Abstract
Context: Polygonum amplexicaule D. Don var. sinense Forb. (Polygonaceae) (PAF) is a well known traditional herb used to treat some diseases, such as fractures, rheumatoid arthritis, muscle injury, and pain. However, its pharmacological mechanism of promoting the healing of fractures is still unknown.
Objective: The present study was designed to investigate the effects of PAF ethanol extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cell in vitro, thereby to illuminate the pharmacological mechanism to promote the healing of fractures.
Materials and methods: The effects of PAF ethanol extracts on MC3T3-E1 cell proliferation and differentiation were detected by using CCK-8, cell cycle, alkaline phosphatase (ALP), and prostaglandin E2 (PGE2) assays in vitro.
Results: The results showed that PAF ethanol extracts significantly stimulated cell proliferation at 0.1–100 μg/mL and the proportion of cells in S-phase increased from 16.33 to 27.29% in osteoblastic MC3T3-E1 cells. Moreover, PAF ethanol extracts increased ALP expression in MC3T3-E1 cells at the concentration from 0.1 to 100 μg/mL and inhibited PGE2 production induced by TNF-α in osteoblasts at the concentrations ranging from 10 to 100 μg/mL in MC3T3-E1 osteoblasts.
Discussion and conclusion: These results indicated that PAF directly stimulates cell proliferation and differentiation of osteoblasts; therefore, this study preliminarily explored the pharmacological mechanism of PAF to promote the healing of bone rheumatism and various fractures.
Introduction
In recent years, traditional Chinese medicines (TCMs) have become important sources for exploration of potential therapeutic agents because of their tremendous diversity. Various strategies have been employed to investigate the phytochemical and pharmacological effects of TCMs based on their therapeutic potentials (CitationBrixen et al., 2004). Polygonum amplexicaule D. Don var. sinense Forb. (Polygonaceae) (PAF) is a famous herbal drug used effectively to treat some diseases such as fractures, rheumatism, osteoporosis, muscle injuries, and pain. PAF has been demonstrated to be an effective herb for the treatment of rheumatism and fractures in Chinese folk medicine. PAF has also been reported to effectively treat atherosclerosis in China. The antibiotic and antiviral effects of PAF were also examined, and positive results were obtained in China. With respect to the chemical composition of P. amplexicaule, CitationRen et al. (2009) isolated nine compounds from the root tubers of the herb, and CitationYang et al. (2007) isolated 21 compounds from its essential oil. Approximately 30 compounds were found during our research (data not shown). However, its pharmacological mechanism of promoting the healing of fracture is still unknown.
The MC3T3-E1 osteoblastic cell line is a widely adopted model for the research of osteogenesis in vitro (CitationQuarlers et al., 1999). By use of the MC3T3-E1 cell model, several medical herbs such as Icariin (CitationZhao et al., 2010), Actaea racemosa (Ranunculaceae) (CitationChan et al., 2008), safflower seeds (Asteraceae) (CitationKim et al., 2008), Ulmus davidiana Planch (Ulmaceae) (CitationKang et al., 2006), Drynaria fortunei (Drynariaceae) (CitationJeong et al., 2004), Drynariae Rhizoma (Drynariaceae) (CitationJeong et al., 2005), soybean isoflavones (CitationChoi et al., 2001; CitationSuh et al., 2003), and Homalomena occulta (Homalomenae) (CitationHu et al., 2008) have been proved to be functional to promote osteogenesis. The purpose of this study is to explore the pharmacological mechanism of PAF to promote the healing of bone fracture by analyzing the proliferation and differentiation of osteoblastic MC3T3-E1 cell.
Materials and methods
Materials
MC3T3-E1 cell line was supplied by Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Tissue culture media and reagents, fetal bovine serum (FBS) were from Hyclone Inc. (Logan City, UT). Diethylstilbestrol (DB) was from Shanghai Sine Kangjie Pharmaceutical Co. Ltd. (Shanghai, China). All other reagents were of analytical grade and purchased from China National Pharmaceutical Industry Corporation. Ltd. (Shanghai, China).
Plant material
The fresh root tubers of PAF were collected by Mr. Chongrong Wang in October 2008 and authenticated by Dr. Dingrong Wan, Professor in Pharmacognosy at the College of Pharmacy, South-Central University for Nationalities (SCUN). The voucher specimen (SCUN0810) was deposited in the Herbarium of the College of Pharmacy, SCUN, China.
Extract and sample preparation
The dried (low temperature drying) root tubers of PAF (10 kg) were ground to coarse powder with a mortar and pestle. The coarse powder was extracted with 95% (v/v 20,000 mL) ethanol for three times at room temperature and concentrated under pressure condition to obtain total ethanol extract 1.185 kg (yield 19%). Ethanol extract sample and DB (as positive control) were weighed and diluted with Dulbecco’s modified Eagle’s medium (DMEM). The concentrations of ethanol extracts and DB were between 0.01 and 100 μg/mL.
Cell culture
MC3T3-E1 cells were cultured in DMEM supplemented with 10% FBS, 100 μg/mL penicillin, and 100 U/mL streptomycin in a mixture of 95% air and 5% CO2 in a humidified incubator at 37°C.
Cell proliferative CCK-8 assay
The cells (2 × 104 cells/well) were seeded in 96-well plates and cultured for 24 h to obtain adherent monolayer cells. After washing twice with phosphate-buffered saline (PBS), the cells were treated with various concentrations of PAF ethanol extracts for 1–7 days, and cell proliferation was evaluated by Cell Count Kit-8 assay (CCK-8; Beyotime Inst Biotech., China) according to the manufacturer’s instructions (CitationKim et al., 2010). In brief, 10 μL CCK-8 was added to each well (100 μL medium). After incubation for 2 h at 37°C, the absorbance at 450 nm was measured by an enzyme-linked immunosorbent assay (ELISA) plate reader. The experiments were carried out in five replicates and the results were expressed as mean ± standard deviation (SD).
Cell cycle analysis
The cells (2 × 105 cells/well) were seeded in six-well plates and cultured for 24 h to obtain adherent monolayer cells. The adherent cells were cultured for another 24 h in serum-free medium for synchronization then washed twice with 1× PBS; the cells were cultured with various concentrations of PAF ethanol extracts. The cells were harvested and fixed with 70% cold ethanol at 4°C overnight, then washed with PBS and centrifuged at 2000 rpm for 3 min. Next, the cells were incubated with 100 μL RNase (1 mg/mL) for 30 min at 37°C, then 50 μL (1 mg/mL) propidium iodide (PI; Jingmei, Shenzhen, China) was added and the cells were incubated at 4°C for 30 min in the dark. The cell cycle was detected by flow cytometry (CitationKanazawa et al., 2008; CitationPozharski, 2010) (BD Biosciences, San Jose, CA). The data were analyzed by Modfit software (Verity Software House, Topsham, ME).
Alkaline phosphatase activity assay
MC3T3-E1 cells were cultured to grow to 80–100% confluent. The medium was replaced with phenol red-free alpha minimum essential medium (α-MEM) containing 5% charcoal–dextran-treated FBS (CD-FBS; Gibco, Grand Island, NY). Cells were cultured with various concentrations of ethanol extracts from PAF, supplemented with 10 mM β-glycerophosphate (β-GP) (G9422; Sigma Chemical Co., St. Louis, MO), which was added to initiate in vitro cells differentiation (CitationChoi, 2005; CitationAmos et al., 2008). After 3 days, the medium was removed and the monolayer cells were gently washed twice with PBS. The cells were harvested to 1.5-mL tubes and centrifuged for 20 min at 3000 rpm. Supernatant was discarded, and PBS (pH 7.2–7.4) was added to prepare a solution with 1 × 107 cells/mL. Freeze–thaw cycles were repeated three times to break the membrane of the cells to release intracellular components. Then the solution was centrifuged for 15 min at 12,000 rpm. The supernatant was harvested for the measurement of alkaline phosphatase (ALP) concentration by use of an ELISA kit (R&D System Inc., Minneapolis, MN). The experiments were carried out in five replicates and data were expressed as means ± SD.
Prostaglandin E2 assay
The cells were cultured according to the method mentioned above except that the β-GP was replaced with 10–10 M tumor necrosis factor alpha (TNF-α). The content of prostaglandin E2 (PGE2) in the supernatant was measured with an ELISA kit (R&D System Inc.) according to the manufacturer’s instructions (CitationKajii et al., 1999). Immediately after the color development, the absorbance was read at 450 nm. The experiment was carried out in five replicates and data were expressed as means ± SD.
Statistical analysis
All experimental results were performed with at least three replicates. Results were expressed as mean ± SD. Differences between groups were examined for statistical significance by Student’s t-test, and P < 0.05 was considered statistically significant.
Results
The effect of PAF ethanol extracts on the proliferation of MC3T3-E1 cells
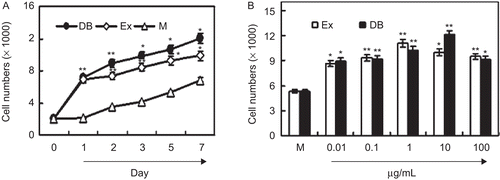
We detected the effects of the ethanol extracts of PAF on the proliferation of MC3T3-E1 cells. After the treatment with 1 µg/mL PAF extract for the indicated days, the numbers of MC3T3-E1 cells were measured with a CCK-8 assay. The results showed that the extract of PAF significantly promoted cell proliferation within 7 days compared with the medium groups (). The dose–response curves indicated that the extract (0.1–100 μg/mL) significantly promoted the proliferation of MC3T3-E1 cells with the optional concentration at 1 μg/mL (P < 0.01, ). These results suggested that the herb PAF was able to promote the proliferation of osteoblastic MC3T3-E1 cells.
Figure 1. PAF ethanol extracts increase the proliferation of MC3T3-E1 cells. (A) PAF ethanol extracts induced time-dependent cell growth. MC3T3-E1 cells in 96-well plates were cultured with 1 µg/mL PAF extract for 7 days. Cell numbers were measured with a CCK-8 assay. (B) PAF ethanol extracts induced dose-dependent cell growth. Cells in 96-well plates were cultured with indicated ethanol extract for 3 days. Cell numbers were measured by a CCK-8 assay. Each point represents the mean ± SD of five determinations. *P < 0.05, **P < 0.01 compared with controls by Student’s t-test. “EX” means ethanol extracts; “DB” means diethylstilbestrol (as positive control).
The effect of PAF ethanol extracts on cell cycle progression
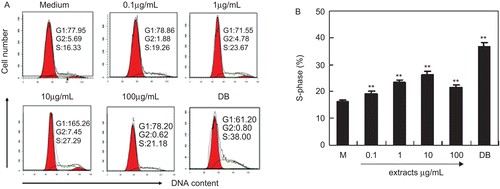
Cell proliferation is regulated by cell cycle progression. MC3T3-E1 cells were treated with various concentrations of ethanol extracts, and cell cycle analysis was performed by PI staining and flow cytometry. The results showed that ethanol extracts dose-dependently up-regulated the cell proportion in S-phase significantly ( and ). When cells were treated with 0.1–10 μg/mL of ethanol extracts, the proportion of cells in S-phase increased from 16.33 to 27.29% ( and ). These results indicated that PAF ethanol extracts promoted cell proliferation by regulating cell cycle progression.
Figure 2. PAF ethanol extracts promotes cell cycle progression in MC3T3-E1 cells. Cells were seeded in six-well plates and cultured with indicated extract and 1 µg/mL DB for 3 days. Cell cycle was analyzed by PI staining. (A) The profile of cell cycle analysis. (B) The percentage of cells in S-phase. Results were expressed as means ± SD of five determinations. **P < 0.01 compared with controls by Student’s t-test. “DB” means diethylstilbestrol (as positive control).
The effect of ethanol extracts on ALP expression in MC3T3-E1 cells
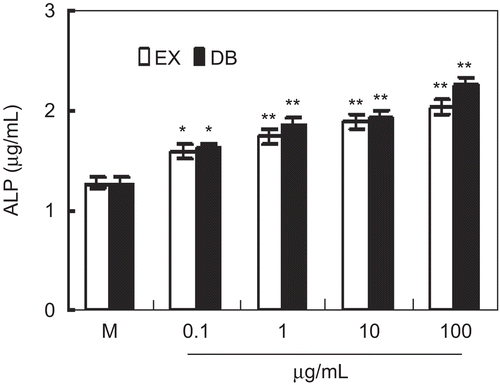
The healing of fracture is related to the differentiation of osteoblasts. In this study, the effect of ethanol extracts on the expression of ALP, a marker for osteoblast differentiation, was detected in MC3T3-E1 cell. The results showed that the treatment of MC3T3-E1 cells with 0.1–100 μg/mL of extract significantly up-regulated the expression of ALP (), which was similar to that of the positive control DB. Therefore, the herb PAF has a markedly positive effect on the differentiation of MC3T3-E1 cells.
Figure 3. The effect of ethanol extracts on alkaline phosphatase (ALP) expression in MC3T3-E1 cells. Cells were cultured with indicated ethanol extracts or DB for 3 days in the presence of 10 mM β-glycerophosphate (β-GP). ALP concentration was detected by ELISA. Results were expressed as means ± SD of five determinations. *P < 0.05, **P < 0.01 compared with controls by Student’s t-test. “EX” means ethanol extracts; “DB” means diethylstilbestrol (as positive control).
The effect of ethanol extracts on PGE2 production in MC3T3-E1 cells
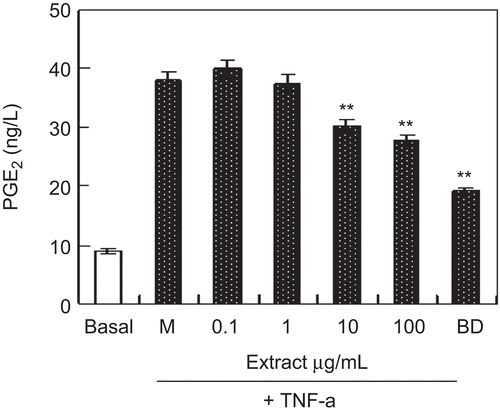
PGE2 is an endogenous factor to stimulate osteolysis through the EP4 receptor (CitationTsutsumi et al., 2009). We examined the effect of ethanol extracts on PGE2 production in MC3T3-E1 cells. As shown in , the treatment of MC3T3-E1 cells with TNF-α increased the expression of PGE2, but ethanol extracts (10–100 μg/mL) significantly inhibited the expression of PGE2 induced by TNF-α.
Figure 4. The effect of ethanol extracts on prostaglandin E2 (PGE2) production in MC3T3-E1 cells. Cells were cultured with indicated ethanol extracts and 1 µg/mL DB for 3 days in the presence of 10−10 M TNF-α. PGE2 concentration was measured by ELISA. Results were expressed as means ± SD of five determinations. **P < 0.01 compared with control by Student’s t-test. “DB” means diethylstilbestrol (as positive control).
Discussion
To investigate the pharmacological mechanism of PAF to promote the healing of fractures and rheumatism, we selected the well-recognized osteoblastic MC3T3-E1 cell line as in vitro model. As the healing of fractures and rheumatism is related to the proliferation and the differentiation of osteoblastic cell, we examined cell growth in osteoblastic MC3T3-E1 cells by CCK-8 assay in vitro. The results showed that the low dosage (1–10 μg/mL) of ethanol extracts stimulated cell growth. At the same time, the results of the cell cycle assay demonstrated that PAF ethanol extract effectively promoted cell cycle progression, the proportion of cells in S-phase of cells significantly increased (from 16.33 to 27.29%). These results indicated that PAF ethanol extract promoted cell proliferation by regulating cell cycle progression. Moreover, the effect of ethanol extract on cell growth in osteoblastic MC3T3-E1 cells was comparable with DB (as positive control).
ALP is the most widely recognized biochemical marker for osteoblastic activity (CitationYamaguchi & Gao, 1998). Although its precise mechanism of action is poorly understood, this enzyme is believed to play an important role in bone metabolism (CitationMizutani et al., 1998). Therefore, we examined the effects of extract from the root tubers of PAF on the ALP activity of osteoblastic MC3T3-E1 cells. The results showed that the treatment of MC3T3-E1 cells with 0.1–100 μg/mL of ethanol extract significantly up-regulated the expression of ALP, and the effect of ethanol extracts on ALP expression was similar to that of the positive control DB.
PGE2 is an endogenous factor to stimulate osteolysis through the EP4 receptor (CitationTsutsumi et al., 2009). It is known that some bone-resorbing agents like TNF-α (Kwan et al., 2004; CitationKitaura et al., 2005; CitationSon et al., 2006) act on osteoblasts and stimulate PGE2 release from osteoblasts (CitationIgarashi et al., 1997). The released PGE2 acts on stroma cells and enhances factors that support the differentiation from stem cells to osteoclasts to augment osteolysis. Furthermore, it is known that the presence of PGE2 caused a significant decrease in bone ALP activity and a corresponding increase in bone acid phosphatase activity. In the present study, EG inhibited PGE2 production induced by TNF-α in osteoblasts, suggesting that EG may inhibit the osteolysis by down-regulating the release of PGE2 induced by TNF-α. This result partly elucidated the pharmacological mechanism of PAF to promote the healing of bone fractures and rheumatism.
TCMs have been developed for over 5000 years and are known to have lower toxicity, showing an advantage over chemical synthesized medicine, although the precise pharmacological mechanism of these agents remained to be explored. PAF is known for its functions in promoting tissue regeneration and granulation. It has been used in the treatment of fractures, rheumatism, and bone injuries. This medicinal herb has been used for hundred of years, and its safety and efficacy are well-established through a long history of human use, but the pharmacological mechanism is still unknown. In this study, we found that PAF extract promoted the proliferation and differentiation of osteoclasts, which play important roles for osteogenesis. These results partly elucidated the pharmacological mechanism of PAF to promote the healing of bone fractures and rheumatism.
Acknowledgements
This work was supported by the Fund of the Natural Science Foundation of Wuhan City (SZY09005) and Fund for New Century Excellent Talents from Ministry of Education, P.R. China (NCET-07-0336) and Fund for the Natural Science Foundation of SCUEC (CZY10015). The authors are thankful to Professor Dingrong Wan in the Pharmacognosy, the College of Pharmacy, South-Central University for Nationalities to authenticate the herb PAF. The authors are thankful to Dr. Guangzhong Yang for instructing isolation and identification of chemicals.
Declaration of interest
This scientific treatise is an original research, and there is no plagiarism. Biodiversity was not harmed when samples of this medicinal herb were collected.
References
- Amos AF, Trevor WS, Robert AS. (2008). Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol, 587, 35–41.
- Brixen KT, Christensen PM, Ejersted C, Langdahl BL. (2004). Teriparatide (biosynthetic human parathyroid hormone 1-34): A new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol, 94, 260–270.
- Chan BY, Lau KS, Jiang B, Kennelly EJ, Kronenberg F, Kung AW. (2008). Ethanolic extract of Actaea racemosa (black cohosh) potentiates bone nodule formation in MC3T3-E1 preosteoblast cells. Bone, 43, 567–573.
- Choi EM. (2005). The licorice root derived isoflavone glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharmacol, 70, 363–368.
- Choi EM, Suh KS, Kim YS, Choue RW, Koo SJ. (2001). Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry, 56, 733–739.
- Hu YM, Liu C, Cheng KW, Sung HH, Williams LD, Yang ZL, Ye WC. (2008). Sesquiterpenoids from Homalomena occulta affect osteoblast proliferation, differentiation and mineralization in vitro. Phytochemistry, 69, 2367–2373.
- Igarashi K, Hirafuji M, Adachi H, Shinoda H, Mitani H. (1997). Effects of bisphosphonates on alkaline phosphatase activity, mineralization, and prostaglandin E2 synthesis in the clonal osteoblast-like cell line MC3T3-E1. Prostaglandins Leukot Essent Fatty Acids, 56, 121–125.
- Jeong JC, Lee JW, Yoon CH, Kim HM, Kim CH. (2004). Drynariae Rhizoma promotes osteoblast differentiation and mineralization in MC3T3-E1 cells through regulation of bone morphogenetic protein-2, alkaline phosphatase, type I collagen and collagenase-1. Toxicol in Vitro, 18, 829–834.
- Jeong JC, Lee JW, Yoon CH, Lee YC, Chung KH, Kim MG, Kim CH. (2005). Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J Ethnopharmacol, 96, 489–495.
- Kajii T, Suzuki K, Yoshikawa M, Imai T, Matsumoto A, Nakamura S. (1999). Long-term effects of prostaglandin E2 on the mineralization of a clonal osteoblastic cell line (MC3T3-E1). Arch Oral Biol, 44, 233–241.
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. (2008). Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun, 375, 414–419.
- Kang SK, Kim KS, Byun YS, Suh SJ, Jim UH, Kim KH, Lee IS, Kim CH. (2006). Effects of Ulmus davidiana Planch on mineralization, bone morphogenetic protein-2, alkaline phosphatase, type I collagen, and collagenase-1 in bone cells. In Vitro Cell Dev Biol Anim, 42, 225–229.
- Kim HS, Shin TH, Yang SR, Seo MS, Kim DJ, Kang SK, Park JH, Kang KS. (2010). Implication of NOD1 and NOD2 for the differentiation of multipotent mesenchymal stem cells derived from human umbilical cord blood. PLoS ONE, 5, e15369.
- Kim KW, Suh SJ, Lee TK, Ha KT, Kim JK, Kim KH, Kim DI, Jeon JH, Moon TC, Kim CH. (2008). Effect of safflower seeds supplementation on stimulation of the proliferation, differentiation and mineralization of osteoblastic MC3T3-E1 cells. J Ethnopharmacol, 115, 42–49.
- Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. (2005). M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest, 115, 3418–3427.
- Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. (2004). IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev, 15, 49–60.
- Mizutani K, Ikeda K, Kawai Y, Yamori Y. (1998). Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun, 253, 859–863.
- Pozharski E. (2010). Fluorometric analysis of individual cationic lipid-DNA complexes. Methods Mol Biol, 606, 385–391.
- Ren HC, Wan DR, Zhou ZM. (2009). Studies on chemical constituents of P. amplexicaule. Chin J Chin Materia Medica, 34, 183–185.
- Quarlers JD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. (1999). Distinct proliferative and differentiated stages of mouse MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res, 27, 683–689.
- Son YO, Kook SH, Choi KC, Jang YS, Jeon YM, Kim JG, Lee KY, Kim J, Chung MS, Chung GH, Lee JC. (2006). Quercetin, a bioflavonoid, accelerates TNF-alpha-induced growth inhibition and apoptosis in MC3T3-E1 osteoblastic cells. Eur J Pharmacol, 529, 24–32.
- Suh KS, Koh G, Park CY, Woo JT, Kim SW, Kim JW, Park IK, Kim YS. (2003). Soybean isoflavones inhibit tumor necrosis factor-alpha-induced apoptosis and the production of interleukin-6 and prostaglandin E2 in osteoblastic cells. Phytochemistry, 63, 209–215.
- Tsutsumi R, Xie C, Wei X, Zhang M, Zhang X, Flick LM, Schwarz EM, O’Keefe RJ. (2009). PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res, 24, 1753–1762.
- Yamaguchi M, Gao YH. (1998). Inhibitory effect of genistein on bone resorption in tissue culture. Biochem Pharmacol, 55, 71–76.
- Yang ZJ, Li BL, Tian XH, Liang B. (2007). Chemical constituents of the essential oils of Pteroxygonum giraldii and Polygonum amplexicaule. ACTA Bot Boreal-Occident Sin, 6, 1261–1264.
- Zhao J, Ohba S, Komiyama Y, Shinkai M, Chung UI, Nagamune T. (2010). Icariin: A potential osteoinductive compound for bone tissue engineering. Tissue Eng Part A, 16, 233–243.
