Abstract
Background: The traditional herbal medicinal formula Da-Cheng-Qi decoction (DCQD) has long been used to treat pancreatitis in China; however, the underlying mechanisms remain unclear.
Aim: To investigate whether DCQD is beneficial to the patients with lung injury in severe acute pancreatitis (SAP); if it is, then to explore the lung protective effect of DCQD and the mechanism involved in rats.
Methods: DCQD was enema administered to 70 patients for 7 days. Mortality, (multi)organ failure during admission were observed, blood samples for laboratory analysis were drawn on admission, on Days 3, 7, and 14 of the treatment. We also experimentally examined the function of two doses of DCQD in SAP rat models. IL-1β, IL-6, and IL-10 mRNA expression in rat lungs was measured quantitatively by the RT-PCR method and confirmed by morphometric studies of the lungs.
Results: It was demonstrated that the administration of DCQD did shorten the average time that patients suffered acute respiratory distress syndrome (ARDS). Compared with untreated rats, the lungs of rats treated with DCQD showed significantly lower levels of proinflammatory cytokine IL-1β and IL-6 mRNA. Rats treated with DCQD had lower mean pathological lung lesion scores than those in SAP rats.
Conclusion: DCQD has good prospects in the treatment for SAP because it did shorten the average time that patients suffered ARDS in the clinic. It exerts therapeutic effects on this disease through inhibiting the production of inflammatory mediators, decreasing the anti-inflammatory factors, and mitigating the pathological damage of the lung injury in SAP model.
Introduction
Severe acute pancreatitis (SAP) is an important medical problem worldwide. Although its management has evolved over the past decades, SAP still has a mortality rate up to 30% (CitationHayden & Wyncoll, 2008). Death usually occurs from massive inflammatory responses causing severe lung injury. Acute respiratory distress syndrome (ARDS) is the most frequent systemic complication and is a leading cause of death in the early stage of SAP (CitationBhatia et al., 2005). The mechanisms by which SAP induces lung injury remain unclear, but an increase in systemic levels of cytokines in SAP has been noted in both clinical and experimental studies (CitationAvecillas et al., 2006; CitationFrossard et al., 2008). Inflammation is characterized by the release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) (CitationPereda et al., 2006). The potential inhibitors of proinflammatory cytokines have been considered as potential anti-inflammatory agents. The therapy of SAP, to date, has primarily been supportive. Specific therapeutic modalities aimed at controlling the pathophysiological mechanisms that lead to the systemic inflammatory response in SAP are still needed.
Da-Cheng-Qi decoction (DCQD), which was first described in AD 200 in the prestigious medical treatise of China, Shang Han Lun., has been widely used in China and East Asia for thousands of years. DCQD is a cocktail of Dahuang (Radix et Rhizoma Rhei), Houpu (Magnolia officinalis Rehd.), Zhishi (Fructus Aurantii Immaturus), and Mangxiao (Natrii Sulfas), with crude herbs mixed in a weight ratio of 1:1:1:1. Although the aqueous extract of the formula contains a large amount of compounds, pharmacokinetic studies from our laboratory and others showed that rhein, emodin, magnolol, honokiol, aloe emodin, naringin, naringenin, hesperidin, rheochrysidin, and chrysophanol are 10 main components detected in serum after oral administration of DCQD (CitationKatakai et al., 2002; CitationWenfu et al., 2008; CitationXu et al., 2008; CitationYu et al., 2009). Several components of DCQD have already been demonstrated to be involved in this effect. Rhein, aloe emodin (CitationMa et al., 2009), emodin (CitationDing et al., 2008) all have been reported to have anti-inflammatory effects in vivo and in vitro. Clinical and experimental studies have observed that treatment with DCQD has beneficial effects in preventing the occurrence of paralytic ileus and bacterial translocation in SAP (CitationTang et al., 2005; CitationXia et al., 2005; CitationQing et al., 2007). The mechanisms involved were reported as inhibiting inflammation and the inflow of Ca2+ in colonic smooth muscle, reducing the levels of serous endotoxin, attenuating the histopathological changes of intestinal mucosa (CitationChen et al., 2000; CitationChen and Pan, 2009). SAP is an inflammatory acute abdomen with multiple organ dysfunction including paralytic ileus and ARDS in the early time. So, it was our intent to explore whether DCQD is beneficial to patients with lung injury in SAP, and whether DCQD exhibits anti-inflammatory effects and has a lung protective effect in rats. This study was designed to explore the mechanism involved.
Materials and methods
Preparation of DCQD extract
Dahuang, houpu, zhishi, and mangxiao were purchased from Chengdu Green Herbal Pharmaceutical Co. Ltd. (Chengdu, China). The crude drugs were identified and the voucher specimen (Department of Integrated Traditional Chinese and Western Medicine, West China Hospital, China) of the prescription for this study was an aliquot from the same batch. The crude components of the formula were extracted twice by refluxing with boiling distilled water (1:12, g/mL) for 1 h. The extract was filtered, concentrated, freeze-dried, and stored at 4°C until use. The extraction yield was ~23% (w/w, dried extract/crude herb). The dried extract was dissolved in distilled water before use.
Quantitative analysis of marker compounds
The spray-dried prescriptions were mixed with distilled water and diluted to 6 mg/mL. The mixture was centrifuged at 3000 rpm for 10 min and the supernatant solution was obtained. Then 10 μL of this solution was injected into the high-performance liquid chromatography (HPLC) system for analysis. All separations were performed on a Waters HPLC system equipped with a binary pump, autosampler, and Waters 2487 dual λ absorbance detector (Waters, Chengdu, China). A RP-C18 HPLC column (150 × 4.6 mm, S-5 µm, 12 nm) and a guard column (GL Sciences, Japan) were used.
The mobile phase was a mixture of solvent (A) 100% methanol and solvent (B) 0.2% aqueous acetic acid (pH 3.12, 1:500, v/v) (A–B: 20 min 36:64; 19 min 65:35; 21 min 70:30). The flow rate was 1.0 mL/min and a 10-min interval was given between sample injections. The column effluents were simultaneously monitored at 254 nm (for aloe emodin, rhein, chrysophanol, and emodin) and 280 nm (for honokiol, magnolol, naringin, and hesperidin). The reference compounds including rhein, emodin, magnolol, honokiol, aloe emodin, naringin, hesperidin, and chrysophanol were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Human studies
The 120 patients with predicted SAP based on an Acute Physiology and Chronic Health Evaluation (APACHE II) score ≥8 were enrolled within 72 h of disease onset at the Traditional Chinese and Western Medicine Department of West China Hospital. All patients or their legal representatives gave written informed consent. This study was carried out in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines. Approval for the study was granted by the Ethical Review Board of Human Research of West China Hospital. No positive control drug in the treatment of SAP was recommended in most of the clinical studies to date. So, our study was prospective, placebo-controlled, and single-blinded. Individuals who had significant medical histories of cardiac, hepatic, renal, pulmonary, digestive, hematological, neurological, endocrine, or psychiatric illness were excluded. Patients were divided into the DCQD group and control group based on their admission time. Patients who were admitted on odd days made up the DCQD group (n = 70, 35 males) and patients who were admitted on even days comprised the control group (n = 50, 20 males). In the DCQD group, the mean age was 49.26 ± 14.37 years (range 21–76 years). In the control group, the mean age was 47.6 ± 10.6 years (range 25–77 years). Members of the DCQD group received an enema (200 mL) of DCQD solution twice a day for 7 days; the solution was prepared by dissolving freeze-dried powder (0.8 g of crude drugs per kilogram of body weight, which is the therapeutic dose in clinic). The same regimen was followed in controls, except that the DCQD solution was replaced with 200 mL of saline. Patients received standard medical care, which included non-per os nutrition, gastrointestinal decompression, life support, total parenteral nutrition, and drug therapy. Blood samples for laboratory analysis were drawn on admission, and on Days 3, 7, and 14 of the treatment. At the above time points, the sequential organ failure assessment (SOFA) score was computed for six organ systems (respiratory, coagulation, hepatic, cardiovascular, neurological, and renal) by collecting the raw data and converting them to SOFA scores as described (CitationFerreira et al., 2001). SOFA values range from 0 to 4, with the following interpretation: 0, normal; 1 or 2, dysfunction; 3 or 4, failure. A standard baseline (intravenous) contrast-enhanced computed tomographic (CT) scan was done 7 days after admission to detect pancreatic necrosis. One experienced radiologist, unaware of treatment allocation, read all CT scans to assess the CT severity index.
The primary endpoint was mortality during admission and 60-day follow-up, (multi) organ failure during admission, duration of (multi)organ failure after randomization. Based on the Atlanta classification (CitationBradley, 1993), organ failure was defined when any of the following was observed: PaO2 below 60 mm Hg despite FIO2 of 30%, the need for mechanical ventilation (pulmonary insufficiency), serum creatinine over 177 mmol/L after rehydration, the need for hemofiltration or hemodialysis (renal failure), systolic blood pressure below 90 mm Hg despite adequate fluid resuscitation, or need for vasopressor support (mainly noradrenalin and dopamine) due to cardiocirculatory insufficiency, adapted from the Atlanta classification. Multiorgan failure was defined as failure of at least two organ systems on the same day. Organ failure duration was defined as the time from onset of any organ failure after randomization until recovery of organ function; patients who died were excluded.
Animal studies
Animal experiments were approved by the Animal Ethics Committee of Sichuan University and carried out in accordance with their guidelines. Male Sprague-Dawley rats (250–300 g) that fasted overnight but had free access to water were anesthetized with pentobarbitone sodium at a dose of 50 mg/kg (i.p.). A midline laparotomy was performed and acute necrotizing pancreatitis was induced by injection of 3% sodium taurocholic acid into the subcapsular region of the pancreas. Then the abdomen was closed in two layers. Sham operation group only underwent laparotomy. DCQD, emodin, or vehicle was administered by enema into the intestines immediately after the model induction. Rats were randomized into four groups: (1) SAP control group, which was given vehicle (0.9% NaCl, 1 mL/kg); (2) SAP + DCQDH group, which received DCQD extract (15 g/kg); (3) SAP + DCQDL group, which received DCQD extract (7.5 g/kg); and (4) sham-operated control group. The rat dose of DCQD (15/7.5 g/kg) in the present study was converted according to our previous study of human (DCQD, 0.8 g/kg). That is, the rat is approximately 10 or 20 times the doses of human. It is consistent with literature (CitationPinkel, 1958). The rats were killed 12 h after drug administration. Plasma was harvested from the collected blood and kept at −20°C. Lung tissue (50–100 mg) was removed by surgery, immediately snap-frozen in liquid nitrogen, and stored at −70°C. Then the rats were perfused via the pulmonary artery with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Other parts of the lungs were immediately removed and immersed in the same fixative for 24 h at room temperature, then processed, and embedded in paraffin. Separate groups were used for lung tissue sampling (n = 10) and blood sampling (n = 10).
Plasma amylase assays
Plasma amylase levels were determined at 37°C by means of an enzymatic assay (Sigma, St. Louis, MO) with a spectrophotometer according to the manufacturer’s instructions. All plasma samples were assayed in duplicate, and the results were averaged at the end of each experiment.
RNA preparation
Total RNA of lung tissue was extracted using TRIZOL (Invitrogen, Shanghai, China) according to the manufacturer’s protocols. The integrity of the isolated RNA was verified by denaturing electrophoresis in agarose containing formamide. Ethidium bromide staining showed similar levels of 5S, 18S, and 28S RNA bands.
RT-PCR
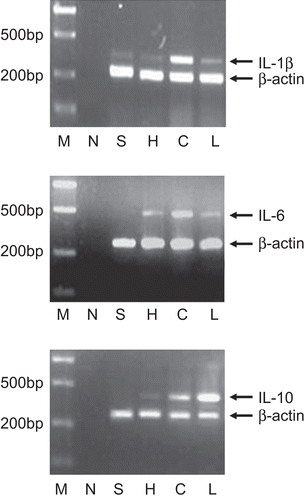
cDNA was synthesized from 1 μg of freshly prepared RNA using an advantage RT-PCR kit (Jingmei, Shenzhen, China). Reverse transcription was performed with 0.5 μg/L oligo (dT)18 primer (1 μL) in a final volume of 20 μL containing 10 mM dNTP (2 μL), recombinant RNase inhibitor (1 μL), and MMLV reverse transcriptase (1 μL). The mixture was incubated at 42°C for 1 h, and then heated to 72°C for 10 min. cDNA amplification was carried out with Taq DNA polymerase (Takara, Shanghai, China) according to the supplier’s instructions. The primer sequences of IL-1β, IL-6, IL-10, and β-actin are indicated in . The cDNA rat β-actin was amplified as a control gene. The PCR conditions were 29 cycles of 95°C, 2 min; 94°C, 30 sec; 52°C, 40 sec; 72°C, 40 sec. The PCR products were separated on 2% agarose gels in 1 × TAE buffer and visualized with ethidium bromide. The stained images were recorded with an image analyzer (Bio Doc-It TM System, UVifec, EEC), and the band intensity was quantified using densitometric analysis by Scion image (Scion Corp., Frederick, MD). The mRNA level was expressed as a ratio to β-actin.
Table 1. The sequence of primer.
Morphometric studies of the lungs
The lungs were fixed by 4% formaldehyde. Tissue sections (6 μm thick) were prepared by paraffin embedding. After hematoxylin and eosin (H&E) staining, slides were observed with a light microscope. All the sections were graded by experienced investigators who had no knowledge about the conditions of the specimens. Ten visual fields were observed randomly in a slide under 400× magnification. The scoring system was classified into five grades: 0, normal; 1, lung edema only; 2, lung edema, alveolar wall slightly incrassate, with a few infiltrated inflammatory cells; 3, lung edema, alveolar wall obviously incrassate, with infiltration of inflammatory and red cells; 4, lung edema, alveolar wall obviously incrassate, with infiltration of inflammatory cells and massive hemorrhage.
Statistical analysis
All data were expressed as mean ± SD. Differences in clinical parameters between the control and DCQD groups at baseline and different time points were assessed using a t-test. One-way ANOVA was used to analyze group differences in the animal studies. Differences with a P < 0.05 were considered to be statistically significant.
Results
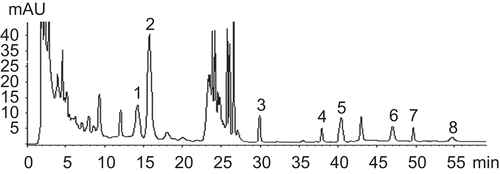
Analysis of chemical components in DCQD
According to the HPLC method used, the content (mg/g) and retention time (Rt, min) of the eight marker compounds of DCQD were as follows: Rhein 0.88 ± 0.05 (Rt = 37.355), emodin 2.39 ± 0.16 (Rt = 49.060), magnolol 1.06 ± 0.07 (Rt = 47.373), honokiol 1.25 ± 0.08 (Rt = 40.579), aloe emodin 1.72 ± 0.08 (Rt = 30.065), naringin 3.89 ± 0.07 (Rt = 14.484), hesperidin 11.27 ± 0.23 (Rt = 16.429), and chrysophanol 0.52 ± 0.03 (Rt = 54.463). The HPLC trace of DCQD is shown in . In spite of other unknown chemical components included in DCQD, hesperidin is the most abundant.
Human studies
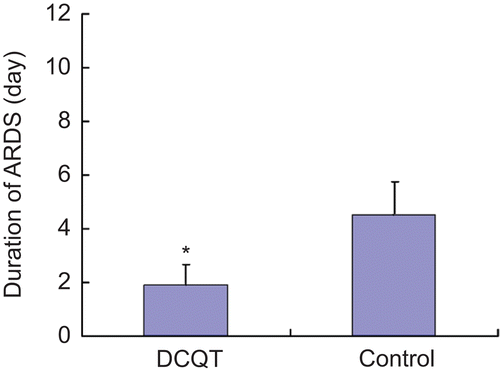
Baseline characteristics of the 120 subjects who completed follow-up are summarized in . There were no significant differences in patient characteristics or illness severity among the two treatment groups at baseline (P > 0.05). Clinical outcomes of human studies were compared between pancreatitis patients receiving DCQD or placebo. The two groups showed no significant differences in early-stage mortality (within 14 days after disease onset) or late-stage mortality (beyond 14 days after onset, up to 2 months). Our clinical outcomes show that DCQD did not affect the overall severity of pancreatitis (based on SOFA scores) or patient mortality during 2 months. The SOFA score is valuable for assessing the overall disease process, but it does not allow detailed examination of specific organ systems. Therefore, we used organ failure indicators to look at all six of the organ systems individually in the two patient groups. Our results show that clinically detectable signs of lung injury are seen in up to 70% of all the patients. In the most severe form of lung injury, ARDS developed. Although DCQD at the dose used in this study did not affect the overall percent of patients with ARDS (17.5% in DCQD group, compared with 21.3% in controls P > 0.05), it did shorten the average time that patients suffered ARDS: the mean duration of ARDS in DCQD patients was 1.90 ± 0.62, compared with a mean duration of 4.50 ± 1.68 in controls (P < 0.05). ().
Table 2. Baseline characteristics.
Plasma amylase in SAP rats
In SAP, necrosis of the pancreatic cells leads to elevated levels of plasma amylase. Plasma amylase was significantly higher in the SAP model than in rats of the sham operation group. In rats given DCQD, amylase in serum was lower than that observed in control rats (P < 0.05). However, no significant differences were found between DCQDH and DCQDL groups in terms of plasma amylase level ().
Table 3. Effect of Da-Cheng-Qi decoction (DCQD) on plasma amylase in ANP rats (n = 10).
IL-1β, IL-6, and IL-10 mRNA expression in rat lungs
In SAP, activated pancreatic macrophages release inflammatory cytokines (e.g., IL-1, IL-6), as a response to local tissue damage. These cytokines would be expected to act locally to aggravate the pancreatitis and could act systemically to damage distant organs. IL-10 is a potent anti-inflammatory cytokines, which inhibits inflammatory cytokine release. We found that the mRNA expression of IL-6 and IL-10 were undetectable in normal lungs, although a low level of mRNA expression of IL-1β in normal lungs was detected. As expected, mRNA expression of the three interleukins increased in SAP rats, and we measured the dynamic changes of proinflammatory cytokines in lung tissue. Levels of mRNA encoding IL-1β, IL-6, and IL-10 increased to the highest level 6–8 h after taurocholic acid injection and then decreased (CitationDing et al., 2008). Compared with untreated SAP rats, the lungs of rats treated with SAP showed significantly lower levels of proinflammatory cytokine IL-1β and IL-6 mRNA ().
Figure 3. Effect of two dosage of Da-Cheng-Qi decoction (DCQD) on mRNA expression of IL-1β, IL-6, and IL-10 in severe acute pancreatitis (SAP) rats’ lungs. M: marker, N: the negative control, no cDNAs being added during PCR reaction; S: sham group; H: DCQD (15 g/kg) treatment group; C: control group; L: DCQD (7.5 g/kg) treatment group.
Effects of DCQD on pathological lesion score in SAP rat lungs
As expected, alveolar wall incrassation, edema, lung inflammatory cell infiltration, and hemorrhage were found in SAP rat’s lung, which are defined as interstitial inflammation (). The mean histological scores of SAP group were significantly higher than those in sham operation animals. In the SAP rats treated with DCQD, the main changes were mild edema of the alveolar walls and no obvious alveolar blood stasis. As shown in , the mean pathological lung lesion scores of these two treated groups were lower than those in SAP rats.
Table 4. Pathological score in severe acute pancreatitis (SAP) rats’ lungs with and without treated by Da-Cheng-Qi decoction (DCQD) (n = 10).
Discussion
Although it is widely accepted that inflammation plays a central role in the pathogenesis of pancreatitis-associated lung injury, specific therapeutic modalities aimed at controlling the pathophysiological mechanisms that lead to the systemic inflammatory response in SAP are lacking. Clinical evidence did not support that corticosteroid could improve the cardiac and pulmonary function in ARDS (CitationDeal et al., 2008). So, phytotherapy targeting inflammation might be useful as a novel anti-inflammatory therapeutic agent for lung injury in SAP.
In our present study, we have demonstrated the beneficial effect of DCQD against lung injury in SAP patients. The average time that patients suffered ARDS in SAP was shortened. At the same time, we noted that after treatment with DCQD, pathological lesion score in SAP rat lungs was decreased, and amylase levels in plasma were also decreased. Compared with untreated SAP rats, the lungs of rats treated with SAP showed significantly lower levels of proinflammatory cytokine IL-1β and IL-6 mRNA. These results suggest that DCQD may have beneficial effects in decreasing severity of acinar cell damage in pancreatitis. The animal experimental findings reported here also support the clinical observation, suggesting that DCQD treatment significantly protected rats against acute pancreatitis-induced lung injury.
The mechanism underlying lung injury induced by acute pancreatitis is not clearly understood, but it has been known that activated enzymes and diverse proinflammatory mediators generated in the pancreas and activated leukocytes contribute to the lung complications (CitationBhatia & Moochhala, 2004). When SAP occurs, pancreatins such as trypsin, pancrelipase, and amylopsin will be activated and excessively released, resulting in necrosis of pancreas and tissues around it. For example, amylase is released from acinar cells during acute pancreatitis, and concentration in the serum is used to confirm the diagnosis of pancreatitis. The absorbed necrotic tissue and bulk toxic substances will cause severe systemic inflammatory reaction. The inflammatory mediators are TNF-α, IL-6, IL-1β, NO, endotoxin, and so on. As one of the final common mediators in cascade reaction of inflammatory mediators, NO can be regarded as a study index for the changes of SAP state. TNF-α is an important cytokine participates in SAP inflammatory cascade reaction (CitationSaidalikhodzhaeva et al., 2002). In particular, circulating levels of IL-6 and cytokine IL-1β play an important role in lung injury due to acute pancreatitis. These cytokines act both locally and systemically to aggravate the disease. Blocking these cytokines resulted in dramatic improvement of the disease (CitationPaszkowski et al., 2002; CitationJiang et al., 2004). IL-10, a major anti-inflammatory cytokine, is able to inhibit the synthesis and activities of many proinflammatory cytokines such as IL-1 and TNF-α (CitationRongione et al., 1997; CitationCook et al., 2001). In the present study, the lungs of rats treated with SAP showed significantly lower levels of proinflammatory cytokine IL-1β and IL-6 mRNA. At the same time, DCQD increased the mRNA expression of IL-10. These data support DCQD regulated systemic proinflammatory media/anti-inflammatory media balance in rats. The regulatory effects of DCQD on the local lung injury not only inhibit the producing of IL-6 and cytokine IL-1β, but also increase the IL-10 level to reestablish the proinflammatory actors/anti-inflammatory factors balance so as to inhibit the local excessive immune response. Our results suggest that DCQD may show potential as an anti-inflammatory and play a beneficial role in treating pulmonary injury in SAP.
The lungs of rats treated with DCQD showed that the therapeutic effects helping to resolve ARDS elicited by DCQD is likely to be multifactorial and involve its anti-inflammatory mechanisms at least. DCQD is a hot water extract from a mixture of four herbs. What is the predominant protective component of the herbal cocktail? Although DCQD appears to exert its protective effect against lung injury, the underlying pulmonary complications mechanism induced by acute pancreatitis is complex. The beneficial effect mechanism of DCQD against lung injury and anti-inflammatory remains to be fully elucidated.
On the other hand, although our findings suggest that posttreatment of patients with DCQD had a protective effect on the lung injury of SAP by shortening the mean duration of ARDS, it did not improve overall survival. It may reflect mortality due to other complications, since SAP is associated with several systemic complications, including infection (CitationGötzinger et al., 2002; CitationConnor et al., 2005); this may also indicate that the dose used in this clinical study is ineffective, and that future studies should consider different doses.
In conclusion, traditional Chinese herbal medicine DCQD appears to treat SAP by more than one mechanism, essentially involving lung protection and possibly as anti-inflammatory medicine with good prospects.
Acknowledgement
This work was supported by a grant (No. 97050432) from key constructing clinical discipline project of health ministry of China and partly supported by Sichuan University Foundation for Young Scholars to Jianlei Zhao (No. 0040104132001).
Declaration of interest
The authors report no conflicts of interest.
References
- Avecillas JF, Freire AX, Arroliga AC. (2006). Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: Incidence, diagnosis, and outcomes. Clin Chest Med, 27, 549–57; abstract vii.
- Bhatia M, Moochhala S. (2004). Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol, 202, 145–156.
- Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. (2005). Pathophysiology of acute pancreatitis. Pancreatology, 5, 132–144.
- Bradley EL 3rd. (1993). A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg, 128, 586–590.
- Chen H, Wu X, Guan F. (2000). [Protective effects of tongli gongxia herbs on gut barrier in rat with multiple organ dysfunction syndrome]. Zhongguo Zhong Xi Yi Jie He Za Zhi, 20, 120–122.
- Chen J, Pan Z-J. (2009). Effects of Chinese medicine Dachengqi decoction on function of pulmonary alveolar macrophages of rat’s acute lung injury induced by acute hemorrhagic necrotizing pancreatitis. J Chin Mod Surg, 6, 74–78.
- Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. (2005). Early and late complications after pancreatic necrosectomy. Surgery, 137, 499–505.
- Cook JW, Karakozis S, Kim D, Provido H, Gongora E, Kirkpatrick JR. (2001). Interleukin-10 attenuates proinflammatory cytokine production and improves survival in lethal pancreatitis. Am Surg, 67, 237–241; discussion 241.
- Deal EN, Hollands JM, Schramm GE, Micek ST. (2008). Role of corticosteroids in the management of acute respiratory distress syndrome. Clin Ther, 30, 787–799.
- Ding Y, Zhao L, Mei H, Zhang SL, Huang ZH, Duan YY, Ye P. (2008). Exploration of Emodin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur J Pharmacol, 590, 377–386.
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. (2001). Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA, 286, 1754–1758.
- Frossard JL, Steer ML, Pastor CM. (2008). Acute pancreatitis. Lancet, 371, 143–152.
- Götzinger P, Sautner T, Kriwanek S, Beckerhinn P, Barlan M, Armbruster C, Wamser P, Függer R. (2002). Surgical treatment for severe acute pancreatitis: Extent and surgical control of necrosis determine outcome. World J Surg, 26, 474–478.
- Hayden P, Wyncoll D. (2008). Severe acute pancreatitis. Curr Anaesth Crit Care, 19:1–7.
- Jiang CF, Shiau YC, Ng KW, Tan SW. (2004). Serum interleukin-6, tumor necrosis factor alpha and C-reactive protein in early prediction of severity of acute pancreatitis. J Chin Med Assoc, 67, 442–446.
- Katakai M, Akamaru T, Tani T. (2002). [An analysis of the frequency of formulations and crude drugs described in Shan-Han-Lun]. Yakushigaku Zasshi, 37, 28–35.
- Ma BL, Ma YM, Yan DM, Zhou H, Shi R, Wang TM, Yang Y, Wang CH, Zhang N. (2009). Effective constituents in Xiexin Decoction for anti-inflammation. J Ethnopharmacol, 125, 151–156.
- Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. (2002). Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg, 235, 68–76.
- Pereda J, Sabater L, Aparisi L, Escobar J, Sandoval J, Viña J, López-Rodas G, Sastre J. (2006). Interaction between cytokines and oxidative stress in acute pancreatitis. Curr Med Chem, 13, 2775–2787.
- Pinkel D. (1958). The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res, 18, 853–856.
- Qing HQ, Jian W, Guo GL, Xian ZhW. (2007). Da-Cheng-Qi-Tang promotes the recovery of gastrointestinal motility after abdominal surgery in humans. Dig Dis Sci, 52, 1562–1570.
- Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. (1997). Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology, 112, 960–967.
- Saidalikhodzhaeva OZ, Iuldashev NM, Daniiarov AN, Muratova UZ. (2002). [Pancreatic enzyme activity in early phases of acute experimental pancreatitis in rats]. Ross Fiziol Zh Im I M Sechenova, 88, 526–529.
- Tang WF, Wan MH, Zhu L, Chen GY, Xia Q, Huang X. (2005). [Immuno-modulatory effect of somatostatin combined with traditional Chinese medicine on severe acute pancreatitis at early stage: A randomized control trial]. Zhong Xi Yi Jie He Xue Bao, 3, 103–107.
- Wenfu T, Meihua W, Zhengyan Z. (2008). Simultaneous determination of eight major bioactive compounds in Dachengqi Tang by high-performance liquid chromatography. Chin Med, 3, 1–6.
- Xia Q, Yuan L, Yang XN, Tang WF, Jiang JM. (2005). Comparison of integrated Chinese and Western medicine with and without somatostatin supplement in the treatment of severe acute pancreatitis. World J Gastroenterol, 11, 1073–1076.
- Xu F, Liu Y, Zhang Z, Song R, Dong H, Tian Y. (2008). Rapid simultaneous quantification of five active constituents in rat plasma by high-performance liquid chromatography/tandem mass spectrometry after oral administration of Da-Cheng-Qi decoction. J Pharm Biomed Anal, 47, 586–595.
- Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. (2009). Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 877, 2025–2031.

![Figure 4. Representative photomicrographs [hematoxylin and eosin (H&E) stain; 400×] of lung from rats with different treatments are shown: A: sham group; B: control group; C: Da-Cheng-Qi decoction (DCQD) (15 g/kg) treatment group; and D: DCQD (7.5 g/kg) treatment group.](/cms/asset/f1a6814c-92da-47a4-a768-669b3e0b175b/iphb_a_565059_f0004_b.gif)