Abstract
Context: Styrax, resin of Liquidambar orientalis Mill. (N.O. Hamamelaceae), belongs to resuscitation-inducing aromatic herbs in traditional Chinese medicine and functions in inducing resuscitation and restoring conscientiousness.
Objective: The possible sedative and anticonvulsant activities of styrax on CNS were investigated. The onsets of action of two different routes (oral and intranasal administration) were compared.
Materials and methods: Styrax was tested for sedative, hypnotic, and anticonvulsant effects using locomotor activity evaluation, pentobarbital-induced sleeping time, and pentylenetetrazol (PTZ)-induced convulsion, respectively.
Results: After oral administration (25, 50, 100 mg/kg), styrax prolonged the sodium pentobarbital-induced sleeping time. In comparison with oral administration, intranasal administration (12.5, 25, 50 mg/kg) prolonged the sleeping time at lower dosage. Moreover, styrax (100 and 200 mg/kg) promoted a significant protection against PTZ-induced seizures and mortality 30 min after oral administration. In contrast, 5 min after intranasal administration, styrax promoted significant protection at lower dosages (25 and 50 mg/kg). These data show that styrax had faster onset of action (5 vs. 30 min) and better anticonvulsant efficacy (25, 50 vs. 100, 200 mg/kg) by intranasal route in comparison with that by intragastric route. Styrax decreased the spontaneous locomotor movements at 100 mg/kg during 5–60 min interval after oral administration.
Discussion and conclusion: Styrax has sedative and anticonvulsant activities. Furthermore, styrax has faster onset of action as well as more potent efficacy after intranasal administration at lower dosage than by intragastric route. This result illustrates that intranasal administration may act as a promising alternative to conventional routes of administration.
Introduction
Liquidambar orientalis Mill. (N.O. Hamamelaceae), a herbaceous plant distributed in several regions of Southeast Asia, has medicinal and cosmetic properties and is widely used in the Mediterranean region. Styrax (resin of L. orientalis) (SuHeXiang in Chinese) produced by injuring L. orientalis has good antiseptic properties (CitationDuru et al., 2002). The bark of the tree is mechanically ruptured in early summer, and then stripped as late as autumn. Crude liquid styrax is obtained by pressing the bark in cold water alternating with boiling water. The crude styrax is then dissolved in alcohol, filtered, and collected so as to obtain purified styrax. Styrax has been used as an expectorant, especially in inhalation with warm air vaporizers. Also, it is used as a topical parasiticide, and for the treatment of some skin diseases (CitationDuru et al., 2002). It has a wide application in cosmetics (CitationSağdiç et al., 2005). The bitter taste and odor are typical properties of styrax (CitationDuru et al., 2002).
Styrax is rich in free and combined cinnamic acid (CitationPastorovaw et al., 1997). Purified styrax yields up to 47% total balsamic acids. Its major components include phenylethylene (styrene), cinnamic esters, and vanillin (CitationHovaneissian et al., 2008). Styrax also contains an aromatic liquid (styrocamphene) (CitationHafizoglu, 1982; CitationLuo et al., 1996).
In traditional Chinese medicine, this herb belongs to resuscitation-inducing aromatic herbs, which has a calming ability in frightened condition and mind-tranquilizing activity. It functions in inducing resuscitation and restoring conscientiousness, which is similar to Moschus (Shexiang in Chinese), but weak in potency. In recent years, several medicines containing styrax as the primary ingredient have been approved to be used clinically in China. For example, the prescription Storax Pill (Chinese name, SuHeXiang Wan), which composed of 15 crude herbs including styrax, has been used orally for the treatment of seizures, infantile convulsion, sudden loss of conscientiousness, stroke, and so on (CitationKoo et al., 2004).
Although the central nervous system (CNS) inhibitory effects of the essential oil from Storax Pill has been evaluated (CitationKoo et al., 2004), the sedative and anticonvulsant activities of styrax have not been studied. In order to interpret the pharmacological properties of styrax as resuscitation-inducing aromatic herb, the present study investigated possible sedative and anticonvulsant activities of styrax on CNS.
In the treatment of neurological diseases, intranasal administration has attracted much attention in the past decades because of its noninvasive route that can offer advantages such as rapid absorption, avoidance of first-pass metabolism, ease of convenience, and self-medication (CitationIllum, 2000, Citation2003). Consequently, the nasal route may be important for drugs that are used for the treatment of CNS diseases. Recently, we have studied the analgesic and sedative efficacy of essential oil from Rhizoma Chuanxiong after intranasal administration (CitationGuo et al., 2010). The result demonstrated that Rhizoma Chuanxiong essential oil had faster onset of action as well as better analgesic and sedative efficacy after intranasal administration than that by intragastric administration. Therefore, intranasal administration of styrax may have beneficial therapeutic effects at lower dosage and have a faster onset of action in comparison with that after oral administration. In this article, the sedative effect and anticonvulsant activity of styrax after intranasal administration were studied and compared with that after oral administration.
Materials and methods
Material and extraction
Crude styrax was purchased from a traditional herb market located in Anhui, China and its identification was carried out by Prof. Chungen Wang, Nanjing University of Chinese Medicine. The voucher specimens (No. NJUTCM-20100401) were deposited in Nanjing University of Chinese Medicine. Crude styrax is a gray, thick liquid with a pleasing odor but a bitter taste. Crude styrax (200 g) was transferred into a 2000-mL distillation flask and dissolved with 1000 mL of 95% ethanol. The solution was filtered and the solvent was removed under reduced pressure to yield the purified styrax (95% yield). Purified styrax forms a brown semi-solid mass that is completely soluble in alcohol.
Animals
ICR male mice (18–22 g) were obtained from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China). The animals were housed in a temperature-controlled (22 ± 1°C) animal room on 12 h light/dark cycles with free access to food and water. Animal welfare and experimental procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996) and the related ethics regulations of Nanjing University of Chinese Medicine (No. SYXK (SU) 2007-0030). For nasal administration, styrax was formulated in normal saline containing 3% (v/v) Tween 80 and administered to mice nasal cavity (10 µL/20 g body weight) using a fine tip attached to a micropipette. The concentrations of styrax were 25, 50, and 100 mg/ml, which corresponded to 12.5, 25, 50 mg/kg, respectively. For oral administration, styrax was formulated in normal saline containing 3% Tween 80 and administered to mice (200 µL/20 g body weight). The concentrations of styrax were 50, 100, 200, and 400 mg/mL, which corresponded to 25, 50, 100, and 200 mg/kg, respectively. For control group, 3% Tween 80 was administered to mice orally or intranasally.
Chemicals and reagents
All reagents used in the experiments were of analytical grade and from commercial sources. Sodium pentobarbital was purchased from Shanghai Xitang Biotechnology Company (Shanghai, China); diazepam was purchased from Tianjin Lisheng Pharmaceutical Co. Ltd. (Tianjin, China); and pentylenetetrazole (PTZ) was purchased from Sigma Chemical (St. Louis, MO). Distilled water was prepared in EPED Superpure water purification system (Nanjing, China).
Pentobarbital-induced sleep in mice
In this test, styrax was administered orally (25, 50, 100 mg/kg) or intranasally (12.5, 25, 50 mg/kg) 1 min before the administration of pentobarbital (40 mg/kg, i.p.). Thereafter, each mouse was observed for the onset of sleep, and mice that lost righting reflex over 1 min were considered to be asleep. The sleeping latency was recorded from the injection of pentobarbital to the sleep onset and the sleeping time was recorded from the loss of righting reflex to recovery (CitationGuo et al., 2010).
Convulsion induced by PTZ
To evaluate the anticonvulsant activity of styrax, mice were pretreated with styrax orally (50, 100, 200 mg/kg) or intranasally (12.5, 25, 50 mg/kg) 5, 15, and 30 min prior to the intraperitoneal injection of PTZ (90 mg/kg), respectively. Different mice groups were used for different time periods. For control group, vehicle (3% Tween 80) was given intranasally. Mice were placed in a clear Plexiglass chamber and observed for 30 min. Latency for the development of clonic seizure, the number and duration of episodes, and mortality protection were determined for 10 mice of each group (CitationZapata-Sudo et al., 2010).
Locomotor activity test in mice
The autonomic activity of mouse was measured with a locomotor-monitoring apparatus. The sedative activity was investigated by determining the spontaneous locomotor activity of mice in an open field of 40 × 40 × 30 cm. The apparatus consisted of a wooden box, with the floor divided into four equal squares (20 × 20 cm). Total locomotor activity was defined as the number of interruptions of the beams recorded in a computer during a 60-min period after administration of styrax (25, 50, and 100 mg/kg orally or 12.5, 25, and 50 mg/kg intranasally). Locomotor activity was determined in each mouse after extract administration. For control group, vehicle (3% Tween 80) was given intranasally and orally, respectively. Prior to assay, mice were placed into a locomotor-monitoring cage and allowed to habituate to the cage for 5 min (0–5 min after the administration). Each mouse was monitored for 1 h. Data were expressed as number of movements (5–60 min) for 10 mice for each group. After each measurement, the locomotor-monitoring cages were carefully cleaned with wet tissue paper (10% ethanol solution) (CitationDeng et al., 2010).
Statistical analysis
Results were expressed as mean ± SD. Statistical analyses were performed by Student’s t-test. P < 0.05 was considered as statistically significant.
Results
Effect of styrax on potentiation of pentobarbital-induced sleep in mice
Effect of styrax on the number of mice falling asleep, sleep latency, and sleep duration time induced by hypnotic dosage of sodium pentobarbital (40 mg/kg) were shown in . The results showed that after oral and intranasal administration, styrax could prolong the sleeping time induced by sodium pentobarbital, but had no effect on the sleep latency. Diazepam, the positive control used in this study, also potentiated pentobarbital hypnosis.
Table 1. Effect of styrax after oral and nasal administration on the onset and duration of sleep in mice induced by hypnotic dosage of pentobarbital (n = 10).
Effect of styrax on PTZ-induced convulsion
Styrax at 100 mg/kg had no effect on PTZ-induced convulsion 5 and 15 min after oral administration (data not shown). Styrax at 100 and 200 mg/kg significantly decreased the mortality rate 30 min after oral administration. At 50 mg/kg, styrax had no effect on PTZ-induced convulsion ().
Table 2. Effect of styrax after oral and nasal administration on convulsions induced by pentylenetetrazol (PTZ) (n = 10).
After intranasal administration, styrax offered a significant protection from PTZ-induced seizures in a dose-dependent manner (). There was a significant decrease in mortality rate 5 min after nasal administration in all doses tested (12.5, 25, and 50 mg/kg) with maximum protection observed at 50 mg/kg (). Only at the highest dose (50 mg/kg), styrax prolonged the latency of the convulsions induced by PTZ (). The duration of seizures was not significantly altered by styrax treatment. Styrax has no effect on the incidence of clonic convulsions. Styrax at 50 mg/kg and 30 min after intranasal administration had no effect on PTZ-induced seizure (data not shown).
According to the above data, in the PTZ-induced convulsion model, styrax had faster onset of action (styrax showed anticonvulsant activity 5 min after intranasal administration and showed anticonvulsant activity 30 min but not 5 min after oral administration) and better anticonvulsant efficacy (the effective dosage was 25, 50 mg/kg for intranasal administration, but 100, 200 mg/kg for oral administration). Furthermore, although intranasal administration has the ability of fast onset of action (5 min), the lasting time was short (styrax has no effect 30 min after intranasal administration).
Effect of styrax on locomotor activity in mice
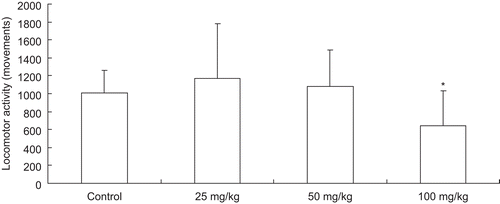
The sedative activity of styrax was investigated by recording the spontaneous locomotor activity of mice in an open field. As shown in , styrax decreased the spontaneous locomotor movements at 100 mg/kg during 5–60 min interval after oral administration. Styrax had no effect on the spontaneous locomotor movements at 50 and 25 mg/kg during 5–60 min interval after oral administration. After intranasal administration, styrax had no effect on the spontaneous locomotor movements at 50, 25, and 12.5 mg/kg during 5–60 min interval (data not shown).
Figure 1. Effect of styrax on spontaneous locomotor activity of mice after oral administration. Styrax (25, 50, 100 mg/kg) or vehicle (3% Tween 80) was administered. Each mouse was determined for 1 h. Data were expressed as number of movements (5–60 min) for 10 mice for each group. Each column represents mean of 10 mice.
Discussion
Nasal drug delivery offers many advantages over oral administration such as the fast onset of action due to the rapid absorption. Consequently, the nasal route may be important for medicines that are used for the treatment of acute central system diseases. In this study, the sedative and anticonvulsant efficacy as well as onset of action of styrax through nasal route was compared with that by oral route.
Styrax showed more potent efficacy after intranasal administration at lower dosage than by intragastric route (12.5, 25, 50 mg/kg vs. 25, 50, 100 mg/kg). After intranasal and oral administration at low dose (12.5 and 25 mg/kg, respectively), the sedative effect of styrax was comparable with that of diazepam (3 mg/kg) ().
Pretreatment of the styrax markedly delayed the appearance of PTZ-induced convulsion and lethality (). PTZ is known to block the action of GABA in the CNS, inducing convulsion (CitationMetcalf, 1979). Therefore, it is possible that styrax inhibited the convulsion by agonistic action to the GABA/benzodiazepine receptor.
Styrax had no effect on locomotor activities if calculated in 5-min time interval period. The total locomotor activity counts within the 55 min time period instead of 5-min time interval period were calculated and compared. Result showed that styrax could significantly decrease locomotor activity at 100 mg/kg during 5–60 min interval after oral administration. Furthermore, after oral and intranasal administration, styrax could prolong the sleeping time induced by sodium pentobarbital, but had no effect on the sleep latency, which showed that styrax might not directly act on the CNS at low dosage (50 and 25 mg/kg, in locomotor activity evaluation), but might delay the metabolism of pentobarbital by inhibiting the hepatic drug-metabolizing enzymes (in sodium pentobarbital-induced sleep time evaluation) (CitationTateoka et al., 1987). “Resuscitation” is a series of actions taken to establish normal breathing, heart rate, color, tone, and response. Our results showed that styrax had sedative effect at 100 mg/kg and prolonged the sleeping time induced by sodium pentobarbital, which was quite opposite to the resuscitation activity of styrax. Moreover, a recent study has showed that styrax could prevent mice from acute hypoxia-induced brain injury (CitationXu et al., 2010) and protect rat against cerebral ischemia–reperfusion injury (CitationNi et al., 2010). Based on this finding, we speculated that styrax exhibits its resuscitation-inducing activity not by directly stimulating or inhibiting CNS activity but might through other ways; the detailed mechanism needs further investigation.
Conclusion
In summary, based on the results described in this article, it is confirmed that styrax has sedative and anticonvulsant activity after oral and intranasal administration. Furthermore, styrax has faster onset of action as well as more potent efficacy after intranasal administration at lower dosage than by intragastric route. This result illustrates that intranasal administration may act as a promising alternative to conventional routes of administration, which would improve the therapeutic efficacy and reduce peripheral side effects. This report also provides more scientific information for further understanding of the clinical use of the herb.
Acknowledgement
We would like to thank Qiang Fang for his assistance in experiments.
Declaration of interest
This work was supported by Jiangsu Provincial TCM Administration Bureau Project (NO. LZ09011), 2009’ Program for Excellent Scientific and Technological Innovation Team of Jiangsu Higher Education and 2009 Program for New Century Excellent Talents by the Ministry of Education (NCET-09-0163), Construction Project for Jiangsu Key Laboratory for High Technology Research of TCM Formulae (BM2010576), Construction Project for Jiangsu Engineering Center of Innovative Drug from Blood-conditioning TCM Formulae.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Deng J, Zhou Y, Bai M, Li H, Li L. (2010). Anxiolytic and sedative activities of Passiflora edulis f. flavicarpa. J Ethnopharmacol, 128, 148–153.
- Duru ME, Cakir A, Harmandar M. (2002). Composition of the volatile oils isolated from the leaves of Liquidambar orientalis Mill. var. orientalis and L. orientalis var. integriloba from Turkey. Flavour Fragr J, 17, 95–98.
- Guo J, Duan JA, Tang Y, Jia N, Li X, Zhang J. (2010). Fast onset of action and the analgesic and sedative efficacy of essential oil from Rhizoma Chuanxiong after nasal administration. Pharmazie, 65, 296–299.
- Hafizoglu H. (1982). Analytical studies on the balsam of Liquidambar orientalis Mill. by gas chromatography and mass spectrometry. Holzforschung, 36, 311–313.
- Hovaneissian M, Archier P, Mathe C, Culioli G, Vieillescazes C. (2008). Analytical investigation of styrax and benzoin balsams by HPLC-PAD-fluorimetry and GC-MS. Phytochem Anal, 19, 301–310.
- Illum L. (2003). Nasal drug delivery—possibilities, problems and solutions. J Control Release, 87, 187–198.
- Illum L. (2000). Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci, 11, 1–18.
- Koo BS, Lee SI, Ha JH, Lee DU. (2004). Inhibitory effects of the essential oil from SuHeXiang Wan on the central nervous system after inhalation. Biol Pharm Bull, 27, 515–519.
- Luo G, Yang R, Lai X, Yang W, Xie S, Zhou H. (1996). [Analysis of cinnamic acid in storax and its original plant by HPLC]. Zhongguo Zhong Yao Za Zhi, 21, 744–5–763.
- Metcalf BW. (1979). Inhibitors of GABA metabolism. Biochem Pharmacol, 28, 1705–1712.
- Ni CX, Zeng N, Tang Q. (2010). Protective effect of resuscitation-inducing aromatic herbs on cerebral ischemia–reperfusion injury in rat. Pharm Clin Chin Mater Med, 5, 64–66.
- Pastorovaw I, de Koster CG, Boom JJ. (1997). Analytical Study of free and ester bound benzoic and cinnamic acids of gum benzoin resins by GC-MS and HPLC-frit FAB-MS. Phytochem Anal, 8, 63–73.
- Sagdiç O, Ozkan G, Ozcan M, Ozçelik S. (2005). A study on inhibitory effects of Sigla tree (Liquidambar orientalis Mill. var. orientalis) storax against several bacteria. Phytother Res, 19, 549–551.
- Tateoka Y, Kimura T, Watanabe K, Yamamoto I, Ho IK. (1987). Mechanism of N,N’-diallylpentobarbital potentiation of pentobarbital-induced sleep in mice. Res Commun Chem Pathol Pharmacol, 57, 173–185.
- Xu FH, Zeng N, Peng Q. (2010). Protective effect of resuscitation-inducing aromatic herbs on acute hypoxia induced brain injury in mice. Pharm Clin Chin Mater Med, 5, 72–74.
- Zapata-Sudo G, Mendes TC, Kartnaller MA, Fortes TO, Freitas NF, Kaplan MA, Sudo RT. (2010). Sedative and anticonvulsant activities of methanol extract of Dorstenia arifolia in mice. J Ethnopharmacol, 130, 9–12.
