Abstract
Context: Potentilla alba L. (Rosaceae) rhizomes have anti-inflammatory, antioxidant, and adaptogenic effects and are used for the treatment of diarrhea and intestinal colic. However, the data concerning the adaptogenic and central nervous system activities of P. alba are fragmentary.
Objectives: To determine the effect of oral administration of dried P. alba extract on the swimming endurance, light/dark exploration, and open-field tests for mice.
Materials and methods: The mice were orally administered Rhodiola rosea extract (RR group); dry extract of P. alba at doses of 12, 36, or 72 mg/kg (groups: PA12, PA36, and PA72); or distilled water (control group) for 7 consecutive days.
Results: The swimming times of the RR, PA36, and PA72 groups were significantly longer than those of the control group. The administration of P. alba significantly increased the light time, latency time, and the number of rearings in a dose-dependent manner. In the open-field test, the P. alba extract at a dose of 12 mg/kg produced a significant increase in the frequency of head dipping and the number of squares crossed and a significant decrease in grooming compared with the control treatment.
Conclusion: The current findings demonstrate that P. alba extracts significantly increased swimming endurance time and have anxiolytic-like action with a predominant locomotor component.
Introduction
Potentilla species have been used for several hundred years. In medieval Europe, physicians and botanists such as H. Bock, L. Fuchs, Paracelsus, Tabernaemontanus, and C. Bauhin described and depicted Potentilla species in their herbal books (CitationTomczyk and Latté, 2009). L. Fuchs mentioned five Potentilla species in his “New Kreuterbuch” (1543), including Potentilla alba L., Potentilla reptans L. and Potentilla neumanniana RCHB (underground parts and leaves); Potentilla anserina L. (stems and leaves); and Potentilla erecta (underground parts and stems and leaves).
There is a lack of information about white cinquefoil—P. alba L. (Rosaceae)—in the scientific literature. The anti-inflammatory, antioxidant, and antimicrobial activities of P. alba have been described (CitationPilipovć et al., 2005; CitationGrujić-Vasić et al., 2006; CitationOszmianski et al., 2007). Phytotherapists recommend P. alba for the treatment of heart diseases, as a stimulator of the central nervous system (CNS), and as an adaptogen (CitationGritsenko and Smik, 1977; CitationShymko and Khishova, 2010).
Adaptogens are medicinal plants that enhance an organism’s state of nonspecific resistance to stress, augmenting resistance to physical, biological, chemical, and psychological stresses. Adaptogens also increase concentration, performance, and endurance during periods of fatigue (CitationBrekhman and Dardymov, 1969; CitationPanossian and Wagner, 2005). The interest in adaptogenic plants has grown markedly, and there is great demand to identify new adaptogenic plants.
It has been reported that P. alba contains a high amount of hydrolysable and condensed tannins (proanthocyanidins), flavonoids (kaempferol and quercetin), polyprenols, phenolcarbonic acids, triterpenes, and polysaccharides (CitationGritsenko and Smik, 1977; CitationOszmianski et al., 2007; CitationTomczyk and Latté, 2009; CitationShikov et al., 2009; CitationTomczyk et al., 2010; CitationShymko and Khishova, 2010).
As a continuation of our studies of medicinal plants with adaptogenic activities (CitationShikov et al., 2008, Citation2010), the purpose of the current work was to determine the effect of oral administration of P. alba dry extract on the swimming endurance, light/dark exploration, and open-field tests for mice.
Materials and methods
Plant material and preparation of the extracts
Rhizomes of 4-year-old P. alba plants were obtained from Ginseng Ltd., Russia. The samples were air dried, ground to a powder using an excelsior mill, and stored in closed vessels. The plant was identified by Dr. Vera Kosman, and voucher specimens (PA409) have been deposited in the herbarium of the St. Petersburg Institute of Pharmacy (St. Petersburg, Russia). The dried sample of P. alba was ground in a coffee grinder and then extracted with water (medicinal plant:water = 1:15, w/v) at 90°C in water bath for 1.5 h. The extract was concentrated under reduced pressure at 50 ± 5°C and lyophilized to obtain a powder, which was used as the test sample. The powder was dissolved in distilled water before oral administration to the mice.
Rhodiola rosea L. (Crassulaceae) liquid extract (Kamelia, Moscow region, Russia), standardized by salidroside (0.6%), was dealcoholized and then made up to the volume in a 100 ml volumetric flask and used as a reference extract with known adaptogenic activity (CitationPanossian et al., 2010).
Animals
Male Balb/c mice weighing 21–24 g were obtained from the Russian Academy of Medical Sciences (Rappolovo, Russia). Mice were housed in groups of eight per standard cage and were maintained under standard laboratory conditions (temperature 19–25°C, relative humidity 50–70%, 12 h light/12 h dark cycle) with free access to a solid pellet (Volosovo, Russia) diet and water ad libitum throughout the study. All procedures used in the current study were approved by the Institutional Ethics Committee on the Use of Animals, complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985) and follows the Principles of Good Laboratory Practice (GOST R 53434-2009, identical to OECD GMP). Each animal was used just once, and all efforts were made to use the minimum number of animals required to obtain consistent experimental data. All studies were recorded using a video camera. The video recordings were later evaluated by an observer, who had no knowledge of the treatment that each animal had received, and the dose responses were evaluated.
Adaptogenic activity: swimming to exhaustion test
The ability of adaptogens to increase work capacity and physical performance in rodents has often been assessed using the swimming test under different experimental conditions (CitationPanossian et al., 1999; CitationPerfumi and Mattioli, 2007; CitationPanossian and Wikman, 2008).
After an adaptation period of 2 weeks, the mice were randomly divided into five groups (n = 6 per group) that received i.g. administration once daily for 7 consecutive days of vehicle, R. rosea extract or P. alba extract at 12, 36, or 72 mg/kg body weight (BW). The forced swimming capacity of mice was measured 40 min after drug administration on day 7 using a glass cylinder (45 cm in height, 20 cm in diameter) filled 25 cm high with water (26 ± 2°C). The mice were loaded with a steel washer weighing approximately 5% of their body weight, which was attached to the tail. It has been reported that this arrangement forces the mouse to maintain continuous rapid leg movement (CitationJung et al., 2004). The swimming to exhaustion time was used as the measure of forced swimming capacity. The mice were assessed to be exhausted when they failed to rise to the surface of the water to breathe within a 7-s period. CitationKamakura et al. (2001) reported that a period of longer than 7 s resulted in frequent drowning, and a period of less than 5 s reduced the reproducibility of the test. Each animal was used only once.
Within 5 min from completion of the test, blood samples were collected from the tail tip. After restraining the mouse, 1.5 μl of blood for glucose estimation was collected from the tail vein and placed directly on a strip placed in the glucometer (One Touch Horizon, Life Scan Inc., Milpitas, CA; Johnson and Johnson Company). The lactate concentration was measured using an Accutrend Lactate Portable Analyzer and BM Lactate strips (Roche Diagnostics GmbH, Mannheim, Germany). Immediately after the blood had been collected, the liver was removed, frozen in liquid nitrogen, and stored at −70°C until analysis for glycogen content. The glycogen content was measured spectrophotometrically using the glucose oxidase method as described elsewhere (CitationChun and Yin, 1998).
Anxiolytic-like activity
A relevant test system to detect anxiety-related behavior in mice is the light/dark exploration test, which uses the aversion of rodents to large brightly lit spaces. The apparatus consisted of a Perspex box (20 × 50 × 20 cm) divided into a light and a dark chamber of the same size; the chambers were connected by a small door. The dark chamber was entirely black and covered with a solid black plastic top. The light chamber was entirely white and open and was illuminated by a 60-W light bulb. The mice (n = 30) were divided into five groups that received i.g. administration once daily for 7 consecutive days of vehicle, R. rosea extract or P. alba extract at 12, 36, or 72 mg/kg BW. On day 7, 1 h after the administration of extract/vehicle, each animal was placed at the center of the illuminated chamber, facing the dark area, and was allowed to explore the whole apparatus for 5 min. The time spent in the light compartment, the latency time until the first passage from the light chamber into the dark chamber, and the number of entries into each compartment were registered.
Locomotor and anxiolytic-like activities
The open-field test is a classical system that is routinely used to evaluate general locomotor activity and anxiety-related behavior of animals (CitationPrut and Belzung, 2003). Open-field activity was measured in a Plexiglas cage (20 × 40 × 40 cm) that was divided into nine squares with 18 holes (two holes in each square). Mice (n = 30) were divided into five groups that received i.g. administration for 7 consecutive days of vehicle, R. rosea extract or P. alba extract at 12, 36, or 72 mg/kg BW. On day 7, 1 h after the administration of extract/vehicle, the test was initiated by placing a single mouse in the middle of the arena and letting it move freely for 3 min. The observed behavioral parameters were as follows: horizontal activity (the number of squares crossed) and the vertical activity (rearing). The central time, grooming, and in holes head dips were indicators of the emotional reactivity of the mouse (CitationMeyer et al., 2006). Therefore, these behaviors were taken as measures of anxiety. The apparatus was cleaned with a detergent and dried after occupancy by each mouse. The animals were used only once in this test.
Statistical analysis
Data were analyzed using Statistica version 6.0 (Statsoft, Moscow, Russia). The results are expressed as the mean values ± standard error of the mean. The significance of the difference in the mean between the control group and each treatment group was determined using Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
The body weight of the animals was recorded before the experiment and after 7 days, and the weight gain was computed. There was no significant difference with respect to body weight between the control group and each treatment group in all experiments. In the current study, P. alba extracts and R. rosea extract had no significant effect on the body weight or weight gain compared with the control group.
Adaptogenic activity: swimming to exhaustion test
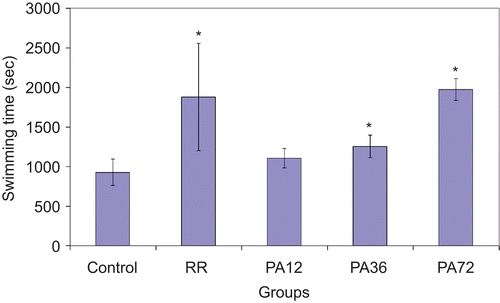
The forced swimming capacities are shown in . Statistical analysis revealed that the effect of P. alba extracts on the swimming to exhaustion times of mice was dose dependent. The swimming to exhaustion times for the RR, PA36, and PA72 groups were 1880 ± 677 s, 1255 ± 144 s, and 1974 ± 140 s, respectively; these times were significantly longer than those of the control group (P < 0.05). The swimming times of the PA12 group was 1107 ± 120 s, and the difference with respect to the control group was not statistically significant (P > 0.05).
Figure 1. Effect of extracts on the swimming endurance of mice. RR, Rhodiola rosea; PA12, Potentilla alba at 12 mg/kg; PA36, P. alba at 36 mg/kg; and PA72, P. alba at 72 mg/kg. Values with an asterisk are significantly different from those of the control group as assessed by Student’s t-test at P < 0.05.
The biochemical parameters of blood are shown in . The serum glucose level was significantly higher in the RR, PA12, and PA36 groups than in the control group (P < 0.05), although the difference between the control group and the PA72 group was not significant. The blood lactate level of the RR group was lower than that of the control group, whereas in the P. alba groups, the lactate levels were slightly higher. However, the difference in the blood lactate level between the control group and each of the other treatment groups was not statistically significant.
Table 1. Effect of extracts on the biochemical parameters of the blood.
The liver glycogen content tended to be lower in the RR, PA12, and PA36 groups. These groups also showed a small increase in the swimming time; the swimming time was significantly higher in the PA72 group, which showed the longest swimming time.
Anxiolytic-like activity
The results of the light/dark test are shown in . The administration of P. alba increased the time spent in the light compartment (light time), the latency time until the first passage from the light compartment into the dark compartment (latency time), and the number of entries in a dose-dependent manner. For all parameters, the post hoc analysis revealed a statistically insignificant effect at all the doses tested.
Table 2. Effect of extracts on the behavioral parameters in the light/dark test.
Locomotor and anxiolytic-like activities
The overall effects of per os administration of P. alba on the spontaneous motor activity of the mice in the open-field test are summarized in . Statistical analysis revealed that the P. alba extract induced an increase in the central time, the number of squares crossed, and the frequency of head dipping. The post hoc analysis revealed a statistically significant effect only at a dose of 12 mg/kg (P < 0.05), whereas no significant effects were seen for the medium and high doses (36 and 72 mg/kg; P > 0.05). A statistically significant dose-dependent decrease in the number of groomings was registered for all P. alba−treated groups. The number of rearings displayed by mice was not different among the various groups of animals, except for the significant decrease (in 1.9 times) in R. rosea–treated group compared with the control group.
Table 3 . Effect of extracts on spontaneous locomotor activity and anxiety-related parameters in the open field test.
Discussion
P. alba rhizomes have been used in traditional medicine for several hundred years. However, data on the adaptogenic and CNS effects of this plant are rare and fragmentary. In this study, we examined the effects of P. alba on the CNS and its adaptogenic activity. Typically, the pharmacological assessment of adaptogens includes the evaluation of stimulation, tonic, and stress-protective activities. The most important feature in the pharmacological profile of adaptogens is that they increase the resistance of animals to physical exhaustion and other stresses, such as anxiety (CitationPanossian and Wikman, 2005; CitationPanossian and Wagner, 2005). The results of the current study provide evidence that the dried P. alba extract is able to induce adaptogenic, stimulating, and anxiolytic-like effects in mice.
The current data demonstrate that, after 7 days of consecutive oral administration, P. alba can significantly increase the swimming time in a dose-dependent manner. Generally, it is assumed that a decrease in the glucose level indicates the consumption of energy and that an increase in the lactate level indicates a fatigued condition during prolonged exercise (CitationJung et al., 2004). The blood lactate level was determined primarily as an index of anaerobic metabolism during swimming (CitationDawson et al., 1971). The rapid increase in the blood lactic acid level may be a reflection of an oxygen debt that is mounting at a high rate. Swimming to exhaustion induces a significantly increased blood lactate level, and the rate at which lactate accumulates in the blood showed an inverse relationship with the swimming time of the mice after administration of Paecilomyces japonica (Ascomycete) and Grifola frondosa (Fr.) S.F. Gray (Polyporaceae) extracts (CitationJung et al., 2004). It has been well established that during prolonged exercise the development of fatigue is closely related to the depletion of glycogen stores in liver tissue (CitationAvakian and Evonuk, 1979). Infusions prepared using fermented leaves of Bergenia crassifolia L. (Saxifragaceae) significantly enhanced the maximum swimming capacity of mice by increasing glucose utilization and by decreasing the lactate level compared with the control treatment (CitationShikov et al., 2010). Although R. rosea and P. alba at a dose of 12 mg/kg exhibited a prolongation effect on swimming times and an increase in the glucose level, neither had a significant effect on the changes in the lactate and glycogen levels compared with the control treatment in the current study. Only at the higher dose of 72 mg/kg of P. alba was a significant increase in the glycogen level registered, while the glucose level of these mice tended to decrease but was not different from that of the controls. The swimming time of mice in the 72 mg/kg P. alba group was the highest.
Our results suggest that P. alba at the low and middle doses administered for 7 consecutive days made mice resistant to physical fatigue by increasing glucose production, which is formed in muscle by glycogenolysis. Glycogen stored in muscle is a major source of fuel, and the depletion of these stores has been shown to correlate closely with exhaustion. Two stimuli of muscle glycogenolysis are known: (i) muscle contractions, during which calcium released from the sarcoplasmic reticulum is believed to increase phosphorylase a activity by activating phosphorylase b kinase; and (ii) epinephrine, which by increasing cyclic adenosine monophosphate generation sets in motion the cascade reaction leading to the conversion of phosphorylase b to phosphorylase a (CitationSoderling and Park, 1974). Recently, it was demonstrated that an extract of R. rosea significantly prolonged the duration of exhaustive swimming in rats and stimulated adenosine triphosphate (ATP) synthesis in muscle during exercise (CitationAbidov et al., 2003). CitationPerfumi and Mattioli (2007) have speculated that, in addition to the enhancement of the catecholaminergic system, the ability of R. rosea to increase the swimming time involves an improvement in cellular energy metabolism, based in part on ATP.
To explain the complex mechanisms underlying the adaptogen activity of P. alba extract, the antioxidant effects of P. alba should also be considered (CitationOszmianski et al., 2007). Indeed, it is known that exercise generates free radicals when it is exhaustive and that P. alba is rich in phenolic compounds that can protect the nervous system from oxidative damage by such free radicals (CitationKelly, 2001). However, the test used here did not allow us to determine the exact mechanism, and further appropriate studies are needed.
The analysis of anxiety-related parameters revealed marked alterations in the anxiety of the P. alba–treated mice. P. alba showed anxiolytic-like activities that counteracted anxiety in mice subjected to an aversive stimulus in two experimental models. The light/dark test is a common test system to detect anxiety-related behavior (CitationPerfumi and Mattioli, 2007; CitationPitsikas et al., 2008). In this experiment, mice treated with R. rosea and P. alba at a dose of 72 mg/kg spent more time in the light chamber of the box than did the control animals. However, the effects of both extracts were not significant.
The open-field test is used to evaluate the animal’s emotional state. Animals removed from their acclimatized cage and placed in a novel environment express anxiety and fear and show alterations in all or some parameters, such as a decrease in ambulation and in the exploration time in the center of the open field with increase peripheral movement. These parameters are attenuated by anxiolytics (CitationWoode et al., 2010). The administration of P. alba at a dose of 12 mg/kg induced a significant increase in the head-dip frequency (2.6 times) and in the number of squares crossed (1.3 times) and a significant decrease in grooming (in 2.1 times) compared with the control treatment (). A number of studies have evaluated the head-dip frequency as an indicator of anxiety: low values seem to reflect high anxiety conditions, whereas high values indicate a low anxiety level (CitationTakeda et al., 1998; CitationCasarrubea et al., 2010). This factor is also a measure of exploratory activity. Grooming is an important component of a rodent’s behavioral repertoire, and it is the initial behavioral response to stressful situations and is used by animals to lower arousal (CitationKalueff and Tuohimaa, 2004). The grooming was significantly decreased in a dose-dependent manner in the P. alba–treated mice. This result suggests a decrease in anxiety. All observations support our hypothesis that the administration of P. alba extracts stimulate exploratory activity and produce an anxiolytic effect.
It is known that numerous pathways are involved in the pathophysiology of anxiety states and that a great number of neurotransmitters participate in the underlying mechanisms of this disease. However, the behavioral tests used here did not allow us to determine the exact mechanisms responsible for the anxiolytic effect of P. alba, and therefore, further appropriate studies are needed.
Conclusion
The current findings, to our knowledge, demonstrate for the first time that P. alba extracts significantly increased swimming endurance time and that they have anxiolytic-like action with a predominant locomotor component. The pharmacological mechanism(s) that might account for the effects of P. alba has yet to be determined. Further studies will be required to assess the generality of the current findings with respect to behavioral paradigms.
Declaration of interest
The authors have declared that there is no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abidov M, Crendal F, Grachev S, Seifulla R, Ziegenfuss T. (2003). Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles. Bull Exp Biol Med, 136, 585–587.
- Avakian EV Jr, Evonuk E. (1979). Effect of Panax ginseng extract on tissue glycogen and adrenal cholesterol depletion during prolonged exercise. Planta Med, 36, 43–48.
- Brekhman II, Dardymov IV. (1969). New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol, 9, 419–430.
- Brown RP, Gerbarg PL, Ramazanov Z. (2002). Rhodiola rosea: A phytomedicinal overview. Herbal Gram, 56, 40–52.
- Casarrubea M, Sorbera F, Magnusson M, Crescimanno G. (2010). Temporal patterns analysis of rat behavior in hole-board. Behav Brain Res, 208, 124–131.
- Chun Y, Yin ZD. (1998). Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J Clin Microbiol, 36, 1081–1082.
- Dawson CA, Nadel ER, Horvath SM. (1971). Arterial blood and muscle lactates during swimming in the rat. J Appl Physiol, 30, 322–327.
- Fuchs L. (1543). New Kreuterbuch, Basel: Kapitel 98. (“Von Tormentill”).
- Gritsenko OM, Smik GK. (1977). Phytochemical study of P. alba. Farm Z (Kiev), 1, 88–89.
- Grujić-Vasić J, Pilipović S, Bosnić T, Redžić S. (2006). Antimicrobial activity of different extracts from rhizome and root of Potentilla erecta L. Raeuschel and Potentilla alba L. Rosaceae. Acta Med Acad, 35, 9–14.
- Jung K, Kim IH, Han D. (2004). Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol, 93, 75–81.
- Kamakura M, Mitani N, Fukuda T, Fukushima M. (2001). Antifatigue effect of fresh royal jelly in mice. J Nutr Sci Vitaminol, 47, 394–401.
- Kelly GS. (2001). Rhodiola rosea: a possible plant adaptogen. Altern Med Rev, 6, 293–302.
- Kalueff AV, Tuohimaa P. (2004). Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc, 13, 151–158.
- Meyer L, Caston J, Mensah-Nyagan AG. (2006). Seasonal variation of the impact of a stressful procedure on open field behaviour and blood corticosterone in laboratory mice. Behav Brain Res, 167, 342–348.
- Oszmianski J, Wojdyło A, Lamer-Zarawska E, Swiader K. (2007). Antioxidant tannins from Rosaceae plant roots. Food Chem, 100, 579–583.
- Panossian A, Wikman G. (2005). Effect of adaptogens on the central nervous system. Arquivos Brasileiros de Fitomed Cie, 2, 108–130.
- Panossian A, Wagner H. (2005). Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res, 19, 819–838.
- Panossian A, Wikman G. (2008). Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol, 118, 183–212.
- Panossian A, Wikman G, Sarris J. (2010). Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine, 17, 481–493.
- Panossian A, Wikman G, Wagner H. (1999). Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine, 6, 287–300.
- Perfumi M, Mattioli L. (2007). Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res, 21, 37–43.
- Pilipovć S, Grujić-Vasić J, Ibrulj A, Redžić S, Bosnić T. (2005). Antiinflammatory effect of rhizome and root of Potentilla erecta (L.) Raeuschel and Potentilla alba L. (Rosaceae). Abstract P164, p. 192, 53. Joint meeting of the Society of Medicinal Plant Research, Florence.
- Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, Sakellaridis N. (2008). Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine, 15, 1135–1139.
- Prut L, Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol, 463, 3–33.
- Shikov AN, Pozharitskaya ON, Faustova NM, Pan’ko NM, Makarov VG. (2009). Phytochemical composition of cultivated rhizome of Potentilla alba L. Book of abstracts of 57th International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research, 16–20 August 2009.Addendum. P. A5.
- Shikov AN, Pozharitskaya ON, Makarova MN, Dorman HJD, Makarov VG, Tikhonov VP, Galambosi B, Hiltunen R. (2008). Examination of adaptogenic effect of infusions of Bergenia crassifolia black and fermented leaves in the forced swimming test. Planta Med, 74, 908.
- Shikov AN, Pozharitskaya ON, Makarova MN, Dorman HJD, Makarov VG, Hiltunen R, Galambosi B. (2010). Adaptogenic effect of black and fermented leaves of Bergenia crassifolia L. in mice. J Funct Foods, 2, 71–76.
- Shymko OM, Khishova OM. (2010). Standardization of grass Potentilla alba. Vestnik Pharmacii, 1, 17–23.
- Soderling TR, Park CR. (1974). Recent advances in glycogen metabolism. Adv Cyclic Nucleotide Res, 4, 283–333.
- Takeda H, Tsuji M, Matsumiya T. (1998). Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol, 350, 21–29.
- Tomczyk M, Bazylko A, Staszewska A. (2010). Determination of polyphenolics in extracts of Potentilla species by high-performance thin-layer chromatography photodensitometry method. Phytochem Anal, 21, 174–179.
- Tomczyk M, Latté KP. (2009). Potentilla–a review of its phytochemical and pharmacological profile. J Ethnopharmacol, 122, 184–204.
- Woode E, Boakye-Gyasi E, Amidu N, Ansah C, Duwiejua M. (2010). Anxiolytic and antidepressant effects of a leaf extract of Palisota hirsute K. Schim. (Commelinaceae) in mice. Int J Pharmacol, 6, 1–17.
