Abstract
Context: Ichnocarpus frutescens (L.) R.Br. (Apocynaceae) is used to treat diabetes and hyperlipidemia in folk medicine.
Objective: The crude methanol extract and fractions of I. frutescens were investigated for antihyperlipidemic effect.
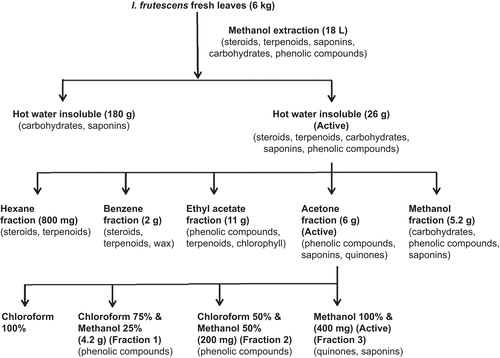
Materials and methods: Fresh leaves of I. frutescens were extracted with methanol and fractionated with hexane, benzene, ethyl acetate, acetone, and methanol. The active acetone fraction was subfractionated, which resulted in active fraction 3. The antihyperlipidemic effects of the methanol extract and fractions of I. frutescens were studied in triton WR-1339-induced and high-fat diet (HFD) obese animals. Further, lipid absorption and excretion were studied.
Results and discussion: The methanol extract significantly reduced total cholesterol (TC) by 29.63% and triglyceride (Tg) by 51.10% at 400 mg/kg in triton WR-1339-induced animals and significantly reduced TC (27.81%) and Tg (37.03%) at 400 mg/kg in HFD animals. Fraction 3 showed significant reduction in TC (25.03%) and Tg (58.05%) at 200 mg/kg. Feeding of HFD consisting 3% of fraction 3 increased feces weight and Tg level in mice. Fraction 3, showed significant decrease in plasma Tg level at the second hour, after oral administration of the lipid emulsion to rats.
Conclusion: The observed properties apparently validate the folk medicinal use of this plant in amelioration of hyperlipidemia.
Introduction
Hyperlipidemia is an important risk factor in the initiation and progression of atherosclerotic lesions (CitationHarrison et al., 2003) and considered as a predisposing factor in the development of cardiovascular disease (CitationSmith et al., 2004). The Lipid Research Clinics Program and the Helsinki Heart Study have clearly demonstrated that when plasma lipids are lowered by hypocholesterolemic agents, the clinical complications of atherosclerosis can be diminished (CitationWarnica, 2004). In obesity, increased visceral fat mass and insulin resistance occurs together and statistics show that 60–90% of all patients with type-2 diabetes are obese (CitationCamp et al., 2002; CitationGolay & Ybarra, 2005). Furthermore, the prevalence of obesity is increasing along with the demand for effective and safe anti-obesity agents, including herbal medicinal products (CitationSung et al., 2009). The search for new agents capable of reducing and regulating serum total cholesterol (TC) and triglyceride (Tg) levels has gained momentum over the years, resulting in numerous reports on significant activities of natural agents (CitationJahromi et al., 1993). Traditional medicine is still the mainstay of about 75–80% of the world population, mainly in the developing countries. India, having a very old and rich tradition of folk medicine for centuries, has provided effective remedies in a simple way to various ailments.
Ichnocarpus frutescens (L.) R.Br. (Apocynaceae) is a profusely branched straggling shrub with oblong leaves distributed commonly in scrub jungles of South-East Asia up to 1100 MSL; it is a ready colonizer on cleared slopes (CitationMatthew, 1991). The vernacular names of this plant are “Udarkodi” in Tamil and “Krsnasariba” in Sanskrit (CitationSivarajan & Balachandran, 1994). The leaves of this plant contain apigenin, luteolin, vitexin, isovitexin, vanilic, syringic, sinapic acids (CitationDaniel & Sabnis, 1978), triterpene, and glycosides (CitationMinocha & Tandon, 1980). The stem contains friedelin, friedelinol, lupeol acetate, and oleonolic acid (CitationVerma & Gupta, 1988). The roots are used as a diuretic and diaphoretic (CitationNadkarni, 1964). The whole plant is used to treat burning sensation, fever, nephrolithiasis, leprosy, and general weakness (CitationWarrier et al., 1995). The Siddis of Uttara Kannada district of Karnataka, India (CitationBhandary et al., 1995) and the tribals of Madhya Pradesh, India (CitationYousuf et al., 2004) use the flowers and roots to treat diabetes. The antidiabetic effect of the aqueous extract of the roots (CitationBarik et al., 2008) and the methanol extract of the leaves (CitationSubash et al., 2008) were reported. Since, there are only very few reports available on the antihyperlipidemic effect of this plant, we have considered it worth investigating.
Materials and methods
Plant material and bioassay-guided fractionation of the active crude extract
Leaves of I. frutescens were collected in Chennai, Tamil Nadu. The plant material was authenticated by the taxonomist at the Department of Botany, Loyola College, Chennai, Tamil Nadu, India. A voucher specimen (ERIS-2) was deposited in the herbarium of the Entomology Research Institute.
Fresh leaves of I. frutescens (6 kg) were ground, extracted with methanol (18 L) by cold percolation, and concentrated under vacuum. The crude methanol extract was separated as hot water soluble (180 g) and hot water insoluble (35 g) parts. The hot water insoluble part (26 g) which showed activity was fractionated in silica gel column (60–120 mesh; RANKEM, New Delhi, India) with hexane (4.6 L), benzene (4 L), ethyl acetate (10.4 L), acetone (11.8 L), and methanol (2.8 L); the yields were 3, 7.6, 42.3, 23, and 20%, respectively. The active acetone (6 g) fraction was subfractionated in silica gel column with 100% chloroform (no eluate obtained), methanol: chloroform at 1:3 (fraction 1), methanol: chloroform at 1:1 (fraction 2), and 100% methanol (fraction 3); the yield of the fractions were 70, 3.3, and 6.6%, respectively (CitationKasetti et al., 2010; CitationSheeja et al., 2010).
Experimental animals
Male albino rats (n=6) Wistar strain weighing 160–180 g and male mice Swiss albino (n=6) weighing 30 ± 1 g were kept in polypropylene cages with free access to standard pellet diet (Pranav Agro Industries Ltd., Pune, Maharashtra, India) and water. Their housing was maintained at a temperature of 24 ± 1°C with a 12-h light/dark cycle and the humidity of 55 ± 10%. This study received clearance from the Institutional Animal Ethical Committee (IAEC-ERI-LC-16) following Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines.
Fixation of doses
Toxic effect of the crude methanol extract was tested at 1, 2, and 4 g/kg (CitationRoux et al., 2004) in normal male mice weighing 30 ± 5 g. Each group containing six animals were deprived of food and water; then they were treated orally with the extract dissolved in a vehicle (0.2% polysorbate-80, 0.5% sodium carboxy methyl cellulose, 0.9% sodium chloride, 0.9% benzyl alcohol, and 97.2% distilled water) (CitationLee, 2001). Behavioral alterations such as tremor, convulsions, jumping, respiration, loss of balance, pupil dilation, salivation, sedation, and rolling gait were observed periodically for 48 h. No adverse effects or mortality was observed; hence, dose ranges of 100–400 mg/kg were used for bioassays (CitationOliveira et al., 2008).
Triton WR-1339-induced hyperlipidemic animals
Acute hyperlipidemia was induced in male Wistar rats weighing 180–200 g, by injecting triton WR-1339 (Sigma) (200 mg/kg bw, i.p) dissolved in phosphate-buffered saline (pH 7.4) (CitationVogel & Vogel, 1997). Throughout the study, the animals had free access to feed and water (CitationLevine & Saltzman, 2007). The animals were divided into six groups, containing six each. Hyperlipidemia was induced in Groups II to VI. Group I served as normal control, treated with vehicle; Group II served as triton WR-1339 control, treated with vehicle; Group III served as positive control, treated with fenofibrate (65 mg/kg) (CitationHarnafi et al., 2007); and Groups IV to VI were treated with methanol extract at 100, 200, and 400 mg/kg, respectively. Treatment was given orally immediately after triton WR-1339 injection. Blood samples were collected from retro-orbital sinus after 18 h of treatment in ethylenediaminetetraacetic acid (EDTA) containing tubes; plasma was separated and stored at −18°C until determination.
Male mice Swiss albino weighing 30 ± 1 g were rendered hyperlipidemic by injecting triton WR-1339 (400 mg/kg bw, i.p) (CitationWeidong et al., 2007) in phosphate-buffered saline (pH 7.4). Immediately after induction, the treatment was given with vehicle/fractions (100 and 200 mg/kg) and fenofibrate (200 mg/kg) (CitationXie et al., 2007). Animals were euthanized after 18 h of triton WR-1339 injection and blood was collected in EDTA-containing tubes; plasma was separated and stored at −18°C until determination.
High-fat diet induced hyperlipidemic animals
Male Wistar rats weighing 150–170 g, were made hyperlipidemic by being fed on high-fat diet (HFD) [composed of standard rat chow-68%, Dalda (saturated fat)-30%, and cholesterol-2% by weight] (CitationGuido & Joseph, 1992) for 15 days. The animals were divided into five groups, containing six each. HFD was fed to Groups II to V. Group I served as normal control fed with normal pellet diet, treated with vehicle; Group II served as HFD control, treated with vehicle; Group III served as positive control, treated with fenofibrate (65 mg/kg); Group IV and V were treated with methanol extract at 200 and 400 mg/kg, respectively. Treatment was given orally between 12.00 noon and 2.00 p.m., once daily for 15 days. At the end of the study, animals were fasted overnight and sacrificed between 9.00 a.m. and 11.00 a.m. to minimize diurnal variations. Blood was collected in EDTA-containing tubes. Liver samples were sliced quickly, snap-frozen in liquid N2 and stored at −70°C.
Fat excretion in feces of mice
Male mice (3 weeks old) were fed HFD or HFD containing 1 or 3% fraction 3, or 0.025% orlistat for 5 days. Wet weight and Tg content in the feces obtained after 24 h were recorded using the commercial Tg test kit (CitationLi et al., 2005).
Plasma Tg levels after oral administration of lipid emulsion to rats
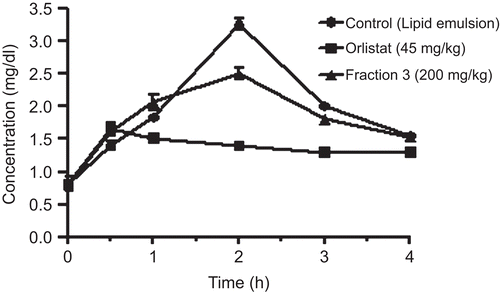
Male Wistar rats weighing 150–160 g were fasted overnight and treated orally with the lipid emulsion (1 mL) plus fraction 3 (200 mg/kg bw) or the lipid emulsion plus orlistat (45 mg/kg bw). Lipid emulsion consisted of 6 mL of corn oil, 80 mg of cholic acid, 2 mg of cholesteryloleate, and 6 mL of saline. Blood samples were taken from the tail vein at 0, 0.5, 1, 2, 3, and 4 h after administration of the lipid emulsion with or without fraction 3 or orlistat using a capillary tube, and centrifuged at 5500g for 5 min. The plasma Tg concentration was determined using a commercial triacylglycerol assay kit (CitationKwon et al., 2003).
Biochemical analysis
TC and Tg levels were estimated by CHOD-PAP and GPO-POD methods (Ecoline, Merck, Mumbai, Maharashtra, India), respectively. High-density lipoprotein cholesterol (HDL-C) level was estimated by CHOD-PAP method after precipitation with phosphotungstic acid. Low-density lipoprotein cholesterol (LDL-C) level was calculated using the formula of CitationFriedwald et al. (1972). Plasma lipoperoxide level was estimated by TBA-reacting method (CitationSlater, 1984). Total lipids in liver were extracted by the method of CitationFolch et al. (1957). TC and Tg in the liver extract were estimated by the method of CitationHenley (1957) and CitationFoster and Dunn (1973), respectively. Retroperitoneal fat pads were removed, weighed and expressed in percent of the body weight.
Statistical analysis
Statistical evaluation of the data was done by one-way analysis of variance followed by the Student’s t-test. The results were expressed as mean ± SEM using Graph Pad Prism (version 5.0). Statistical significance was defined at p ≤ 0.05.
Results
Preliminary phytochemical analysis
The preliminary phytochemical analysis of the methanol extract and bioassay-guided fractions of I. frutescens were shown in .
Methanol extract in triton WR-1339-induced animals
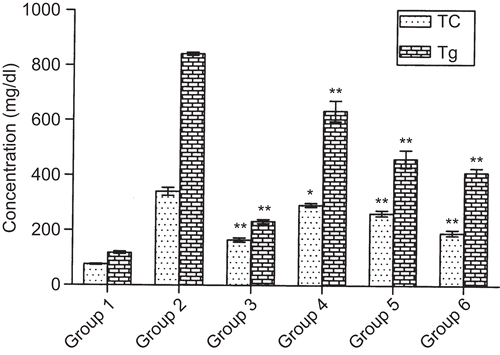
Acute administration of triton WR-1339 showed a marked increase in plasma levels of TC and Tg compared to normal control group. Treatment with methanol extract caused significant decrease in TC level by 14.26, 24.52, and 29.63% at 100, 200, and 400 mg/kg, respectively, and in Tg level by 24.63, 45.33, and 51.10% at 100, 200, and 400 mg/kg, respectively. The fenofibrate-treated group had shown reduced levels of TC and Tg by 51.56 and 72.34%, respectively, at 65 mg/kg ().
Figure 1. Effect of Ichnocarpus frutescens methanol extract and fenofibrate on plasma lipid levels in triton induced hyperlipidemic rats. Values are mean ± SEM of six rats *p ≤ 0.01, **p ≤ 0.001. Normal control (Group 1), hyperlipidemic control (Group 2), hyperlipidemic + fenofibrate 65 mg/kg treated (Group 3), hyperlipidemic + methanol extract 100 mg/kg treated (Group 4), hyperlipidemic + methanol extract 200 mg/kg treated (Group 5), and hyperlipidemic + methanol extract 400 mg/kg treated (Group 6).
Fraction 3 in triton WR-1339-induced animals
The active methanol extract was separated into hot water soluble and hot water insoluble parts. The hot water insoluble part, with significant antihyperlipidemic effect (), was separated into hexane, benzene, ethyl acetate, acetone, and methanol fractions. The acetone fraction with significant antihyperlipidemic effect () was subfractionated; among the three fractions obtained, fraction 3 exhibited significant reduction in TC (19.84 and 25.03% at 100 and 200 mg/kg) and in Tg (53.30 and 58.05% at 100 and 200 mg/kg). LDL-C level was reduced by 29.26 and 34.51% at 100 and 200 mg/kg, respectively; a significant increase in HDL-C/TC ratio by 24.39 and 29.26% at 100 and 200 mg/kg, respectively was also seen. The fenofibrate-treated group had shown significant reduction in TC, Tg, and LDL-C levels by 61.69, 69.05, and 70.30%, respectively, and in turn increased HDL-C/TC ratio by 41.46%, significantly at 200 mg/kg (). Fraction 3 gave positive results for quinones. It also formed lather with normal saline indicating the presence of saponins.
Table 1. Effect of the methanol extract of I. frutescens on plasma lipid levels of high-fat diet induced hyperlipidemic rats.
Figure 2. Effect of hot water soluble and hot water insoluble parts of methanol extract and fenofibrate on plasma lipid levels in triton-induced hyperlipidemic rats. Values are mean ± SEM of six rats **p ≤ 0.001. Normal control (Group 1), hyperlipidemic control (Group 2), hyperlipidemic + fenofibrate 200 mg/kg treated (Group 3), hyperlipidemic + hot water soluble part 200 mg/kg treated (Group 4), hyperlipidemic + hot water insoluble part 200 mg/kg treated (Group 5).
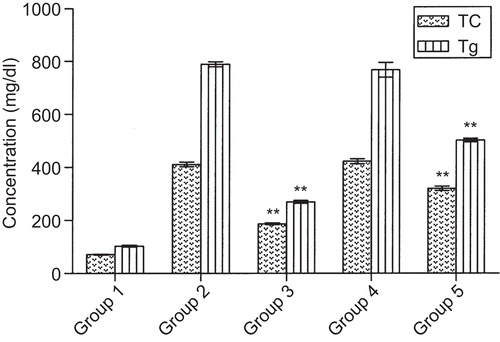
Figure 3. Effect of fractions of Ichnocarpus frutescens and fenofibrate on plasma lipid levels in triton induced hyperlipidemic rats. Values are mean ± SEM of six rats *p ≤ 0.01, **p ≤ 0.001. Normal control (Group 1), hyperlipidemic control (Group 2), hyperlipidemic + fenofibrate 200 mg/kg treated (Group 3), hyperlipidemic + hexane fraction 200 mg/kg treated (Group 4), hyperlipidemic + benzene fraction 200 mg/kg treated (Group 5), and hyperlipidemic + ethyl acetate fraction 200 mg/kg treated (Group 6), hyperlipidemic + acetone fraction 200 mg/kg treated (Group 7), hyperlipidemic + methanol fraction 200 mg/kg treated (Group 8).
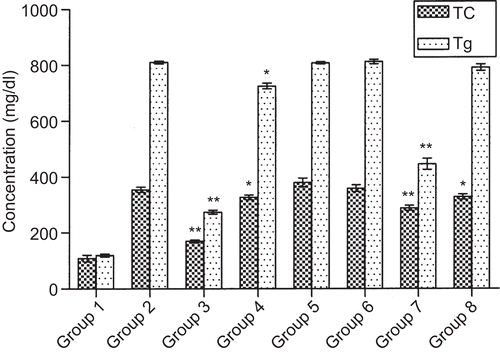
Methanol extract in HFD fed animals
Data in show the effect of I. frutescens methanol extract on lipid levels of HFD induced hyperlipidemic group. Plasma levels of TC and Tg were consistently higher in the case of HFD fed group, compared with the basal diet fed group. After 15 days of treatment, the methanol extract at 200 mg/kg showed reduction in TC (21.23%) and Tg (35.07%); at 400 mg/kg it showed significant reduction in TC (27.81%) and Tg (37.03%). There was a decrease in LDL-C level by 28.28 and 37.33% at 200 and 400 mg/kg, respectively. On the other hand, fenofibrate-treated group showed significant reduction in TC (36.01%), Tg (47.60%) and LDL-C (49.91%) at 65 mg/kg. The HDL-C concentration, however, was significantly increased in methanol extract and fenofibrate-treated groups compared with the HFD fed control group. In addition, the methanol extract reduced lipoperoxide level in plasma and TC and Tg levels in the liver. The retroperitoneal fat mass was found reduced in methanol extract and fenofibrate-treated groups ().
Table 2. Effect of the methanol extract of I. frutescens on the activities of biochemical parameters in high-fat diet induced hyperlipidemic rats.
Table 3. Effect of I. frutescens fractions on plasma lipid levels in triton WR-1339-induced hyperlipidemic mice.
Fat excretion in feces of mice fed HFD plus fraction 3 or orlistat
Mice fed HFD for 5 days showed reduced feces weight at day 5 compared with the normal control group. Mice fed HFD plus 3% fraction 3, or 0.025% orlistat for 5 days showed significantly higher Tg level at day 5 compared with HFD group ().
Table 4. Effect of fraction 3 of Ichnocarpus frutescens and orlistat on fat excretion in feces of mice fed with high-fat diet.
Effect of fraction 3 on plasma Tg levels after oral administration of lipid emulsion
The plasma Tg levels were markedly increased at the second hour after oral administration of lipid emulsion in the control group. Fraction 3 (200 mg/kg) and orlistat (45 mg/kg) significantly decreased the Tg levels compared with the control group ().
Discussion
Although the common definitions of insulin resistance still define it in terms of the effects of insulin on glucose metabolism, in recent years the traditional “glucocentric” view of insulin resistance has shifted to a lipocentric perspective (CitationSesti, 2006). The presence of different hypolipidemic drugs in the market is sometimes associated with severe side effects (CitationGhatak & Asthana, 1995). Hence, efforts in finding safer and more efficient antihyperlipidemic drugs have led to the study of medicinal plants. In this study, attempts were made to evaluate the antihyperlipidemic activity of I. frutescens in triton-induced and HFD fed animal models.
It is well known that high serum TC, Tg, and LDL-C levels are the primary risk factors for vascular diseases, and high serum level of HDL-C confers a protective effect against its development. CitationSchurr et al. (1972) demonstrated that parenteral administration of triton in adult rats increases blood TC and Tg levels reaching its maximum at 20 h. This effect occurs due to the fact that triton WR-1339, a non-ionic detergent, suppresses the action of lipases and blocks the uptake of lipoprotein from circulation by extrahepatic tissues resulting in an increase in the levels of circulating lipid (CitationVishnu et al., 2008). Many medicinal plants, such as Phyllanthus niruri (CitationKhanna et al., 2002) and Ocimum basilicum (CitationHicham et al., 2008), have been assessed for their hypolipidemic effect in triton WR-1339-induced hyperlipidemic model. The data from our study showed that the increased serum TC and Tg levels were significantly reduced at the eighteenth hour by the treatment with methanol extract and fraction 3 of I. frutescens in a dose-dependent manner in triton WR-1339-induced animals. The results are correlated with fenofibrate, a broad-spectrum lipid-lowering drug of the fibrate class that inhibits the synthesis of TC and Tg as well as enhances their elimination (CitationMcKenney et al., 2006). The hypolipidemic action of I. frutescens extract was markedly higher for Tg than for TC. The underlying mechanisms are not elucidated in this study; however, the restoration of catabolic metabolism of very-low-density lipoprotein could be due to an increased stimulation of the lipolytic activity of plasma lipoprotein lipase (CitationPérez et al., 1999).
To study the etiology of hyperlipidemia related metabolic disturbances, the HFD-induced animal model was preferred (CitationBocan, 1998). Hypolipidemic effect was studied extensively in guggul lipids and synthetic derivatives of guggulsterone (CitationBhavna et al., 2009). CitationKumarappan et al. (2007) have earlier reported the hypolipidemic effect of hydroalcoholic extract of I. frutescens in streptozotocin induced diabetic rats. In our study, appropriate hyperlipidemic animal models were used. The aqueous extract and swertiamarin (an active lead) of Enicostemma littorale (CitationVihas et al., 2005; CitationHitesh et al., 2009), aqueous extract of O. basilicum (CitationHicham et al., 2009), and aqueous extract and butanol fraction of Eclipta prostrata (CitationKumari et al., 2006; CitationDae et al., 2008) showed hypolipidemic effect in animal models. Rats fed with HFD for 15 days showed a consistent decrease in blood lipid levels on treatment with the methanol extract. The incorporation of 2% cholesterol in the HFD showed elevated TC level in the plasma of HFD control group. This condition was, however, reversed by treatment with methanol extract of I. frutescens in a significant manner. Serum TC can be lowered at several metabolic points including decrease in resorption of endogenous cholesterol or an increase in the rate of secretion into intestinal tract. The methanol extract of I. frutescens did not show any inhibition in HMG-CoA reductase activity (data not shown). The decrease in plasma TC level may be due to its excretion as fecal bile acid and neutral sterol, which needs to be elucidated in future study. LDL is known as “bad cholesterol” as it transfers cholesterol from the liver to circulation. There was a significant increase in serum LDL levels in HFD group, which was decreased in the methanol extract treated group. The results suggest that the methanol extract might show its effect at LDL receptor’s upregulation or gene transcription level and thereby facilitate removal of cholesterol from the circulation (CitationKrause & Hartman, 1984). The levels of plasma thiobarbituric acid reactive substance showed a significant reduction in methanol extract treated group, thereby indicating a decreased rate of lipid peroxidation. HDL-C in serum implies the activity of lecithin cholesterol acyltransferase, which plays a key role in lipoprotein metabolism and may contribute to the regulation of blood lipids (CitationZhang et al., 2004). Our study showed a significant increase in HDL-C levels in the methanol extract treated group.
HFD produces an increase in Tg levels due to lipoprotein lipase triacylglycerol hydrolysis, so that the accumulation in the liver becomes more evident (CitationFeoli et al., 2003). In contrast, the effect of I. frutescens can be attributed to a reduction in the hepatic synthesis of lipids, which decreases the concentration of Tg both in plasma and in the liver. Feeding of HFD, consisting 3% of fraction 3 for 5 days showed increased feces weight and Tg level compared with HFD group. We examined the effect of fraction 3 on the plasma Tg levels after oral administration of lipid emulsion to rats. The results showed a significant decrease in Tg level at the second hour. Orlistat, used as reference control, inhibits pancreatic lipase and other gastrointestinal lipases, thereby decreasing the digestibility of dietary fat that limits Tg absorption (Tg must be hydrolyzed to monoacyl glycerols before being absorbed). Fraction 3 had the potential to limit lipid absorption and thus reduce the body fat in animals.
Conclusion
In conclusion, the observed properties apparently validate the folk medicinal use of this plant to treat obesity. Fraction 3 of I. frutescens positively modified the lipoprotein profile in plasma and minimized lipid absorption at intestinal region indicating good therapeutic potential in amelioration of hyperlipidemia.
Acknowledgements
The authors are grateful to Dr. K. Balakrishna, (Central Drug Research Institute for Siddha, Chennai, India) for assisting in phytochemistry standardization. The authors are grateful to Entomology Research Institute for financial assistance.
Declaration of interest
The authors report no declarations of interest.
References
- Barik R, Jain S, Qwatra D, Joshi A, Tripathi GS, Goyal R. (2008). Antidiabetic activity of aqueous root extract of Ichnocarpus frutescens in streptozotocin-nicotinamide induced type-II diabetes in rats. Indian J Pharmacol, 40, 19–22.
- Bhandary MJ, Chandrashekar KR, Kaveriappa KM. (1995). Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J Ethnopharmacol, 47, 149–158.
- Bhavna S, Rajani S, Swati S, Chandrajeetbalo M, Partha R. (2009). Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol, 47, 2631–2639.
- Bocan TM. (1998). Animal models of atherosclerosis and interpretation of drug intervention studies. Curr Pharmacol Des, 4, 37–52.
- Camp HS, Ren D, Leff T. (2002). Adipogenesis and fat cell function in obesity and diabetes. Trend Mol Med, 8, 442–447.
- Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, Lim JK, Kim JS. (2003). Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem, 67, 1451–1456.
- Dae IK, Sung HL, Jin HC, Hyun SL, Mi HY, Gun SL. (2008). The butanol fraction of Eclipta prostrata (Linn) effectively reduces serum lipid levels and improves antioxidant activities in CD rats. Nutr Res, 28, 550–554.
- Daniel M, Sabnis SD. (1978). Chemotaxonomical studies on Apocyanaceae. Indian J Exp Biol, 16, 512–513.
- Feoli AM, Roehrig C, Rotta LN, Kruger AH, Souza KB, Kessler AM, Renz SV, Brusque AM, Souza DO, Perry ML. (2003). Serum and liver lipids in rats and chicks fed with diets containing different oils. Nutrition, 19, 789–793.
- Folch J, Lees M, Sloane-Stanley GH.(1957). A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem, 226, 497–509.
- Foster LB, Dunn RT. (1973). Stable reagents for determination of serum triglyceride by a colorimetric Hantzsch condensation method. Clin Chem, 19, 338–340.
- Friedwald WI, Levy RI, Fredrickson DS. (1972). Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative centrifuge. Clin Chem, 18, 499–502.
- Ghatak A, Asthana OP. (1995). Recent trends in hyperlipoproteinemias and its pharmacotherapy. Indian J Pharmacol, 27, 14–9.
- Golay A, Ybarra J. (2005). Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab, 19, 649–663.
- Guido S, Joseph T. (1992). Effect of chemically different calcium antagonists on lipid profile in rats fed on a high fat diet. Indian J Exp Biol, 30, 292–294.
- Harnafi H, Bouanani Nel H, Aziz M, Serghini Caid H, Ghalim N, Amrani S. (2007). The hypolipidaemic activity of aqueous Erica multiflora flowers extract in triton WR-1339 induced hyperlipidaemic rats: A comparison with fenofibrate. J Ethnopharmacol, 109, 156–160.
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. (2003). Role of oxidative stress in atherosclerosis. Am J Cardiol, 91, 7A–11A.
- Henley AA.(1957). The determination of serum total cholesterol. The Analyst, 82, 286–287.
- Hicham H, Hana SC, Nour EHB, Mohammed A, Souliman A. (2008). Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in triton WR-1339-induced hyperlipidemic mice. Food Chem, 108, 205–212.
- Hicham H, Mohammed A, Souliman A. (2009). Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. e-SPEN, Eur e-J Clin Nutr Metab, 4, e181–e186
- Hitesh V, Mandapati R, Vasudevan S, Harish P, Ramesh G. (2009). Swertiamarin: A lead from EnicostemmalittoraleBlume. For anti-hyperlipidaemic effect. Eur J Pharmacol, 617, 108–112.
- Jahromi F, Ray AB, Chansouria JPN. (1993). Antihyperlipidemic effect of flavonoids from Pterocarpusmarsupium. J Nat Prod, 56, 989–994.
- Kasetti RB, Rajasekhar MD, Kondeti VK, Fatima SS, Kumar EG, Swapna S, Ramesh B, Rao CA. (2010). Antihyperglycemic and antihyperlipidemic activities of methanol:water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food Chem Toxicol, 48, 1078–1084.
- Khanna AK, Rizvi F, Chander R. (2002). Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol, 82, 19–22.
- Krause BR, Hartman AD. (1984). Adipose tissue and cholesterol metabolism. J Lipid Res, 25, 97–110.
- Kumarappan CT, Rao TN, Mandal SC. (2007). Polyphenolic extract of Ichnocarpus frutescens modifies hyperlipidemia status in diabetic rats. J Cell Mol Biol, 6, 175–187.
- Kumari CS, Govindasamy S, Sukumar E. (2006). Lipid lowering activity of Eclipta prostrata in experimental hyperlipidemia. J Ethnopharmacol, 105, 332–335.
- Lee KM. (2001). Overview of drug product development. Curr Protoc Pharmacol, 7, 1–10.
- Levine S, Saltzman A. (2007). A procedure for inducing sustained hyperlipemia in rats by administration of a surfactant. J Pharmacol Toxicol Methods, 55, 224–226.
- Li KH, Yi NZ, Masayuki Y, Hiromichi O, Yoshiyuki K. (2005). Anti-obesity effects of chikusetsusaponins isolated from Panax japonicas rhizomes. BMC Complement Altern Med, 5, 9.
- Matthew KM. (1991). An Excursion Flora of Central Tamil Nadu. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd.
- McKenney JM, Farnier M, Lo KW, Bays HE, Perevozkaya I, Carlson G, Davies MJ, Mitchel YB, Gumbiner B. (2006). Safety and efficacy of long-term co-administration of fenofibrate and ezetimibe in patients with mixed hyperlipidemia. J Am Coll Cardiol, 47, 1584–1587.
- Minocha PK, Tandon RT. (1980). A new triterpene glycoside from the stem of Ichnocarpus frutescens. Phytochemistry, 19, 2053–2055.
- Nadkarni AK. (1964). The Indian Materia Medica. 1. Bombay: Popular Prakashan, 674.
- Oliveira HC, dos Santos MP, Grigulo R, Lima LL, Martins DT, Lima JC, Stoppiglia LF, Lopes CF, Kawashita NH. (2008). Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol, 115, 515–519.
- Pérez C, Canal JR, Campillo JE, Romero A, Torres MD. (1999). Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother Res, 13, 188–191.
- Roux S, Sable E, Porsolt RD. (2004). Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effect on behavior and physiological function. Curr Protoc Pharmacol, 10, 1–23.
- Schurr PE, Schultz JR, Parkinson TM. (1972). Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids, 7, 68–74.
- Sesti G. (2006). Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab, 20, 665–679.
- Sheeja E, Joshi SB, Jain DC. (2010). Bioassay-guided isolation of anti-inflammatory and antinociceptive compound from Plumbago zeylanica leaf. Pharm Biol, 48, 381–387.
- Sivarajan VV, Balachandran I. (1994). Ayurvedic Drugs and their Plant Resources. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd.
- Slater TF. (1984). Overview of methods used for detecting lipid peroxidation. Meth Enzymol, 105, 283–293.
- Smith SC Jr, Jackson R, Pearson TA, Fuster V, Yusuf S, Faergeman O, Wood DA, Alderman M, Horgan J, Home P, Hunn M, Grundy SM. (2004). Principles for national and regional guidelines on cardiovascular disease prevention: A scientific statement from the World Heart and Stroke Forum. Circulation, 109, 3112–3121.
- Subash BP, Ignacimuthu S, Agastian P. (2008). Insulin secretagogue effect of Ichnocarpus frutescence leaf extract in experimental diabetes: A dose-dependent study. Chem Biol Interact, 172, 159–171.
- Sung HT, Ting YC, Jiun RC, I-Hsin L, Ching CW. (2009). Hypolipidemic effects of three purgative decoctions. eCAM, 1–9.
- Verma RK, Gupta MM. (1988). A new sorboside from Ichnocarpus frutescens. Section B—Organic chemistry including Medicinal Chemistry. Indian J Chem, 27, 283–284.
- Vihas TV, Hiren M, Jyoti VT, Sarita G. (2005). Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol, 101, 277–282.
- Vishnu K, Mohammad MK, Ashok KK, Ranjana S, Sushma S, Ramesh C, Farzana M, Abbas AM, Jitendra KS, Raj KS. (2008). Lipid lowering activity of anthocephalusindicus root in hyperlipidemic rats. eCAM, 1–6.
- Vogel G, Vogel WH. (1997). Influence of lipid metabolism, In: Drug Discovery and Evaluation: Pharmacological Assay. Berlin: Springer-Verlag, 604.
- Warnica JW. (2004). The CARE trial: The effect of prevastatin on coronary events after myocardial infarction in patients with average cholesterol levels, In: Gaw A, Packard CJ, Shepard J, eds. Statins-The HMG-CoA reductase inhibitors in perspective, second edition. London: Martin and Duntitz, 123–143.
- Warrier PK, Nambiar VPK, Ramankutty C. (1995). Indian Medicinal Plants Vol.3. New Delhi, India: Orient Longman Publishers, 1995.
- Weidong X, Wei W, Hui S, Dongming X, Guoping C, Lijun D. (2007). Hypolipidemic mechanisms of Ananascomosus L. leaves in mice: Different from fibrates but similar to statins. J Pharmacol Sci, 103, 267–274.
- Xie W, Wang W, Su H, Xing D, Cai G, Du L. (2007). Hypolipidemic mechanisms of Ananascomosus L. leaves in mice: Different from fibrates but similar to statins. J Pharmacol Sci, 103, 267–274.
- Yousuf SH, Tripathi L, Bhattacharya S. (2004). Antidiabetic plants used by tribals in Madhya Pradesh. Nat Prod Radi, 3, 427–428.
- Zhang AH, Gao S, Fan JL, Huang W, Zhao TQ, Liu G. (2004). Increased plasma HDL cholesterol levels and biliary cholesterol excretion in hamster by LCAT overexpression. FEBS Lett, 570, 25–29.