Abstract
Context: Cancer is a major public health problem in India and many other parts of the world. Its two main characteristics are uncontrolled cell growth and metastasis. Natural products represent a rich source of compounds that have found many applications in various fields of medicines and therapy including cancer therapy. Effective ingredients in several plant-derived medicinal extracts are terpenoid compounds and many terpenes have biological activities and are used for the treatment of human diseases.
Objectives: This review attempted to collect all available published scientific literature of eight naturally occurring terpenoids and their effect on inhibition of tumor progression.
Methods: The present review is about eight potent naturally occurring terpenoids that have been studied for their pharmacological properties in our lab and this review includes 130 references compiled from all major databases.
Results: Literature survey revealed that triterpenoids, such as glycyrrhizic acid, ursolic acid, oleanolic acid, and nomilin, the diterpene andrographolide, and the monoterpenoids like limonene and perillic acid had shown immunomodulatory and antitumor activities. All of them could induce apoptosis in various cancer cells by activating various proapoptotic signaling cascades. Many of these terpenoids found to inhibit metastatic progression and tumor-induced angiogenesis. The molecular mechanisms that involved in these activities include inhibition of various oncogenic and anti-apoptotic signaling pathways and suppression or nuclear translocation of various transcription factors including nuclear factor kappa B (NF-κB).
Conclusion: The chemopreventive and chemoprotective effects of these compounds point toward their possible role in modern anticancer therapies.
Introduction
Cancer is a hyperproliferative disorder that involves transformation, dysregulation of apoptosis, proliferation, invasion, angiogenesis, and metastasis. Extensive research during the last 30 years has revealed much about the biology of cancer. Natural products have been an important source of chemotherapeutics for many years; more than half of effective cancer drugs can be traced to natural in origins. Development of naturally derived anticancer drugs, therefore, is crucial, and isolation of novel compounds has become an important part of cancer research. Drugs used to treat most cancers are those that can block cell signaling, including growth factor signaling, inflammation, drug resistance, cell cycle, metastasis, angiogenesis, and apoptosis. Numerous reports have suggested that plants and their components mediate their effects by modulating several of these recently identified therapeutic targets (CitationAggarwal et al., 2006; CitationMa and Wang, 2009). Cancer chemoprevention by naturally occurring substances, especially by those occurring in vegetable foods and medicinal plants, seems to be a promising approach and various phytochemicals isolated from these sources have shown potent chemopreventive activity in animal experiments. Terpenoids have been shown to possess chemopreventive activities in animal models.
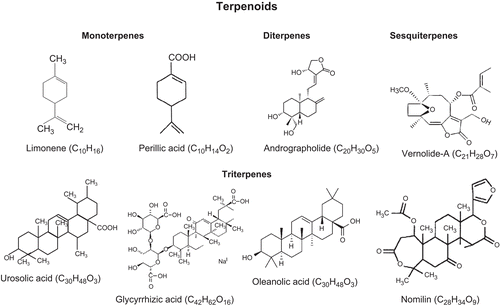
Terpenoids are minor but ubiquitous components of our diet, and are considered relatively nontoxic to humans. These compounds, therefore, have the potential of being used as cancer chemopreventive agents (CitationAkihisa et al., 2003). Terpenoids, also referred to as terpenes, are the largest group of natural compounds. They are plant secondary metabolites along with alkaloids and flavonoids. Many terpenes have biological activities and are used for the treatment of human diseases. Terpenoids are formed from five-carbon isoprene units (C5H8) and are also called isoprenoids. Based on the number of the building blocks, terpenoids are commonly classified as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), and triterpenes (C30) () (CitationWang et al., 2005). These terpenoids display a wide range of biological activities. In this review, we focus on the usefulness of selected terpenoids that have anticancer properties.
Glycyrrhizic acid
Glycyrrhizic acid (18β-GL or GL), a glycoside of glycyrrhetenic acid, is the main bioactive ingredient of licorice Glycyrrhiza glabra L. (Fabaceae), an important medicinal plant in Ayurvedic system of medicine. It is an herbal drug with a broad spectrum of pharmacological effects and multiple sites of action. CitationFiore et al. (2005) have reviewed the therapeutic use of licorice from ancient times to the present. Licorice has been used to treat several diseases of the cardiovascular, gastrointestinal, and respiratory systems (CitationFiore et al., 2005). Hypertension can be a side effect of the excessive use of licorice. The major constituent of glycyrrhizin is glycyrrhizic acid, which can inhibit nuclear factor kappa B (NF-κB) signaling (CitationKang et al., 2005; CitationCherng et al., 2006). The calcium-mediated activation of the NF-κB system was suppressed by glycyrrhizic acid. Glycyrrhizic acid has been shown to inhibit glutamate-induced cytotoxicity in primary neurons (CitationCherng et al., 2006).
Several immunomodulatory activities have been attributed to glycyrrhizin (CitationOhuchi et al., 1981; CitationKobayashi et al., 1993; CitationZhang et al., 1993; CitationKondo and Takano, 1994; CitationRaphael and Kuttan, 2003). Our laboratory had already reported the immunomodulatory effect of glycyrrhizic acid in normal and tumor-bearing mice (CitationRaphael and Kuttan, 2003, Citation2008). Glycyrrhizic acid could enhance the total WBC count and the maximum count was observed only on the ninth day. Glycyrrhizic acid showed 36.8% and 15.9% increase in bone marrow cellularity and number of α-esterase-positive cells, respectively. It could also enhance the antibody production and number of antibody-producing cells. Glycyrrhizic acid could inhibit
the delayed-type hypersensitivity (DTH) reaction remarkably. A 95% of inhibition of DTH reaction was observed in glycyrrhizic acid treated group compared with the normal untreated group of animals. The cell-mediated immune response also found enhanced by the treatment of glycyrrhizic acid. Glycyrrhizic acid can augment the NK cell activity. Administration of glycyrrhizic acid clearly enhanced the antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent complement-mediated cytotoxicity (ACC) in B16F-10 metastatic melanoma-bearing mice.
The elevated level of GM-CSF in tumor-alone treated control animals was reduced by the treatment with glycyrrhizic acid (20.3 pg/mL). The level of interleukin (IL)-2 was enhanced by the treatment with glycyrrhizic acid compared with untreated tumor-bearing control animals (CitationRaphael and Kuttan, 2008). Glycyhrrtinic acid was found as an inducer of type 2 antagonistic CD41 T cells in vivo and in vitro (CitationUtsunomiya et al., 1995; CitationNakajima et al., 1996). It also stimulated macrophage-derived NO production, and was able to up-regulate inducible nitric oxide synthase (iNOS) expression through NF-κB transactivation in murine macrophages (CitationJeong and Kim, 2002). Both of them could induce interferon (IFN) activity and augment natural killer (NK) cell activity (CitationAbe et al., 1982). Glycyrrhizic acid also had inhibitory effects on tumor necrosis factor (TNF)-α-induced IL-8 production in intestinal epithelial cells (CitationKang et al., 2005). Glycyrrhizin could selectively activate extrathymic T cells in the liver and in human T-cell lines and glycyrrhizic acid enhanced Fas-mediated apoptosis without alteration of caspase-3-like activity (CitationKimura et al., 1992; CitationIshiwata et al., 1999).
Glycyrrhizin inhibited reactive oxygen species (ROS) generation by neutrophils that are the potent mediator of tissue inflammation in the in vitro system (CitationAkamatsu et al., 1991; CitationWang and Nixon, 2001). Two mechanisms have been suggested for the anti-inflammatory effects of β-glycyhrritinic acid: First, it inhibits glucocorticoid metabolism and potentiates their effects. This potentiation was reported in skin and lung after co-administration of them with β-glycyhrritinic acid (CitationTeelucksingh et al., 1990; CitationSchleimer, 1991). Since β-glycyhrritinic acid is a potent inhibitor of 11β-hydroxysteroid dehydrogenase (CitationWalker and Edwards, 1991), it causes an accumulation of glucocorticoids with anti-inflammatory properties. Oral administration of β-glycyhrritinic acid or glycyrrhizin confirmed this result (CitationMacKenzie et al., 1990). Second, it inhibits classical complement pathway activation and its activity is dependent on its conformation (CitationKroes et al., 1997). Thus, it is suggested that co-medication of it with hydrocortisone in the treatment of inflammatory lung disease will be useful (CitationSchleimer, 1991; CitationAsl and Hosseinzadeh, 2008).
It is already reported that glycyrrhizic acid induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells (CitationHibasami et al., 2005) and prostate cancer (PC) cell lines DU-145 and LNCaP (CitationThirugnanam et al., 2008). Glycyrrhizic acid also reported to inhibit TNF-mediated apoptosis in the human hepatoblastoma cell line HepG2 (CitationYoshikawa et al., 1999). Glycyrrhizic acid was found to modulate critical end points of oxidative stress induced apoptosis and could be beneficial against liver diseases where oxidative stress is known to play a crucial role (CitationTripathi et al., 2009). CitationMoro et al. (2008) showed that glycyrrhizin and its metabolite glycyrhhetinic acid inhibit Smad3-mediated type 1 collagen gene transcription and suppress experimental murine liver fibrosis. Glycyrrhetinic acid did not affect gene expression of TGF-β receptors or Smad proteins, but inhibited nuclear accumulation of Smad3 in activated hepatic stellate cells (CitationMoro et al., 2008).
CitationRahman and Sultana (2007) demonstrated the chemopreventive activity on 12-O-tetradecanoyl phorbol-13-acetate-induced coetaneous oxidative stress and tumor promotion in Swiss albino mice. Studies using 1249 patients, in 2006, found that a long-term glycyrrhizin injection therapy could reduce hepatocellular carcinogenesis rate in patients with IFN-resistant active chronic hepatitis C (CitationIkeda et al., 2006).
Ursolic acid
Ursolic acid (UA) is a pentacyclic triterpenoid compound, which is derived from berries, leaves, flowers, and fruits of medicinal plants, such as Boerhaavia diffusa L. (Nyctaginaceae), Rosemarinus officinalis L. (Lamiaceae), Eriobotrya japonica Lindl. (Rosaceae), Calluna vulgaris L. (Ericaceae), Ocimum sanctum L. (Lamiaceae), and Syzygium cumini L. (Myrtaceae) (CitationLiu, 1995), and has a broad range of biological effects.
UA was found to activate immune system by activating the cell-mediated immune responses in B16F-10 melanoma-bearing mice. Intraperitoneal administration of UA (50 µmol/kg body weight for five consecutive days) was found to produce increased NK cell activity in metastatic tumor-bearing animals. Administration of UA clearly enhanced the ADCC. Intraperitoneal administration of UA was also found to enhance ACC in metastatic tumor-bearing animals. The elevated level of GM-CSF in tumor-alone treated control animals was reduced by the treatment with UA. The highly elevated level of IL-6 in control animals was also reduced by the treatment of UA. The level of IL-2 was enhanced by the treatment with UA compared with untreated tumor-bearing control animals (CitationRaphael and Kuttan, 2008).
One of the important properties of UA is its antioxidant capacity and it can suppress the preneoplastic lesions and has a protective effect against colon carcinogenesis (CitationFurtado et al., 2008). CitationMartin-Aragón et al. (2001) investigated possible protective effects of UA against carbon tetrachloride-induced alterations in antioxidant defense enzymes in vivo and in vitro. The authors suggested that UA may prevent the initiation and propagation of the lipid peroxidation process by scavenging free radicals through conjugation with glutathione, with the consequent control of oxidative damage and tissue protection. Hepatoprotective activity of UA was previously investigated (CitationSaravanan et al., 2006) and the studies showed that in addition to reducing lipid peroxidation markers in plasma, UA increased the levels of circulatory antioxidants such as reduced glutathione, ascorbic acid, and α-tocopherol, thus demonstrating that the protective effect of this agent is probably related to its antioxidant capacity.
UA has been reported to possess a wide range of pharmacological properties and is one of the most promising chemopreventive agents (CitationShih et al., 2004). UA has been shown to suppress tumorigenesis and inhibit tumor promotion (CitationOhigashi et al., 1986; CitationTokuda et al., 1986; CitationNishino et al., 1988). Many of these effects of UA are mediated through suppression of the expression of lipoxygenase, cyclooxygenase-2 (COX-2), matrix metalloproteinase (MMP)-9, and iNOS (CitationNajid et al., 1992; CitationSimon et al., 1992; CitationCha et al., 1996; CitationRingbom et al., 1998; CitationSubbaramaiah et al., 2000), all of which are genes regulated by NF-κB. CitationBonaccorsi et al. (2008) demonstrated the antiproliferative and differentiating effect of human tumor cell lines from melanoma, glioblastoma, and thyroid anaplastic carcinoma. They also reported the UA-mediated inhibition of reverse transcription activity in tumor cells, which was recently shown to be involved in the control of proliferation and differentiation of neoplastic cells.
There are several reports showing the anti-metastatic effects of UA. Triterpenes including UA can augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells and suppress experimental metastasis (CitationYamai et al., 2009). We have already reported that UA could induce apoptosis in B16F-10 melanoma cells by activating p53-induced caspase-3 activation and inhibition of NF-κB-mediated activation of bcl-2 (CitationManu and Kuttan, 2008). NF-κB subunits c-Rel, p65, and p50 were found inhibited by the treatment of UA in B16F-10 melanoma cells. The nuclear translocation of c-fos, ATF-2, and CREB-1 were also inhibited by the treatment of UA. UA could significantly inhibit the production of TNF-α, IL-1β, IL-6, and GM-CSF gene expression and production by B16F-10 melanoma cell in culture in a dose-dependent manner.
CitationLi et al. (2009) demonstrated that the chemosensitization by UA in cancer cells was dependent on the amplified activation of intrinsic pathway (caspase-8–BID–mitochondria–cytochrome c–caspase-3) by augmentation of BID cleavage and activation of Fas/FasL–caspase-8 pathway. UA inhibits the cell proliferation of human lung cancer cell line A549 by blocking the cell cycle progression in the G1 phase (CitationHsu et al., 2004). It also decreased the protein expression of cyclins D1, D2, and E and their activating partners cyclin-dependent kinase (Cdk) 2, Cdk4, and Cdk6 with concomitant induction of p21/WAF1. UA can inhibit proliferation and induce apoptosis of HT-29 colon cancer cells by down-regulating epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase (MAPK) pathway (CitationJian-zhen et al., 2009). In addition, UA and its derivatives have been shown to induce apoptosis in a wide variety of cancer cells including breast carcinoma, melanoma, hepatoma, prostate carcinoma, and acute myelogenous leukemia (CitationEs-Saady et al., 1996; CitationHollósy et al., 2000, Citation2001; CitationKonopleva et al., 2002) through inhibition of DNA replication, activation of caspases (CitationHollósy et al., 2001; CitationKonopleva et al., 2002), inhibition of protein tyrosine kinase caspases (CitationHollósy et al., 2001), and induction of Ca2+ release (CitationBaek et al., 1997). UA was found as a potent inhibitor of proliferation and inducer of apoptosis in both KRAS- and BRAF-mutated human colorectal cancer cells (CitationXavier et al., 2009). UA is able to inhibit key steps of angiogenesis in vitro, including endothelial cell proliferation, migration, and differentiation (CitationCárdenas et al., 2004).
Oleanolic acid
Oleanolic acid (OA) is a triterpenoid compound widely found in natural plants (CitationLiu, 1995), and has been shown to be an active ingredient in producing biological effects. OA has been isolated from >120 plant species (CitationWang and Jiang, 1992).
The effect of naturally occurring triterpenoid, OA on immune system was studied using Balb/c mice. Intraperitoneal treatments with five doses of OA were found to enhance the total white blood cells (WBC) count. In OA-treated animals, the maximum total WBC count was observed on the sixth day and the percentage of increase in the total WBC count was up to 135.75 ± 6.4%. Bone marrow cellularity and α-esterase-positive cells were also enhanced by the treatment with this terpenoid. OA enhanced the specific antibody titer and the number of plaque-forming cells (PFC) in the spleen and also inhibited DTH reaction (CitationRaphael and Kuttan, 2003).
OA inhibited the in vitro immunohemolysis of antibody-coated sheep erythrocytes by guinea pig serum. In further experiments, this reduced immunohemolysis was found to be due to inhibition of the C3-convertase of the classical complement pathway. The threshold concentration for inhibition of C3-convertase was 100 μg/mL. However, higher concentrations of OA showed constant inhibitory effects on immunohemolysis. OA also exhibited weak inhibitory effects on individual components of the complement system (CitationKapil and Sharma, 1994). OA suppressed the production of proinflammatory cytokines and delayed graft-specific immune responses to prolong islet allograft survival (CitationNataraju et al., 2009).
The anti-inflammatory effect of OA was first reported in 1960s. Inhibitory effects of OA on carrageenan-induced rat paw edema and formaldehyde-induced arthritis were already demonstrated (CitationGupta et al., 1969). The anti-inflammatory effects of OA have also been confirmed in later studies (CitationTakagi et al., 1980; CitationYue et al., 1989; CitationSingh et al., 1992). OA displayed anti-inflammatory activity in carrageenan- and dextran-induced edema in rats. It elicited marked anti-arthritic action in adjuvant-induced polyarthritis in rats and mice and in formaldehyde-induced arthritis in rats. OA reduced exudate volume and inhibited leukocyte infiltration in carrageenan-induced pleurisy in rats. It is devoid of any analgesic, antipyretic, or ulcerogenic action. OA did not affect the parturition time in pregnant rats or castor oil-induced diarrhea in rats. Oral LD50 was found to be greater than 2 g/kg in mice and rats (CitationSingh et al., 1992).
OA was found to possess significant anti-inflammatory and complement inhibitory activities in adjuvant-induced arthritis and carrageenan-induced paw edema in rats. The intraperitoneal injection of OA (60 mg/kg, twice a day), before and after Freund’s complete adjuvant challenge and thereafter repeated for several days, significantly reduced footpad thickness of experimental animal models and simultaneously reduced complement activity. OA also produced marked reduction in complement levels and inflammatory effects on carrageenan-induced paw edema in rats when injected intraperitoneally (60 mg/kg, twice a day) (CitationKapil and Sharma, 1995).
It has been reported that OA produce a wide variety of antitumor activity, including decrease in the incidence and multiplicity of azoxymethane-induced intestinal tumor. Treatment of rats with OA (200 ppm) in diet for 3 weeks decreased the incidence and multiplicity of azoxymethane-induced intestinal tumor (CitationYoshimi et al., 1992). The use of triterpenoid, OA has been recommended for skin cancer therapy in Japan (CitationMuto et al., 1990). Pharmaceutical preparation containing OA is patented for the treatment of nonlymphatic leukemia (CitationLiu, 1986).
The effects of OA on the differentiation of F9 teratocarcinoma stem cells were studied. OA caused the morphological change of F9 cells into endoderm cells, as did retinoic acid (RA). Moreover, expression of laminin B1, type IV collagen, and retinoic acid receptor beta (RARβ) increased in OA-treated F9 cells. Dexamethasone, a synthetic glucocorticoid, also induced the morphological change and altered the expression of laminin B1, type IV collagen, and RARβ in F9 cells. In addition, transcription of glucocorticoid receptor was detected after treatment with these three agents. According to southwestern blot analysis, a 94-kDa protein, thought to be a glucocorticoid receptor, was detected in F9 cells treated with these agents. Gel-shift assay identified protein factors binding to the glucocorticoid-responsive element (GRE) in the nuclear proteins from F9 cells treated with OA. The binding activity of the GRE-binding protein disappeared on the addition of unlabeled GRE oligonucleotide. Taken together, these results suggest that OA can induce the differentiation of F9 cells and may regulate the expression of differentiation-specific genes, probably by forming a complex with the glucocorticoid receptor or its analogous nuclear receptor (CitationLee et al., 1994).
OA is capable of inducing apoptosis in tumor cells on one side and preventing malignant transformation of normal cells on the other side. This triterpenoid have the potential to be used clinically either as antitumor or chemopreventive agent (CitationNovotný et al., 2001). The effect of dextrose–OA (the most potent OA derivative) on apoptosis of osteosarcoma cells was evaluated using the Annexin-V method. The cell cycle of dextrose–OA-treated cells was examined by flow cytometry, and the in vivo effects of dextrose–OA were evaluated in a mouse osteosarcoma model. OA had an overall inhibitory effect on the proliferation of osteosarcoma cells (CitationHua et al., 2011).
Nomilin
Citrus limonoids were demonstrated to possess potential biological activities in reducing the risk of certain diseases. Nomilin is a triterpenoid with putative anticancer properties. Immunomodulatory activity of the naturally occurring triterpenoid was studied using Balb/c mice. Treatment of nomilin was found to enhance the total WBC count and the maximum count was observed on the sixth day. In nomilin-treated animals, the percentage of increase in the total WBC count was up to 117.33 ± 17.9%. Bone marrow cellularity and α-esterase-positive cells were also enhanced by the treatment with this terpenoid. Nomilin enhanced the antibody titer and the number of PFC in the spleen and also remarkably inhibited DTH reaction (CitationRaphael and Kuttan, 2003).
The limonoids are bitter principles found in common edible Citrus fruits. Nomilin, when given three times (at 5 and 10 mg per animal) every 2 days, induced increased glutathione S-transferase activity 2.48 and 3.44 times over the control, respectively, in the liver of female ICR/Ha mice. The increases of GST activity in the small intestinal mucosa were 3.00 and 4.17, respectively, over the control. Nomilin, which is the more active enzyme inducer, was found to inhibit benzo[a]pyrene (BP)-induced neoplasia in the forestomach of ICR/Ha mice. The number of mice with tumors was reduced from 100% to 72%, and the number of tumors per mouse was significantly decreased as a result of nomilin treatment. These findings suggest limonoids as a class of regularly consumed natural products and also as an effective chemopreventive agent (CitationLam and Hasegawa, 1989). Effect of nomilin against a series of human cancer cell lines was investigated. The human cancer cell lines included leukemia (HL-60), ovary (SKOV-3), cervix (HeLa), stomach (NCI-SNU-1), liver (Hep G2), and breast (MCF-7). The growth-inhibitory effect of nomilin against MCF-7 cells was significant, and the antiproliferative activity was also dose- and time-dependent. With use of flow cytometry, it was found that nomilin could induce apoptosis in MCF-7 cells (CitationTian et al., 2001).
The effect of Citrus limonoid, nomilin against two human cancer cell lines, SH-SY5Y neuroblastoma and Caco-2 colonic adenocarcinoma, and a noncancerous mammalian epithelial Chinese hamster ovary (CHO) cells was studied. Viability, as quantified by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium reduction and light microscopy, was shortened significantly (P < 0.001) in cancer cells exposed to nomilin. SH-SY5Y cells were more sensitive than Caco-2 cells to the nomilin, whereas noncancerous CHO cells showed hardly any change in cell numbers or cell morphology (CitationPoulose et al., 2006).
Anti-metastatic potential of nomilin and its possible mechanism of action were investigated. Administration of nomilin could inhibit tumor nodule formation in lungs (68%) and markedly increased the survival rate of the metastatic tumor-bearing animals. These results correlated with the biochemical parameters and histopathological analysis. Nomilin showed an inhibition of tumor cell invasion and the activation of matrix metalloproteinases. Treatment with nomilin induced apoptotic response, characterized by an increase of the sub-G1 fraction of cells with chromatin condensation and membrane blebbing, a typical ladder of DNA fragmentation and detection of apoptotic cells by TUNEL assay. Nomilin treatment also exhibited a down-regulated Bcl-2, cyclin D1 expression, and up-regulated p53, Bax, caspase-9, caspase-3, p21, and p27 gene expression in B16F-10 cells. The proinflammatory cytokine production and gene expression were found to be down-regulated in nomilin-treated cells. The study also reveals that nomilin could inhibit the activation and nuclear translocation of anti-apoptotic transcription factors such as NF-κB, CREB, and ATF-2 in B16F-10 cells (CitationPratheeshkumar and Kuttan, 2010a).
Andrographolide
Andrographolide (ANDLE; bicyclic diterpenoid lactone), the principal bioactive chemical constituent of the plant Andrographis paniculata (Barm.f.) Nees (Acanthaceae), has shown credible anticancer potential in various investigations around the globe (CitationLu et al., 1981). Modulation of immune responses is highly relevant in tumor cell destruction. Effect of ANDLE on cell-mediated immune responses in normal and tumor-bearing control animals was studied. Treatment of ANDLE significantly enhanced NK cell activity in normal and tumor-bearing animals. ACC was also found to be increased in ANDLE-treated normal and tumor-bearing animals. An early enhancement of ACC was also observed by the administration of ANDLE in normal as well as tumor-bearing animals. ANDLE administration significantly enhanced the mitogen-induced proliferation of splenocyte, thymocyte, and bone marrow cells. Moreover, treatment of ANDLE significantly elevated the production of IL-2 and IFN-γ in normal and Ehrlich ascites carcinoma-bearing animals (CitationSheeja and Kuttan, 2007).
ANDLE of A. paniculata induced significant stimulation of antibody and DTH response to sheep red blood cells (SRBC) in mice (CitationPuri et al., 1993). The stimulatory effect of ANDLE on cytotoxic T lymphocyte (CTL) production was determined in BALB/c mice by Winn’s neutralization assay using CTL-sensitive EL4 thymoma cells as target cell. ANDLE showed a significant increase in CTL production in both the in vivo and in vitro models. The level of cytokines such as IL-2 and IFN-γ was also found to be enhanced in these animals when they were treated with ANDLE (CitationSheeja et al., 2007).
ANDLE is known to exert significant anti-inflammatory properties, including inhibition of intercellular adhesion molecule-1 expression in monocytes activated by TNF-α (CitationHabtemariam, 1998), suppression of iNOS in RAW264.7 (CitationChiou et al., 2000), COX-2 expression in neutrophils and microglial cells (CitationWang et al., 2004; CitationHidalgo et al., 2005), and IFN-γ and IL-2 production (CitationBurgos et al., 2005; CitationIruretagoyena et al., 2005). It has been proposed that ANDLE exerts its anti-inflammatory effects by inhibiting NF-κB binding to DNA, and thus reducing the expression of proinflammatory proteins in neutrophils (CitationHidalgo et al., 2005). For instance, NF-κB is the molecular target for the anti-inflammatory activity. ANDLE effectively inhibited the nuclear activation of NF-κB by covalent modification of reduced cysteine 62 of p50, to exert its potent anti-inflammatory activity (CitationXia et al., 2004; CitationHidalgo et al., 2005). ANDLE inhibited extensive infiltration of inflammatory cells in lung and decreased airway hyperreactivity. It also down-regulated NF-κB expression in lung and nucleus of airway epithelial cells (CitationLi et al., 2009).
ANDLE has been reported to inhibit gastric cancer, liver cancer, lung cancer, and breast cancer. The antitumor mechanism of ANDLE was versatile; for instance, ANDLE can induce the apoptosis of cancer cell, inhibit the cell cycle, and increase the antitumor activity of lymphocyte (CitationQi et al., 2007). Inhibition of angiogenesis is currently perceived as one of the promising strategies in the treatment of cancer. ANDLE inhibited the migration and invasion of Lovo cells under noncytotoxic concentrations (CitationShi et al., 2009). Intraperitoneal administration of ANDLE significantly inhibited the B16F-10 melanoma cell line-induced capillary formation in C57BL/6 mice. Analysis of serum cytokine profile showed a drastic elevation in the proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and GM-CSF, and the most potent angiogenic factor, vascular endothelial growth factor (VEGF), in angiogenesis-induced animals. Treatment of ANDLE significantly reduced this elevated levels. Moreover, VEGF mRNA level in B16F-10 cell line showed a reduced level of expression in the presence of ANDLE. Serum NO level that was increased in B16F-10 melanoma-injected control animals was also found to be significantly lowered by the administration of ANDLE. Antiangiogenic factors such as TIMP-1 and IL-2 level was elevated in ANDLE-treated angiogenesis-induced animals. In the rat aortic ring assay, ANDLE inhibited the microvessel outgrowth at nontoxic concentrations (CitationSheeja et al., 2007).
In vitro studies demonstrated the capability of ANDLE in inducing cell cycle arrest and apoptosis in a variety of cancer cells at different concentrations (CitationVarma et al., 2009). ANDLE was found to cause G0/G1 cell cycle arrest through induction of p27 and decreasing expression of Cdk4 in some human cancer cells (CitationRajagopal et al., 2003; CitationSatyanarayana et al., 2004). The anticancer activity of this compound is further substantiated by findings using in vivo B16F0 melanoma syngenic and HT-29 xenograft models (CitationRajagopal et al., 2003). The effect of ANDLE on apoptosis is controversial. ANDLE is capable of protecting immune cells (thymocytes) or endothelial cells against apoptosis (CitationChen et al., 2004; CitationBurgos et al., 2005). On the other hand, a couple of very recent reports showed that ANDLE at relatively high concentrations (from 40 to 100 mM) could induce apoptosis in human prostatic adenocarcinoma PC-3 cells (CitationKim and Milner, 2005) or human leukemic HL-60 cells (CitationCheung et al., 2005). It was reported to induce apoptosis in hepatoma Hep3B cells (CitationJi et al., 2007). ANDLE potentiates the cytotoxic effect of 5-fluorouracil (5-FU) in HCC cell line SMMC-7721 through apoptosis. ANDLE alone induces SMMC-7721 apoptosis with p53 expression, Bax conformation, and caspase-3, caspase-8, caspase-9 activation. Surprisingly, the addition of ANDLE to 5-FU induces synergistic apoptosis, which could be corroborated to the increased caspase-8, p53 activity and the significant changes of Bax conformation in these cells, resulting in increased losses of mitochondrial membrane potential, increased release of cytochrome c, and activation of caspase-9 and caspase-3. It induced apoptosis of PC-3 cells (the most sensitive cell line among the cell lines screened) via the activation of caspase-3, up-regulation of bax, and down-regulation of bcl-2. Furthermore, its inhibitory activity on the level of VEGF was also verified by ELISA. CitationZhao et al. (2008) shown that pretreatment with ANDLE significantly enhances TRAIL-induced apoptosis in various human cancer cell lines, including those TRAIL-resistant cells. Such sensitization is achieved through transcriptional up-regulation of death receptor (DR) 4, a DR of TRAIL. ANDLE is capable of activating p53 via increased p53 phosphorylation and protein stabilization, a process mediated by enhanced ROS production and subsequent c-Jun NH(2)-terminal kinase activation. Pretreatment with an antioxidant (N-acetylcysteine) or a c-Jun NH(2)-terminal kinase inhibitor (SP600125) effectively prevented ANDLE-induced p53 activation and DR4 up-regulation and eventually blocked the ANDLE-induced sensitization on TRAIL-induced apoptosis (CitationZhou et al., 2008). Suppression of caspase-8 with the specific inhibitor z-IETD-fmk abrogates largely ANDLE/5-FU biological activity by preventing mitochondrial membrane potential disappearance, caspase-3, caspase-9 activation, and subsequent apoptosis (CitationYang et al., 2009).
ANDLE inhibited Lovo cell growth by G1/S phase arrest, and was exerted by inducing the expression of p53, p21, and p16 that, in turn, repressed the activity of cyclin D1/Cdk4 and/or cyclin A/Cdk2, as well as Rb phosphorylation (CitationShi et al., 2008). The cytotoxic activity of ANDLE was evaluated against Jurkat, PC-3, HepG2, and Colon 205 tumor cells, and normal peripheral blood mononuclear cells (PBMCs). The bioactivity assays showed that ANDLE exhibited IC(50) values of 0.05, 0.07, and 0.05 mm, respectively, and also blocked the cell cycle progression at G0/G1 phase of the Jurkat cell line (CitationGeethangili et al., 2008). ANDLE inhibited in vitro angiogenesis by regulating MMPs and also by inhibiting the nuclear translocation of transcription factors (CitationPratheeshkumar and Kuttan, 2011).
Limonene
Limonene is a naturally occurring monoterpene that serves as a precursor to a host of other oxygenated monocyclic monoterpenes such as carveol, carvone, menthol, perillyl alcohol (POH), and perillaldehyde (CitationKarp et al., 1990). In Citrus fruits (CitationChayet et al., 1977), peppermint, and other plants, d-limonene is formed by the cyclization of geranyl pyrophosphate in a reaction catalyzed by limonene synthase (CitationCroteau and Kjonaas, 1983; CitationAlonso et al., 1992). Limonene then serves as a precursor to a host of other oxygenated monocyclic monoterpenes such as carveol, carvone, menthol, POH, and perillaldehyde (CitationKarp et al., 1990; CitationMcGarvey and Croteau, 1995). d-Limonene is a prevalent flavoring agent in fruit juices, soft drinks, baked goods, ice cream, and pudding. Orange oil, naturally consisting of 90–95% d-limonene, is a commercially available food-flavoring agent. Furthermore, because of its pleasant Citrus fragrance, d-limonene is commonly added to cosmetics, soaps, and other cleaning products.
Limonene has well-established chemopreventive activity against many cancer types. Dietary limonene reduces the incidence of spontaneous lymphomas in p532/2 mice (CitationHursting et al., 1995). Furthermore, when administered either in pure form or as orange peel oil (95% d-limonene), limonene inhibits the development of chemically induced rodent mammary (CitationElegbede et al., 1984; CitationElson et al., 1988; CitationMaltzman et al., 1989; CitationWattenberg and Coccia, 1991), skin (CitationElegbede et al., 1986) liver (CitationDietrich and Swenberg, 1991), lung, and forestomach (CitationWattenberg et al., 1989; CitationWattenberg and Coccia, 1991) cancers. In rat mammary carcinogenesis models, the chemopreventive effects of limonene are evident during the initiation phase of 7,12-dimethylbenz[a]anthracene (DMBA)-2-induced cancer (CitationElson et al., 1988) and during the promotion phase of both DMBA- and nitrosomethylurea (NMU)-induced cancers (CitationElson et al., 1988; CitationMaltzman et al., 1989). Dietary limonene also inhibits the development of ras oncogene-induced mammary carcinomas in rats. There are many reports that the development of azoxymethane-induced aberrant crypt foci in the colon of rats was significantly reduced when they were given 0.5% limonene in the drinking water. CitationLu et al. (2003) demonstrated that d-limonene can inhibit the growth of human gastric cancer cells in vitro through a mechanism of inducing the apoptosis of tumor cells. Therefore, d-limonene has been applied to precaution and treatment in chemical-induced animal model, such as colon cancer, breast cancer, gastric cancer, pancreatic cancer, and hepatic cancer with promising results (CitationBroitman et al., 1996; CitationUedo et al., 1999; CitationStratton et al., 2000; CitationKaji et al., 2001; CitationGuyton and Kensler, 2002; CitationCárdenas et al., 2004; CitationLu et al., 2004).
We have already reported the anti-metastatic potential of limonene. Administration of limonene could remarkably inhibit the tumor nodule formation by 65% and also could increase the lifespan by 50.7%. It could also inhibit the levels of lung collagen hydroxyl proline, hexosamine, and uronic acid showing reduced lung fibrosis. Lungs in the control animals showed infiltration of the neoplastic cells around the main bronchioles extended to the pleura. Metastatic tumor-bearing animals treated with limonene showed a significant reduction in tumor mass. Alveoli and pleura was remarkably tumor cell free in the case of limonene-treated groups. The reduced level of serum sialic acid and serum γ-glutamyl transpeptidase (GGT) in limonene-treated B16F-10-bearing mice showed reduced number of tumor cells in circulation (CitationRaphael and Kuttan, 2003). d-Limonene found to inhibit tumor-specific angiogenesis, which may also be involved in the anti-metastatic mechanism of limonene (CitationLu et al., 2004).
d-Limonene also found to activate the immune system. It could increase the survival of lymphoma-bearing mice, DTH reaction to dinitrochlorobenzene (DNCB), phagocytosis, and microbial activity. In vitro studies indicated that d-limonene increased NO production in peritoneal macrophages obtained from tumor-bearing mice (CitationDel Toro-Arreola et al., 2005). Administration of limonene (100 mmol/kg body weight/dose/animal) was found to increase the total WBC count in Balb/c mice. Administration of limonene increased the total antibody production and number of antibody-producing cells in spleen. Limonene-treated groups showed a significant increase in bone marrow cells compared with normal animals. Moreover, the number of α-esterase-positive cells was also found to be increased significantly in limonene-treated animals compared with the normal, indicating its potentiating effect on the immune system (CitationRaphael and Kuttan, 2003).
Perillic acid
Perillic acid (PA) is a major metabolite of POH, a naturally occurring monoterpene. The immunomodulatory activity of PA was studied in Balb/c mice. Administration of PA (50 µM/kg body weight/dose/animal) was found to increase the total WBC count in Balb/c mice. The maximum total WBC count in PA (14,437 cells/cm3)-treated animals was observed on the ninth day after the drug treatment. Administration of this monoterpene increased the total antibody production, antibody-producing cells in spleen, bone marrow cellularity, and α-esterase-positive cells significantly compared with the normal animals indicating its potentiating effect on the immune system (CitationRaphael and Kuttan, 2003).
PA is effective against a variety of rodent organ-specific tumor models. To establish the molecular mechanism of PA as antiproliferative agent, their effects on cell proliferation, cell cycle, and cell cycle regulatory proteins were studied in HCT 116 human colon cancer cells. PA exerted a dose-dependent inhibitory effect on cell growth correlated with G1 arrest. Analysis of G1 cell cycle regulators expression revealed that monoterpenes increased expression of Cdk inhibitor p21 (Waf1/Cip1) and cyclin E, and decreased expression of cyclin D1, Cdk4, and Cdk2. Results suggest that PA induce growth arrest of colon cancer cells through the up-regulation of p21 (Waf1/Cip1) and the down-expression of cyclin D1 and its partner Cdk4 (CitationBardon et al., 2002). The effect of PA on the proliferation of non-small cell lung cancer (NSCLC, A549, and H520) cells was investigated. PA elicited dose-dependent cytotoxicity, induced cell cycle arrest and apoptosis with increasing expression of bax, p21, and caspase-3 activity in both the cell lines. Combination studies revealed that exposing the cells to an IC50 concentration of PA sensitized the cells to cisplatin and radiation in a dose-dependent manner. These results indicated that PA in combination therapy may have chemotherapeutic value against NSCLC (CitationYeruva et al., 2007). PA has a potential for use as radiosensitizers in chemoradiation therapy of head and neck cancers and should be further studied (CitationSamaila et al., 2004).
The effects of PA on lung metastasis induced by B16F-10 melanoma cells were studied in C57BL/6 mice. Administration of PA (50 µM/kg body weight) remarkably reduced the metastatic tumor nodule formation by 67%. This result correlated with the biochemical parameters such as serum sialic acid, lung collagen hydroxyproline, and uronic acid contents. Serum sialic acid level in control group was 126.8 µg/mL serum, which was significantly lowered in PA-treated animals (53.6 µg/mL serum). Uronic acid level was also inhibited to 39.7% in PA-treated animals. Histopathological studies also correlated with these above results. These results indicate that PA could inhibit the metastatic progression of B16F-10 melanoma cells in mice (CitationRaphael and Kuttan, 2003). PA is a monoterpene substantially suppressed IL-2 and IL-10 production in mitogen-activated T lymphocytes. The effects of PA on cytokine secretion were selective: generation of IL-6 and transforming growth factor-β1 (TGF-β1) was unchanged. In H9 T lymphoma cells, exposure to PA resulted in a dose-dependent depletion of membrane-bound Ras proteins. Unlike hydroxymethylglutaryl-CoA reductase or protein farnesyl transferase inhibitors, PA did not induce a shift of membrane-bound into cytosolic p21ras but depleted total cellular Ras proteins. Triggering of the T-cell receptor (TCR) perturbs the guanine nucleotide-binding cycle of p21ras and in turn induces phosphorylation and activation of MAPK. In PA-treated cells, the levels of phosphorylated but not total MAPK were also decreased in a dose-dependent manner. PA interrupts signaling via the Ras/MAP kinase pathway by depleting farnesylated Ras levels, an effect that may contribute to its inhibition of IL-2 production and T-cell activation (CitationSchulz et al., 1997).
The effect of PA on cell growth, cell cycle progression, and expression of cyclin D1, cell cycle regulatory gene in T-47D, MCF-7, and MDA-MB-231 breast cancer cell lines was investigated. Results revealed that PA caused a dose-dependent inhibition of cell proliferation, which was associated with a fall in the proportion of cells in the S phase and an accumulation of cells in the G1 phase of the cell cycle (CitationBardon et al., 1998). Cell cycle analysis revealed that PA arrested cells in G1 and prevented cells from entering S phase in a manner similar to that induced by the specific 3-hydroxymethylglutaryl-CoA reductase inhibitor, compactin. However, unlike compactin, the PA-induced effects on lymphocyte proliferation were not prevented by addition of mevalonate (CitationSchulz et al., 1994).
Vernolide-A
Vernolide-A (C21H28O7) is a sesquiterpene lactone (SL) present in the plant Vernonia cinerea L. (Asteraceae). Biological evaluation showed that vernolide-A has potent cytotoxicity against human KB, DLD-1, NCI-661, and Hela tumor cell lines (CitationKuo et al., 2003). SLs are the active constituents of a variety of medicinal plants used in traditional medicine for the treatment of inflammatory diseases. Various SLs have been demonstrated to execute their anticancer capability via inhibition of inflammatory responses, prevention of metastasis, and induction of apoptosis. All SLs contain a common functional structure, α-methylene-γ-lactone group, and this important chemical characteristic means that the thiol reactivity of SLs is an underlying mechanism responsible for their bioactivities (CitationZhang et al., 2005).
Our laboratory had investigated the effect of vernolide-A on the induction of apoptosis as well as its regulatory effect on the activation of transcription factors in B16F-10 melanoma cells. Treatment of B16F-10 cells with nontoxic concentration of vernolide-A showed the presence of apoptotic bodies and induced DNA fragmentation in a dose-dependent manner. Cell cycle analysis and TUNEL assays also confirmed the observation. The proapoptotic genes p53, Bax, caspase-9, and caspase-3 found up-regulated in vernolide-A treated cells, whereas the anti-apoptotic gene Bcl-2 was down-regulated. Vernolide-A treatment also showed a down-regulation of cyclin D1 expression and up-regulated p21 and p27 gene expression in B16F-10 melanoma cells. The study also reveals that vernolide-A treatment could alter the production and expression of proinflammatory cytokines and could inhibit the activation and nuclear translocation of p65, p50, and c-Rel subunits of NF-κB, and other transcription factors such as c-fos, activated transcription factor-2, and cyclic adenosine monophosphate response element-binding protein in B16F-10 melanoma cells (CitationPratheeshkumar and Kuttan, 2010b).
The inhibitory effect of vernolide-A on lung metastasis induced by B16F-10 melanoma cells was studied using C57BL/6 mice. Vernolide-A was administered by three different modalities such as simultaneous with tumor, prophylactic to tumor, and after tumor development. Maximum inhibition in the metastasis observed when vernolide-A was administered simultaneously with tumor. There was 89.39% inhibition of lung tumor nodule formation and 88.51% increase in the lifespan of metastatic tumor-bearing animals. Highly elevated levels of lung hydroxyproline, lung uronic acid, lung hexosamine, serum sialic acid, serum GGT, and serum VEGF in the metastatic control animals were found to be significantly lowered in the vernolide-A treated animals. Vernolide-A administration down-regulated the expression of MMP-2, MMP-9, extracellular signal-regulated kinase (ERK)-1, ERK-2, and VEGF in the lung tissue of B16F-10 melanoma-challenged animals. In the in vitro system, vernolide-A showed a significant inhibition of invasion of B16F-10 melanoma cells across the collagen matrix. Vernolide-A treatment also inhibited the migration of B16F-10 melanoma cells across a polycarbonate filter in vitro. Vernolide-A could inhibit MMP-2 and MMP-9 protein expression in gelatin zymographic analysis of B16F-10 cells. 3H-thymidine proliferation assay showed that vernolide-A could inhibit the proliferation of B16F-10 melanoma cells in vitro (CitationPratheeshkumar and Kuttan, 2010c).
Antiangiogenic activity of vernolide-A using in vivo as well as in vitro models was investigated. Vernolide-A significantly inhibited tumor-directed capillary formation. The levels of serum proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and GM-CSF and of serum VEGF, a proangiogenic factor, were found to be elevated in angiogenesis-induced animals, which were significantly reduced by the treatment of vernolide-A in C57BL/6 mice. Administration of vernolide-A significantly enhanced the production of antiangiogenic factors such as IL-2 and TIMP. In vitro studies using rat aortic ring assay showed that vernolide-A at nontoxic concentrations significantly inhibited microvessel sprouting and also exhibited a significant inhibition in the proliferation, migration, and tube formation of endothelial cells, which are key events in the process of angiogenesis. Vernolide-A significantly inhibited the invasion of the collagen matrix by HUVECs in a dose-dependent manner and also showed an inhibition in the activation of procollagenase to active collagenase of metalloproteinases (CitationPratheeshkumar and Kuttan, 2011).
Conclusion
Many of the terpenoid drugs have provided tremendous benefits for patients and for the pharmaceutical industry. Artemisinin and its derivatives comprise a multimillion-dollar market worldwide. Taxol alone is estimated to have annual sales of over $1.8 billion. Terpenoids indisputably continue to be important compounds for drug discovery. Now a days, more and more terpenoids have been getting explored and entered the pharmaceutical industry. Major obstacle in this regard is the quantity of these compounds in their natural sources. Many of these are available at very low levels and a huge number of them still remain unexplored. More and more studies should be conducted to identify, isolate, purify, and make it available for basic and clinical research.
Naturally occurring terpenoids are a diverse class of molecules, which can provide new opportunities for researchers to discover new drugs against cancer and other diseases with minimum side effects. Although terpenoids have been widely used for medicinal purpose in many Asian countries, their biogenesis and pleiotropic actions have not impacted on the practice of western medicines. Even though this class of molecules has members with therapeutic properties including anticancer, antiparasitic, antimicrobial, antiallergenic, antispasmodic, antihyperglycemic, anti-inflammatory, and immunomodulatory properties, still their usefulness in therapy level has not exploited by the modern medicine. Nowadays, attempts to link the Ayurvedic and naturopathic medicines with modern western medicine provide new opportunities in this regard.
The terpenoids like andrographalide, glycyrrhizic acid, UA, OA, perillic acid, nomilin, and limonene were found to have many pharmacological activities. They can activate our immune system by enhancing humoral and cell-mediated immune responses. They can activate anticancer immunity, which may be involved in their anticancer and anti-metastatic potential. They show induction of apoptosis in various cancer cells by activating the intracellular signaling cascade. These terpenoids also show inhibition of various oncogenic expressions and could block various signaling cascades involved in tumor progression and metastasis. The chemopreventive and chemoprotective effects point toward their possible role in modern anticancer therapies.
Acknowledgements
The studies from the author’s laboratory cited in this article were financially supported by grants from Indian Council of Medical Research, New Delhi and Committee for Science Technology and Environment Department of Planning and Economics Affairs, Government of Kerala, India.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Abe N, Ebina T, Ishida N. (1982). Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol Immunol, 26, 535–539.
- Aggarwal BB, Ichikawa H, Garodia P, Weerasinghe P, Sethi G, Bhatt ID, Pandey MK, Shishodia S, Nair MG. (2006). From traditional Ayurvedic medicine to modern medicine: identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin Ther Targets, 10, 87–118.
- Akamatsu H, Komura J, Asada Y, Niwa Y. (1991). Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med, 57, 119–121.
- Akihisa T, Yasukawa K, Tokuda H. (2003). Potentially cancer chemopreventive and anti-inflammatory terpenoids from natural sources. Stud Nat Prod Chem, 29, 73–126.
- Alonso WR, Rajaonarivony JI, Gershenzon J, Croteau R. (1992). Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) and spearmint (Mentha spicata). J Biol Chem, 267, 7582–7587.
- Asl MN, Hosseinzadeh H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res, 22, 709–724.
- Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, Kim KW. (1997). Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer, 73, 725–728.
- Bardon S, Foussard V, Fournel S, Loubat A. (2002). Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett, 181, 187–194.
- Bardon S, Picard K, Martel P. (1998). Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer, 32, 1–7.
- Bonaccorsi I, Altieri F, Sciamanna I, Oricchio E, Grillo C, Contartese G, Galati EM. (2008). Endogenous reverse transcriptase as a mediator of ursolic acid’s anti-proliferative and differentiating effects in human cancer cell lines. Cancer Lett, 263, 130–139.
- Broitman SA, Wilkinson J4th, Cerda S, Branch SK. (1996). Effects of monoterpenes and mevinolin on murine colon tumor CT-26 in vitro and its hepatic “metastases” in vivo. Adv Exp Med Biol, 401, 111–130.
- Burgos RA, Seguel K, Perez M, Meneses A, Ortega M, Guarda MI, Loaiza A, Hancke JL. (2005). Andrographolide inhibits IFN-gamma and IL-2 cytokine production and protects against cell apoptosis. Planta Med, 71, 429–434.
- Cárdenas C, Quesada AR, Medina MA. (2004). Effects of ursolic acid on different steps of the angiogenic process. Biochem Biophys Res Commun, 320, 402–408.
- Cha HJ, Bae SK, Lee HY, Lee OH, Sato H, Seiki M, Park BC, Kim KW. (1996). Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Res, 56, 2281–2284.
- Chayet L, Rojas C, Cardemil E, Jabalquinto AM, Vicuña R, Cori O. (1977). Biosynthesis of monoterpene hydrocarbons from [1–3H]neryl pyrophosphate and [1–3H]geranyl pyrophosphate by soluble enzymes from Citrus limonum. Arch Biochem Biophys, 180, 318–327.
- Chen JH, Hsiao G, Lee AR, Wu CC, Yen MH. (2004). Andrographolide suppresses endothelial cell apoptosis via activation of phosphatidyl inositol-3-kinase/Akt pathway. Biochem Pharmacol, 67, 1337–1345.
- Cherng JM, Lin HJ, Hung MS, Lin YR, Chan MH, Lin JC. (2006). Inhibition of nuclear factor kappaB is associated with neuroprotective effects of glycyrrhizic acid on glutamate-induced excitotoxicity in primary neurons. Eur J Pharmacol, 547, 10–21.
- Cheung HY, Cheung SH, Li J, Cheung CS, Lai WP, Fong WF, Leung FM. (2005). Andrographolide isolated from Andrographis paniculata induces cell cycle arrest and mitochondrial-mediated apoptosis in human leukemic HL-60 cells. Planta Med, 71, 1106–1111.
- Chiou WF, Chen CF, Lin JJ. (2000). Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol, 129, 1553–1560.
- Croteau R, Kjonaas R. (1983). Demonstration that limonene is the first cyclic intermediate in the biosynthesis of oxygenated p-menthane monoterpenes in Mentha piperita and other Mentha species. Arch Biochem Biophys, 220, 79–89.
- Del Toro-Arreola S, Flores-Torales E, Torres-Lozano C, Del Toro-Arreola A, Tostado-Pelayo K, Guadalupe Ramirez-Dueñas M, Daneri-Navarro A. (2005). Effect of d-limonene on immune response in BALB/c mice with lymphoma. Int Immunopharmacol, 5, 829–838.
- Dietrich DR, Swenberg JA. (1991). The presence of alpha 2u-globulin is necessary for d-limonene promotion of male rat kidney tumors. Cancer Res, 51, 3512–3521.
- Elegbede JA, Elson CE, Qureshi A, Tanner MA, Gould MN. (1984). Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis, 5, 661–664.
- Elegbede JA, Maltzman TH, Verma AK, Tanner MA, Elson CE, Gould MN. (1986). Mouse skin tumor promoting activity of orange peel oil and d-limonene: a re-evaluation. Carcinogenesis, 7, 2047–2049.
- Elson CE, Maltzman TH, Boston JL, Tanner MA, Gould MN. (1988). Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis, 9, 331–332.
- Es-Saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C. (1996). MCF-7 cell cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer Res, 16, 481–486.
- Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. (2005). A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol, 99, 317–324.
- Furtado RA, Rodrigues EP, Araújo FR, Oliveira WL, Furtado MA, Castro MB, Cunha WR, Tavares DC. (2008). Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon. Toxicol Pathol, 36, 576–580.
- Geethangili M, Rao YK, Fang SH, Tzeng YM. (2008). Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother Res, 22, 1336–1341.
- Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP. (1969). Anti-inflammatory activity of natural products. I. Triterpenoids. Eur J Pharmacol, 6, 67–70.
- Guyton KZ, Kensler TW. (2002). Prevention of liver cancer. Curr Oncol Rep, 4, 464–470.
- Habtemariam S. (1998). Andrographolide inhibits the tumor necrosis factor-α-induced upregulation of ICAM-1 expression and endothelial–monocyte adhesion. Phytother Res, 12, 37–40.
- Hibasami H, Iwase H, Yoshioka K, Takahashi H. (2005). Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int J Mol Med, 16, 233–236.
- Hidalgo MA, Romero A, Figueroa J, Cortés P, Concha II, Hancke JL, Burgos RA. (2005). Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol, 144, 680–686.
- Hollósy F, Idei M, Csorba G, Szabó E, Bökönyi G, Seprödi A, Mészáros G, Szende B, Kéri G. (2001). Activation of caspase-3 protease during the process of ursolic acid and its derivative-induced apoptosis. Anticancer Res, 21, 3485–3491.
- Hollósy F, Mészáros G, Bökönyi G, Idei M, Seprödi A, Szende B, Kéri G. (2000). Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res, 20, 4563–4570.
- Hsu YL, Kuo PL, Lin CC. (2004). Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci, 75, 2303–2316.
- Hua Y, Zhang Z, Li J, Li Q, Hu S, Li J, Sun M, Cai Z. (2011). Oleanolic acid derivative Dex-OA has potent anti-tumor and anti-metastatic activity on osteosarcoma cells in vitro and in vivo. Invest New Drugs, 29, 258–65.
- Hursting SD, Perkins SN, Haines DC, Ward JM, Phang JM. (1995). Chemoprevention of spontaneous tumorigenesis in p53-knockout mice. Cancer Res, 55, 3949–3953.
- Ikeda K, Arase Y, Kobayashi M, Saitoh S, Someya T, Hosaka T, Sezaki H, Akuta N, Suzuki Y, Suzuki F, Kumada H. (2006). A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: a cohort study of 1249 patients. Dig Dis Sci, 51, 603–609.
- Iruretagoyena MI, Tobar JA, González PA, Sepúlveda SE, Figueroa CA, Burgos RA, Hancke JL, Kalergis AM. (2005). Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J Pharmacol Exp Ther, 312, 366–372.
- Ishiwata S, Nakashita K, Ozawa Y, Niizeki M, Nozaki S, Tomioka Y, Mizugaki M. (1999). Fas-mediated apoptosis is enhanced by glycyrrhizin without alteration of caspase-3-like activity. Biol Pharm Bull, 22, 1163–1166.
- Jeong HG, Kim JY. (2002). Induction of inducible nitric oxide synthase expression by 18beta-glycyrrhetinic acid in macrophages. FEBS Lett, 513, 208–212.
- Ji L, Liu T, Liu J, Chen Y, Wang Z. (2007). Andrographolide inhibits human hepatoma-derived Hep3B cell growth through the activation of c-Jun N-terminal kinase. Planta Med, 73, 1397–1401.
- Jian-zhen S, Yan-yan X, Shu Zheng Q. (2009). Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B, 10, 668–674.
- Kaji I, Tatsuta M, Iishi H, Baba M, Inoue A, Kasugai H. (2001). Inhibition by d-limonene of experimental hepatocarcinogenesis in Sprague-Dawley rats does not involve p21(ras) plasma membrane association. Int J Cancer, 93, 441–444.
- Kang OH, Kim JA, Choi YA, Park HJ, Kim DK, An YH, Choi SC, Yun KJ, Nah YH, Cai XF, Kim YH, Bae KH, Lee YM. (2005). Inhibition of interleukin-8 production in the human colonic epithelial cell line HT-29 by 18 beta-glycyrrhetinic acid. Int J Mol Med, 15, 981–985.
- Kapil A, Sharma S. (1994). Anti-complement activity of oleanolic acid: an inhibitor of C3-convertase of the classical complement pathway. J Pharm Pharmacol, 46, 922–923.
- Kapil A, Sharma S. (1995). Effect of oleanolic acid on complement in adjuvant- and carrageenan-induced inflammation in rats. J Pharm Pharmacol, 47, 585–587.
- Karp F, Mihaliak CA, Harris JL, Croteau R. (1990). Monoterpene biosynthesis: specificity of the hydroxylations of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata), and perilla (Perilla frutescens) leaves. Arch Biochem Biophys, 276, 219–226.
- Kim YS, Milner JA. (2005). Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem, 16, 65–73.
- Kimura M, Watanabe H, Abo T. (1992). Selective activation of extrathymic T cells in the liver by glycyrrhizin. Biotherapy, 5, 167–176.
- Kobayashi M, Schmitt DA, Utsunomiya T, Pollard RB, Suzuki F. (1993). Inhibition of burn-associated suppressor cell generation by glycyrrhizin through the induction of contrasuppressor T cells. Immunol Cell Biol, 71 (Pt 3), 181–189.
- Kondo Y, Takano F. (1994). Nitric oxide production in mouse peritoneal macrophages enhanced with glycyrrhizin. Biol Pharm Bull, 17, 759–761.
- Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M. (2002). Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood, 99, 326–335.
- Kroes BH, Beukelman CJ, van den Berg AJ, Wolbink GJ, van Dijk H, Labadie RP. (1997). Inhibition of human complement by beta-glycyrrhetinic acid. Immunology, 90, 115–120.
- Kuo YH, Kuo YJ, Yu AS, Wu MD, Ong CW, Yang Kuo LM, Huang JT, Chen CF, Li SY. (2003). Two novel sesquiterpene lactones, cytotoxic vernolide-A and -B, from Vernonia cinerea. Chem Pharm Bull, 51, 425–426.
- Lam LK, Hasegawa S. (1989). Inhibition of benzo[a]pyrene-induced forestomach neoplasia in mice by citrus limonoids. Nutr Cancer, 12, 43–47.
- Lee HY, Chung HY, Kim KH, Lee JJ, Kim KW. (1994). Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids. J Cancer Res Clin Oncol, 120, 513–518.
- Li J, Luo L, Wang X, Liao B, Li G. (2009). Inhibition of NF-kappaB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell Mol Immunol, 6, 381–385.
- Liu J. (1995). Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol, 49, 57–68.
- Liu Y. (1986). Pharmaceutical composition for treating nonlymphatic leukemia and its components. Chem Abstr, 106, 90209j.
- Lu XG, Feng BA, Zhan LB, Yu ZH. (2003). [D-Limonene induces apoptosis of gastric cancer cells]. Zhonghua Zhong Liu Za Zhi, 25, 325–327.
- Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH, Xie JH. (2004). Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J Gastroenterol, 10, 2140–2144.
- Lu XL, Zhang SL, Wang ZS. (1981). [Analysis of andrographolide compounds. I. Ion pair high performance liquid chromatographic analysis of andrographolide derivatives (author’s transl)]. Yao Xue Xue Bao, 16, 182–189.
- Ma X, Wang Z. (2009). Anticancer drug discovery in the future: an evolutionary perspective. Drug Discov Today, 14, 1136–1142.
- MacKenzie MA, Hoefnagels WH, Kloppenborg PW. (1990). Glycyrrhetinic acid and potentiation of hydrocortisone activity in skin. Lancet, 335, 1534.
- Maltzman TH, Hurt LM, Elson CE, Tanner MA, Gould MN. (1989). The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis, 10, 781–783.
- Manu KA, Kuttan G. (2008). Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol, 8, 974–981.
- Martin-Aragón S, de las Heras B, Sanchez-Reus MI, Benedi J. (2001). Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol, 53, 199–206.
- McGarvey DJ, Croteau R. (1995). Terpenoid metabolism. Plant Cell, 7, 1015–1026.
- Moro T, Shimoyama Y, Kushida M, Hong YY, Nakao S, Higashiyama R, Sugioka Y, Inoue H, Okazaki I, Inagaki Y. (2008). Glycyrrhizin and its metabolite inhibit Smad3-mediated type I collagen gene transcription and suppress experimental murine liver fibrosis. Life Sci, 83, 531–539.
- Muto Y, Ninomiya M, Fujiki H. (1990). Present status of research on cancer chemoprevention in Japan. Jpn J Clin Oncol, 20, 219–224.
- Najid A, Simon A, Cook J, Chable-Rabinovitch H, Delage C, Chulia AJ, Rigaud M. (1992). Characterization of ursolic acid as a lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and differentiated HL60 leukemic cells. FEBS Lett, 299, 213–217.
- Nakajima N, Utsunomiya T, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. (1996). In vitro induction of anti-type 2 T cells by glycyrrhizin. Burns, 22, 612–617.
- Nataraju A, Saini D, Ramachandran S, Benshoff N, Liu W, Chapman W, Mohanakumar T. (2009). Oleanolic acid, a plant triterpenoid, significantly improves survival and function of islet allograft. Transplantation, 88, 987–994.
- Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, Iwata S, Shibata S. (1988). Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res, 48, 5210–5215.
- Novotný L, Vachálková A, Biggs D. (2001). Ursolic acid: an anti-tumorigenic and chemopreventive activity. Minireview. Neoplasma, 48, 241–246.
- Ohigashi H, Takamura H, Koshimizu K, Tokuda H, Ito Y. (1986). Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett, 30, 143–151.
- Ohuchi K, Kamada Y, Levine L, Tsurufuji S. (1981). Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins Med, 7, 457–463.
- Poulose SM, Harris ED, Patil BS. (2006). Antiproliferative effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells. Nutr Cancer, 56, 103–112.
- Pratheeshkumar P, Kuttan G. (2010a). Nomilin inhibits metastasis via induction of apoptosis and regulates the activation of transcription factors and cytokine profile in B16F-10 cells. Integr Cancer Ther (in press).
- Pratheeshkumar P, Kuttan G. (2011). Andrographolide inhibits human umbilical vein endothelial cell invasion and migration by regulating MMP-2 and MMP-9 during angiogenesis. J Environ Pathol Toxicol Oncol, 30, 33–41.
- Pratheeshkumar P, Kuttan G. (2010b). Vernolide-A, a sesquiterpene lactone from Vernonia cinerea, induces Apoptosis in B16F-10 melanoma cells by modulating p53 and caspase-3 gene expressions and regulating NF-κB mediated bcl-2 activation. Drug Chem Toxicol (in press).
- Pratheeshkumar P, Kuttan G. (2010c). Anti-metastatic potential of vernolide-A, a sesquiterpenoid from Vernonia cinerea L. Hum Exp Toxicol (in press).
- Pratheeshkumar P, Kuttan G. (2011). Vernolide-A inhibits tumor specific angiogenesis by regulating proinflammatory cytokines, VEGF, MMPs and TIMP. Eur J Pharmacol, 656, 10–8.
- Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. (1993). Immunostimulant agents from Andrographis paniculata. J Nat Prod, 56, 995–999.
- Qi CL, Wang LJ, Zhou XL. (2007). [Advances in study on anti-tumor mechanism of andrographolide]. Zhongguo Zhong Yao Za Zhi, 32, 2095–2097.
- Rahman S, Sultana S. (2007). Glycyrrhizin exhibits potential chemopreventive activity on 12-O-tetradecanoyl phorbol-13-acetate-induced cutaneous oxidative stress and tumor promotion in Swiss albino mice. J Enzyme Inhib Med Chem, 22, 363–369.
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. (2003). Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol, 3, 147–158.
- Raphael TJ, Kuttan G. (2003). Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine, 10, 483–489.
- Raphael TJ, Kuttan G. (2008). Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharmacol Immunotoxicol, 30, 243–255.
- Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L. (1998). Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod, 61, 1212–1215.
- Samaila D, Toy BJ, Wang RC, Elegbede JA. (2004). Monoterpenes enhanced the sensitivity of head and neck cancer cells to radiation treatment in vitro. Anticancer Res, 24, 3089–3095.
- Saravanan R, Viswanathan P, Pugalendi KV. (2006). Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci, 78, 713–718.
- Satyanarayana C, Deevi DS, Rajagopalan R, Srinivas N, Rajagopal S. (2004). DRF 3188 a novel semi-synthetic analog of andrographolide: cellular response to MCF 7 breast cancer cells. BMC Cancer, 4, 26.
- Schleimer RP. (1991). Potential regulation of inflammation in the lung by local metabolism of hydrocortisone. Am J Respir Cell Mol Biol, 4, 166–173.
- Schulz S, Bühling F, Ansorge S. (1994). Prenylated proteins and lymphocyte proliferation: inhibition by d-limonene related monoterpenes. Eur J Immunol, 24, 301–307.
- Schulz S, Reinhold D, Schmidt H, Ansorge S, Höllt V. (1997). Perillic acid inhibits Ras/MAP kinase-driven IL-2 production in human T lymphocytes. Biochem Biophys Res Commun, 241, 720–725.
- Sheeja K, Guruvayoorappan C, Kuttan G. (2007). Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int Immunopharmacol, 7, 211–221.
- Sheeja K, Kuttan G. (2007). Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr Cancer Ther, 6, 66–73.
- Shi MD, Lin HH, Chiang TA, Tsai LY, Tsai SM, Lee YC, Chen JH. (2009). Andrographolide could inhibit human colorectal carcinoma Lovo cells migration and invasion via down-regulation of MMP-7 expression. Chem Biol Interact, 180, 344–352.
- Shi MD, Lin HH, Lee YC, Chao JK, Lin RA, Chen JH. (2008). Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem Biol Interact, 174, 201–210.
- Shih YH, Chein YC, Wang JY, Fu YS. (2004). Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci Lett, 362, 136–140.
- Simon A, Najid A, Chulia AJ, Delage C, Rigaud M. (1992). Inhibition of lipoxygenase activity and HL60 leukemic cell proliferation by ursolic acid isolated from heather flowers (Calluna vulgaris). Biochim Biophys Acta, 1125, 68–72.
- Singh GB, Singh S, Bani S, Gupta BD, Banerjee SK. (1992). Anti-inflammatory activity of oleanolic acid in rats and mice. J Pharm Pharmacol, 44, 456–458.
- Stratton SP, Dorr RT, Alberts DS. (2000). The state-of-the-art in chemoprevention of skin cancer. Eur J Cancer, 36, 1292–1297.
- Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. (2000). Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res, 60, 2399–2404.
- Takagi K, Park EH, Kato H. (1980). Anti-inflammatory activities of hederagenin and crude saponin isolated from Sapindus mukorossi Gaertn. Chem Pharm Bull, 28, 1183–1188.
- Teelucksingh S, Mackie AD, Burt D, McIntyre MA, Brett L, Edwards CR. (1990). Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet, 335, 1060–1063.
- Thirugnanam S, Xu L, Ramaswamy K, Gnanasekar M. (2008). Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep, 20, 1387–1392.
- Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. (2001). Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr Cancer, 40, 180–184.
- Tokuda H, Ohigashi H, Koshimizu K, Ito Y. (1986). Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett, 33, 279–285.
- Tripathi M, Singh BK, Kakkar P. (2009). Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes. Food Chem Toxicol, 47, 339–347.
- Uedo N, Tatsuta M, Iishi H, Baba M, Sakai N, Yano H, Otani T. (1999). Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett, 137, 131–136.
- Utsunomiya T, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. (1995). Glycyrrhizin (20 beta-carboxy-11-oxo-30-norolean-12-en-3 beta-yl-2-O-beta-d-glucopyranuronosyl-alpha-d-glucopyranosiduronic acid) improves the resistance of thermally injured mice to opportunistic infection of herpes simplex virus type 1. Immunol Lett, 44, 59–66.
- Varma A, Padh H, Shrivastava N. (2009). Andrographolide: a new plant-derived antineoplastic entity on horizon. Evid Based Complement Alternat Med.
- Walker BR, Edwards CR. (1991). 11beta-Hydroxysteroid dehydrogenase and enzyme-mediated receptor protection: life after liquorice? Clin Endocrinol (Oxf), 35, 281–289.
- Wang B, Jiang Z. (1992). Studies on oleanolic acid. Chin Pharm J, 27, 393–397.
- Wang G, Tang W, Bidigare R. (2005). Terpenoids as therapeutic drugs and pharmaceutical agents. Nat Prod, 197–227.
- Wang T, Liu B, Zhang W, Wilson B, Hong JS. (2004). Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J Pharmacol Exp Ther, 308, 975–983.
- Wang ZY, Nixon DW. (2001). Licorice and cancer. Nutr Cancer, 39, 1–11.
- Wattenberg LW, Coccia JB. (1991). Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone carcinogenesis in mice by d-limonene and citrus fruit oils. Carcinogenesis, 12, 115–117.
- Wattenberg LW, Sparnins VL, Barany G. (1989). Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res, 49, 2689–2692.
- Xavier CP, Lima CF, Preto A, Seruca R, Fernandes-Ferreira M, Pereira-Wilson C. (2009). Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett, 281, 162–170.
- Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin X, Yao X, Ma D, Slungaard A, Hebbel RP, Key NS, Geng JG. (2004). Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol, 173, 4207–4217.
- Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K, Miyoshi T, Kondo K, Bando Y, Ohnishi Y, Tangoku A. (2009). Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer, 125, 952–960.
- Yang L, Wu D, Luo K, Wu S, Wu P. (2009). Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett, 276, 180–188.
- Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. (2007). Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett, 257, 216–226.
- Yoshikawa M, Toyohara M, Ueda S, Shiroi A, Takeuchi H, Nishiyama T, Yamada T, Fukui H, Ishizaka S. (1999). Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol Pharm Bull, 22, 951–955.
- Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, Yamahara J, Mori H. (1992). Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res, 83, 1273–1278.
- Yue D, Bing-qian H, Li-wu T. (1989). Anti-inflammatory effect of oleanolic acid [J]. Chin J Pharmacol Toxicol, 2.
- Zhang S, Won YK, Ong CN, Shen HM. (2005). Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents, 5, 239–249.
- Zhang YH, Isobe K, Nagase F, Lwin T, Kato M, Hamaguchi M, Yokochi T, Nakashima I. (1993). Glycyrrhizin as a promoter of the late signal transduction for interleukin-2 production by splenic lymphocytes. Immunology, 79, 528–534.
- Zhao F, He EQ, Wang L, Liu K. (2008). Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J Asian Nat Prod Res, 10, 467–473.
- Zhou J, Lu GD, Ong CS, Ong CN, Shen HM. (2008). Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol Cancer Ther, 7, 2170–2180.