, HeLa, HT-29 and MCF-7 cell lines. Results are shown as IC50 values in .
Table 2. Growth inhibition activity of different extracts from Artemisia species on AGS, HeLa, HT-29 and MCF-7 cancer cell lines. Data are shown as IC50 values (µg/ml).
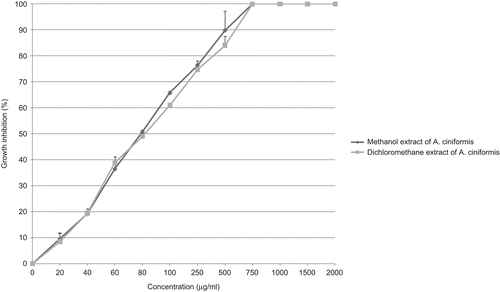
For AGS cell line, isolated extracts inhibited the cell growth with IC50 values ranging from 35 µg/ml (for dichloromethane extract of A. ciniformis Krasch. & Popov ex Poljak.; ) up to more than 2000 µg/ml (for dichloromethane extract of A. diffusa Krasch. ex Poljak.). Methanol extract of A. ciniformis (IC50 value: 60 µg/ml) also had a high growth inhibitory effect on AGS cell line ().
Figure 4. Antiproliferative effect of methanol and dichloromethane extracts from A. ciniformis on AGS cancer cell line.
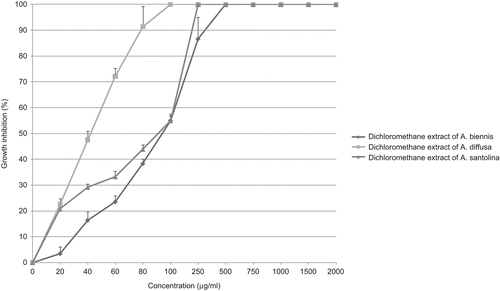
In case of HeLa cell line, obtained extracts showed growth inhibitory activity with IC50 values ranging from 71 µg/ml (for dichloromethane extract of A. diffusa; ) up to more than 2000 µg/ml (for methanol extracts of A. diffusa, A. persica Boiss, A. santolina Schrenk and A. vulgaris L.). It seems most methanol extracts had no remarkable inhibitory effect on the growth of HeLa cells. Ethyl acetate extract of A. ciniformis, dichloromethane extract of A. biennis Willd and dichloromethane extract of A. ciniformis (IC50 values: 73, 74 and 97 µg/ml, respectively) showed considerable growth inhibitory activity ().
Figure 5. Antiproliferative effect of dichloromethane extracts from A. diffusa, A. biennis, A. ciniformis and ethyl acetate extract from A. ciniformis on HeLa cancer cell line.
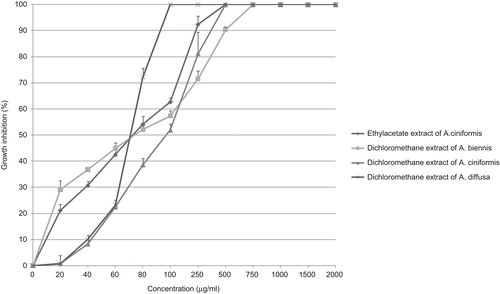
Dichloromethane extracts of A. diffusa, A. santolina and A. ciniformis (IC50 values: 42, 91 and 94 µg/ml, respectively) exhibited more inhibition on HT-29 cell line in comparison to other extracts (). However, methanol extract of A. santolina (IC50 value: more than 2000 µg/ml) had the lowest inhibitory effect on HT-29 cells.
Figure 6. Antiproliferative effect of dichloromethane extracts from A. diffusa, A. ciniformis and A. santolina on HT-29 cancer cell line.
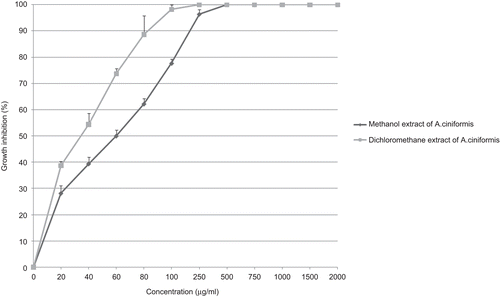
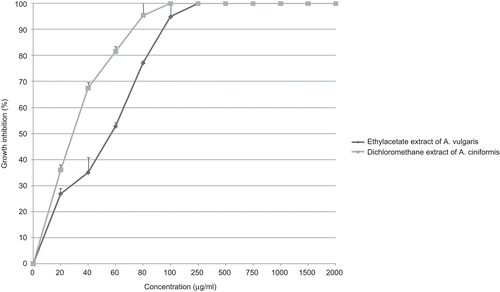
Although MCF-7 cells were best inhibited by A. ciniformis dichloromethane (IC50 value: 29 µg/ml) and A. vulgaris ethyl acetate (IC50 value: 57 µg/ml) extracts (), it was not inhibited by n-hexane extract of A. diffusa (IC50 value: more than 2000 µg/ml).
Figure 7. Antiproliferative effect of dichloromethane extract from A. ciniformis and ethyl acetate extract from A. vulgaris on MCF-7 cancer cell line.
Overall, among all tested extracts on cancer cell lines, A. ciniformis dichloromethane extract seems to be a strong inhibitor for the growth of a wide variety of tumor cell lines including AGS, HeLa, HT-29 and MCF-7 cell lines (IC50 values: 35, 97, 94 and 29 µg/ml, respectively).
We also examined normal fibroblast-like cell line (L929 cell line) for growth inhibitory activity of different extracts of Artemisia species. L929 cell growth was best inhibited by A. ciniformis methanol and dichloromethane extracts (IC50 values: 79 and 82 µg/ml, respectively; ). In addition, A. diffusa n-hexane extract (IC50 value: 84 µg/ml) showed high inhibition on the growth of L929 cells.
Discussion
Cancer is a rising health problem around the world. Natural products have long been used to prevent and treat many diseases, including cancer, and thus, they are considered suitable candidates for the development of anticancer drugs (CitationSmith-Warner et al., 2000).
Various reports have been published on antiproliferative activity of different Artemisia spp. fractions and/or extracts. Essential oil of Artemisia annua could induce apoptosis of cultured SMMC-7721 cells. Artemisinin, a compound isolated from the sweet wormwood (A. annua), has shown to have selective toxicity toward cancer cells in vitro. Moreover, it is given orally to retard breast cancer development in 7,12-dimethylbenz[α]anthracene (DMBA)-treated rats (CitationLi et al., 2004; CitationHenry & Narendra, 2006). Jaceosidin was isolated from Artemisia argyi as a putative oncogene inhibitor. It might be used as a potential drug for the treatment of cervical cancers associated with human papillomavirus and ovary cancer (CitationLee et al., 2005; CitationLv et al., 2008). Whole herbal extracts of A. asiatica has been used in oriental traditional medicine for the treatment of inflammation, cancer and other disorders. Eupatilin, a pharmacologically active ingredient of A. asiatica, exhibited antiproliferative effect on HL-60 and AGS cells (CitationSeo & Surh, 2001; CitationKim et al., 2005). The ability of A. princeps smoke and water extracts to induce apoptosis was evaluated in vitro on human breast cancer MCF-7 cells. Its smoke and water-soluble extracts induce apoptosis via the mitochondrial pathway in breast cancer cells (CitationSarath et al., 2007). Yomogin is an active compound isolated from A. princeps, a traditional oriental medicinal herb, which has been shown to inhibit the proliferation of human promyelocytic leukemia cells through the induction of apoptosis (CitationJeong et al., 2004). Aqueous and methanol herbal extracts from A. capillaries and A. annua L. showed inhibitory activity against different cancer cell lines (CitationSun et al., 2007). Cirsilineol, a compound isolated from A. vestita, inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway (CitationSheng et al., 2008). Smoke and water-soluble extracts from dried leaves of A. princeps induce apoptosis via mitochondrial pathway in human breast cancer MCF-7 cells (CitationSarath et al., 2007). DA-9601, a standardized extract of A. asiatica, blocked TNF-α-mediated inflammatory signals by potentially modulating the p38 kinase pathway and/or a signal leading to NF-κB dependent pathways in gastric epithelial cells (CitationChoi et al., 2006).
Here, we report antiproliferative effects of some Artemisia species from Iran against different cancer cell lines. Although these species have been used in folk medicine, however, this is the first report of their antiproliferative activity. All Artemisia extracts showed degrees of inhibitory effects on cultured cancer cell lines. For AGS, dichloromethane and methanol extracts from A. ciniformis showed the highest inhibitory effects. The highest inhibitory effects on HeLa were for dichloromethane extract from A. diffusa and ethyl acetate extract from A. ciniformis. For HT-29, methanol extract from A. annua and dichloromethane extract from A. diffusa had the highest inhibitory effects. Dichloromethane extract from A. ciniformis and ethyl acetate extract from A. vulgaris showed the highest inhibitory effect on MCF-7 cell line.
Among different extracts from studied Artemisia species, dichloromethane extract from A. ciniformis showed the highest overall inhibitory effect on various cancer cell lines.
Conclusion
This study is the first to show the antiproliferative activity of different extracts from various Artemisia species collected in Iran. These extracts showed varied inhibitory effects on different cancer cell lines. Based on the overall strong inhibition effects of A. ciniformis dichloromethane extract, isolation and studying of its compounds is suggested.
Declaration of interest
This study was supported by a grant (No. 86468) from Mashhad University of Medical Sciences (MUMS) vice president for research, Mashhad, Iran.
References
- Cakir A, Kilic H, Kodali S, Mavi A, Yildirim A. (2005). Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem, 53, 1408–1416.
- Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, Lee WJ, Park JS, Shin CY, Oh TY, Jun CD. (2006). DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol, 12, 4850–4858.
- Emami SA, Aghazari F. (2008). Les Phanerogames endemiques de la flore d’ Iran publication de l’ Universiteâ€2 d’ Iran des Sciences Medicales. Téhéran, 349.
- Emami SA, Taghizadeh Rabe SZ, Iranshahi M, Ahi A, Mahmoudi M. (2010). Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacol Immunotoxicol, 32, 688–695.
- Ghahreman A, Attar F. (1999). Biodiversity of Plant Species in Iran. Tehran: Tehran University Publication, volume 1, 41–42.
- Henry L, Narendra P. (2006). Singh oral artemisinin prevents and delays the development of 7,12-dimethylbenz[α]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett, 231, 43–48.
- Heywood VH, Humphries CJ. (1997). Anthemideae systematic review. In: Heywood VH, Harborn JB, Turner BL, ed. The Biology and Chemistry of the Compositae. London: Academic Press, volume 2, 868.
- Jeong SH, Koo SJ, Ha JH, Ryu SY, Park HJ, Lee KT. (2004). Induction of apoptosis by yomogin in human promyelocytic leukemic HL-60 cells. Biol Pharm Bull, 27, 1106–1111.
- Kim JH, Park MK, Lee JY, Okuda H, Kim S, Hwang WI. (1998). Antioxidant and antitumor effects of Manda. Biochem Arch., 14, 211–219.
- Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh Ph D Professor YJ. (2005). Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol, 24, 261–269.
- Lee HG, Yu KA, Oh WK, Baeg TW, Oh HC, Ahn JS, Jang WC, Kim JW, Lim JS, Choe YK, Yoon DY. (2005). Inhibitory effect of jaceosidin isolated from Artemisiaargyi on the function of E6 and E7 oncoproteins of HPV 16. J Ethnopharmacol, 98, 339–343.
- Li Y, Li MY, Wang L, Jiang ZH, Li WY, Li H. (2004). [Induction of apoptosis of cultured hepatocarcinoma cell by essential oil of Artemisia annul L]. Sichuan Da Xue Xue Bao Yi Xue Ban, 35, 337–339.
- Lv W, Sheng X, Chen T, Xu Q, Xie X. (2008). Jaceosidin induces apoptosis in human ovary cancer cells through mitochondrial pathway. J Biomed Biotechnol, 2008, 394802.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 65, 55–63.
- Mozaffarian V. (2003). A Dictionary of Iranian Plant Names. Tehran: Farhang Moasser Publication, 56–58.
- Mucciaralli M, Maffel M. (2002). Introduction of the genus. In: Wright CW, ed. Artemisia. London: Taylor and Francis, 1–50.
- Otsuka H. (2006). Purification by solvent extraction using partition coefficient. In: Sarker SD, Latif Z, Gray AL, ed. Natural Products Isolation. 2nd ed. Totowa, New Jersey: Humana Press, 269–273.
- Pezzuto JM. (1997). Plant-derived anticancer agents. Biochem Pharmacol, 53, 121–133.
- Podlech D. (1986). In: Rechinger KH, ed. Flora Iranica Akademische Druck-u. Verlagsansalt, Graz, 158, 159–223.
- Polyakov P. (1995). Artemisia. In: Shishkin BK, ed. Flora of the USSR (English translation). Germany: Koeringstein, Bisnen Singh Scientific Books, 29, 488–489.
- Rao CV, Newmark HL, Reddy BS. (2002). Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect Prev, 26, 419–425.
- Sarath VJ, So CS, Won YD, Gollapudi S. (2007). Artemisia princeps var orientalis induces apoptosis in human breast cancer MCF-7 cells. Anticancer Res, 27, 3891–3898.
- Seo HJ, Surh YJ. (2001). Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human promyelocytic leukemia cells. Mutat Res, 496, 191–198.
- Sheng X, Sun Y, Yin Y, Chen T, Xu Q. (2008). Cirsilineol inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway. J Pharm Pharmacol, 60, 1523–1529.
- Smith-Warner SA, Elmer PJ, Tharp TM, Fosdick L, Randall B, Gross M, Wood J, Potter JD. (2000). Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev, 9, 307–317.
- Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. (2007). In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res, 21, 1102–1104.
- Swamy SM, Tan BK. (2000). Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol, 70, 1–7.
- Tan RX, Zheng WF, Tang HQ. (1998). Biologically active substances from the genus Artemisia. Planta Med, 64, 295–302.
- Tutin TG, Persson K. (1976). Artemisia. In: Tutin TG, eds. Cambridge: Flora Europae Cambridge University Press, 1, 178–186.
- Zhang H, Spitz MR, Tomlinson GE, Schabath MB, Minna JD, Wu X. (2002). Modification of lung cancer susceptibility by green tea extract as measured by the comet assay. Cancer Detect Prev, 26, 411–418.
- Zheng GQ. (1994). Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med, 60, 54–57.