Abstract
Context: Prosthechea michuacana W.E. Higgins (LaLlave & Lex) (Orchidaceae) is an orchid that has been used in traditional medicine for the treatment of inflammation, diabetes, wound, and liver disorders. Therefore, we thought it would be worthwhile to study the effect of this orchid on liver damage using mice as model.
Objective: The present study investigates the effect of flavonoids isolated from methanol extract of P. michuacana on carbon tetrachloride (CCl4)-induced liver damage in mice.
Materials and methods: The methanol extract was purified by repeated column chromatography, resulting in the identification of five metabolites whose hepatoprotective effects were evaluated by measuring aspartate transaminase, alanine transaminase, alkaline phosphatase, glutamate, total bilirubin level, lactate dehydrogenase, total serum protein, and lipid peroxidation (thiobarbituric acid reactive substances assay) in CCl4-induced hepatic injury in mice.
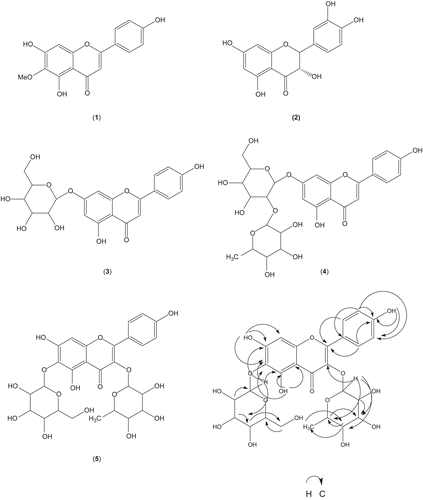
Results: From the bulbs of P. michuacana, four known flavonoids were isolated (scutellarein 6-methyl ether, dihydroquercetin, apigenin 7-O-glucoside, and apigenin-7-neohesperidoside), together with the new flavonol glycoside apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside. Their structures were characterized by 1D and 2D nuclear magnetic resonance experiments. Treatment with flavonoids significantly prevented the biochemical measurable changes induced by CCl4 in the liver. Compounds 1, 4, and 5 were found to exhibit good hepatoprotective effect. These effects were comparable to that of the standard drug silymarin, a well-known hepatoprotective agent.
Discussion: These results demonstrate that flavonoids contained in the bulbs of P. michuacana contribute to the hepatoprotective activity attributed to the plant.
Keywords::
Introduction
Liver is expected not only to perform physiological functions but also to protect against hazards of harmful drugs and chemicals. It is involved with almost all biochemical pathways related to growth, disease fighting functions, nutrient supply, energy provision, and reproduction (CitationWard et al., 1999). Liver has great capacity to eliminate toxic substances and synthesize useful principles. Therefore, damage to the liver inflicted by hepatotoxic agents results in grave consequences (CitationSubramoniam & Pushpangadan, 1999). Most hepatotoxic chemicals damage liver cells mainly by inducing lipid peroxidation and other oxidative damage (CitationDianzani et al., 1991). Oxidative stress, a consequence of an imbalance of pro-oxidants and antioxidants in the organism, has been implicated in several disease states, including inflammation and liver diseases (CitationPoli, 1993). Compounds that block or retard the chain reaction of oxidation are reported to prevent oxidative stress-induced hepatotoxicity (CitationHanda et al., 1986).
Prosthechea michuacana W.E. Higgins (LaLlave & Lex) (Orchidaceae), is a member of the orchid family that has been commonly found available in traditional markets of the Mexican state Oaxaca since the times of its American discovery by C. Colon. This orchid has a great reputation in folk medicine for the treatment of liver disorders and diabetes. Some studies on this plant’s properties reported relaxant and antispasmodic activity (CitationVargas-Solis & Perez-Gutierrez, 2009). In other scientific reports, its anti-inflammatory and wound healing activity has been demonstrated (CitationPerez-Gutierrez & Vargas-Solis, 2009a), and we found antioxidant activity in the constituents of the orchid bulbs (CitationPerez-Gutierrez & Neira-Gonzales, 2010). In previous studies, we evaluated the hepatoprotective effects of a methanol extract of P. michuacana against paracetamol and carbon tetrachloride (CCl4)-induced hepatic damage in albino rats (CitationPerez-Gutierrez & Vargas-Solis, 2009b). The obvious next step for us was to isolate and characterize the flavonoids present in bulb extracts of P. michuacana and to evaluate its protection activity against induced hepatotoxicity, knowing the importance of this strong antioxidants applied in modern liver therapy such as the case of silymarin and catechin (CitationLee et al., 2005), it was the aim of this investigation to isolate and characterize the flavonoids of P. michuacana by 1D and 2D nuclear magnetic resonance (NMR) experiments proceeding from an antihepatotoxic crude extract. Furthermore, the antihepatotoxic activity of the purified components was tested by antagonization of the CCl4 intoxication in mice.
Materials
General experimental procedures
Melting points was measured without correction on an Electrothermal model 9100 (Los Angeles, California, USA). Infrared (IR) spectra were obtained on a Perkin–Elmer FTRI 1720X (Massachusetts, Waltham, USA). A Bruker DRX-600 (Madison, Wisconsin, USA) NMR spectrometer, operating at 599.19 MHz for 1H and 150.86 MHz for l3C, using the UXNMR software package, was used for NMR experiments; chemical shifts are expressed in δ (ppm) using tetramethyl silane as an internal standard. DEPT l3C (Distortionless enhancement by polarization), ID TOCSY (Pulsed field gradient one-dimensional NMR selective), lH- lH DQF-COSY (Double-quantum filtered COSY), and HMBC (Heteronuclear multiple bond correlation) NMR experiments were carried out using the conventional pulse sequences as described in the literature. Mass spectrometery was measured on an A JEOL HX 110 mass spectrometer (Houston, Texas, USA). Column chromatography and preparative thin-layer chromatography (TLC) were performed over silica gel (Merck, Manhattan, New York, USA) (CitationFried & Sherma, 1982) and Sephadex LH-20 (Pharmacia, Bridgewater, New Jersey, USA).
Plant material
P. michuacana bulbs were collected from El Punto, municipio de Santa Catarina Ixtepeji, distrito de Ixtlán, Oaxaca state, México in May of 2007 and were taxonomically authenticated by taxonomists working at the Department of Botany of Escuela Nacional de Ciencias Biologicas, Instituto Politécnico Nacional and a voucher specimen of the plant is stored for reference (No. 6478).
Extraction and isolation
Shade dry bulbs of P. michoacana (2 kg) were ground into power, which was defatted three times with hexane, and the residual material was extracted two times with methanol consecutively (20 L). The extracts were evaporated under reduced pressure. The methanol extract was applied to a silica gel column, eluting with CHCl3/EtOAc (7:1). The fractions were collected and combined based on their TLC profiles to produce nine pooled fractions, which were subjected to antioxidant and antihepatotoxic tests. Fractions obtained were numbered in order of elution with Roman numerals (FI–FIX). FIII was the fraction that showed more activities which was subjected to silica gel column chromatography eluted with ethyl ether/ethyl acetate (7:1). The fractions (40 mL each) were combined according to TLC monitoring into six portions (FIII-1-1 to FIII-1-6). Active fraction FIII-1-3 was purified further using mixtures of chloroform/ethyl ether (6:2) and chloroform/acetone (4:1) by silica gel column chromatography. Final purification of the active subfractions FIII-1-3-2 was achieved by preparative TLC on 1 mm thick silica gel plates eluted with hexane/chloroform/methanol (2:7:1) and visualized under ultraviolet light at 254 nm. The following metabolites were obtained: 1 (109 mg), 2 (92 mg), and 3 (79 mg). Fraction FIII-1-3-4 was subjected to preparative TLC, where five fractions were obtained (FIII-1-3-4-1 to FIII-1-3-4-5). FIII-1-3-4-1 was subjected to Sephadex LH-20, where it gave 4 (98 mg), while fraction FIII-1-3-4-3 was further purified by column chromatography (CHCl3-MEOH, 8:2) to give 5 (112 mg).
Apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside (5). Yellow powder; Mp 223–224°C; IR (KBr): νmax 3420 (OH), 1651 (C=O), 1606, 1552 (aromatic rings), 1492, 1191 and 1092; High resolution electron ionization mass spectral (HREIMS), m/z ([M+H])+ 610.456 calculated for C27H30O16,610.498; 1H NMR (DMSO-d6, 300 MHz) δ: 7.14 (d, J = 9.0 Hz, H-2′,H-6′), 6.92 (d, J = 9.0 Hz, H-3′,H-5′), 6.96 (s, H-8), 12.6 (s, OH-5); Glc: 4.93 (d, J = 7.5 Hz, H-1″), 4.30 (dd, J = 8.5, 7.5 Hz, H-2″), 3.60 (t, J = 8.5 Hz, H-3″), 3.54 (t, J = 8.5 Hz, H-4″), 3.39 (m, H-5″), 3.76 (dd, J = 12.1, 4.5 Hz, H-6″), Rha: 5.18 (d, J = 7.0 Hz, H-1″′), 3.98 (dd, J = 1.5, 2.5 Hz, 2′″), 3.75 (dd, J = 2.5, 9.5 Hz, 3′″), 3.40 (t, J = 9.5 Hz, 4′″), 4.1 (m, 5′″), 1.21 (d, J = 6.5 Hz, 6′″); 13C NMR (DMSO-d6, 300 MHz) δ: 159.8 (C-2), 135.7 (C-3), 178.7 (C-4), 158.6 (C-5), 108.8 (C-6), 163.8 (C-7), 94.7 (C-8), 155.3 (C-9), 105.1 (C-10), 122.7 (C-1′), 128.1 (C-2′, C-6′), 116.2 (C-3′, C-5′), 161.4 (C-4′); Glc: 101.1 (C-1″), 73.6 (C-2″), 75.2 (C-3″), 69.6 (C-4″), 77.3 (C-5″), 60.7 (C-6″); Rha: 103.2 (C-1″′), 72.9 (C-2″′), 73.6 (C-3″′), 74.0 (C-4″′), 70.1 (C-5″′), 18.3 (C-6″′).
Hydrolysis of compound 5
Acid hydrolysis of the glycosides was performed according to the method of CitationNgounou et al. (2000). Compound 5 (4 mg) was dissolved in 50% aqueous methanol, before 2 M hydrochloric acid (5 mL) was added. The solution was refluxed for 7 h at 60°C, the reaction mixture then being concentrated under reduced pressure. The residue was dissolved in 10 mL of water, and the aglycon was extracted with chloroform. The 1H NMR spectrum of compound 5 was also consistent with that of apigenin. The sugar in the water-soluble portion was, compared with standard sugars on a TLC plate (silica gel 60; Merck,) by using BuOH-EtOAc-iso-PrOH-AcOH-H2O (4:5:2:3:1). Spots were detected with the aniline-diphenylamine reagent (CitationNgounou et al., 2000).
Experimental animals
The study was conducted with male CD1 strain albino mice, initially 8 weeks old and weighing about 25–30 g. Before and during the experiment, animals were fed standard laboratory mice diet and water ad libitium. The animals were acclimatized for a period of 3 days in the new environment before initiation of experiment. Mice were cared for under a protocol approved by the Animal Research Ethics Committee of our institute and according to the guidelines of the National Institutes of Health (CitationAnonymous, 1985).
Induction of liver injury
Mice were fasted for 24 h prior to experiments, but were allowed free access to water. Animals were divided into six groups six animals each. Group I remained untreated, received normal saline and was kept as a control group. Groups II, III, IV, V, and VI received 0.125 mL of CCl4 in corn oil (50%, v/v) per 100 g body weight intraperitoneally. Group II received only CCl4 treatment. Group III, IV, and V were treated with 1, 4, and 5, respectively, at a dose of 20 mg/kg, p.o. Groups VI was administered silymarin at a dose of 20 mg/kg, p.o. Isolates and silymarin were administered daily for 7 days. After completion of the experimental period, the animals were fasted overnight and sacrificed by CO2 asphyxiation. Blood was collected from the abdominal aorta for biochemical studies.
Serum was separated by centrifugation at 2500g for 15 min and tested for alkaline phosphatase (ALP) (CitationBessay et al., 1946), and total bilirubin (TB) level (CitationJendrassik & Grof, 1938), lactate dehydrogenase (LDH) (CitationHenderson, 1986). Aspartate transaminase (AST), and alanine transaminase (ALT) were measured using a kit (Asan Pharm Co., Hwaseong-si, Gyeonggi-do, South Korea). Lipid peroxidation level was measured with thiobarbituric acid reactive substances assay (TBARS) as a marker of lipid peroxidation using a modification of the method described by Ohkawa (1979). Total serum protein (TSP) was determined by the Biuret reaction (CitationGornallet et al., 1994).
Statistical analysis
For each set of experiments, where two or more than two groups were compared, an analysis of variance test was used to determine the significance of the differences. Differences between the control and CCl4-treated group were compared for significance using Student’s t-test for non-paired samples. p-Values < 0.05 were consider as significant.
Results
The methanol extract was subjected to repeated column chromatographic analyses; each fraction was tested for hepatoprotective activity. Chromatography purification of the active fractions led to the identification of five flavones 1–5 (). Compounds were characterized by comparing their spectroscopic data with those reported in the literature and by comparison with a reference material.
Table 1. Fractions obtained during the bio-fractionation of methanol extract of Prosthechea michuacana.
Pretreatment of the flavonoids 1, 4, and 5 to such CCl4-treated rats reduced the enhanced level of serum of hepatic enzymes as follows: AST by 53, 60, and 47%, ALT by 54, 59, and 51%, TB by 47, 51, 44%, and ALP by 30, 39, and 25%, respectively ( and ). The significant decrease in TSP by 37, 43, and 26% was observed indicating the antihepatotoxic activity (). The elevated level of lipid peroxidation products (TBARS), an indicator of oxidative stress in CCl4-intoxicated mice, was clearly depressed by oral administration of flavonoids 1, 4, and 5 () at a dose of 20 mg/kg. There was a significant (p < 0.001) restoration of these enzyme levels AST, ALT, and LDH on pretreatment of the flavonoids ( and ). The effect was comparable with that of standard drug silymarin.
Table 2. Hepatic protection of scutellarein 6-methyl ether (1), apigenin-7-neohesperidoside (4) and apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside (5) from Prosthechea michuacana on TB (total bilirubin level) and TSP (total protein) in CCl4-induced liver injury in mice.
Table 3. Hepatoprotective effects of scutellarein 6-methyl ether (1), apigenin-7-neohesperidoside (4) and apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside (5) from Prosthechea michuacana on AST (aspartate transaminase), ALT (alanine transaminase), ALP (alkaline phosphatase), LDH (lactate dehydrogenase) and TBARS (lipid peroxidation) in mice intoxicated with CCl4.
Discussion
Broad fractionation of the methanol crude extract on silica gel yielded active fractions which conducted and resulted in the isolation of the known flavones (1) scutellarein 6-methyl ether (CitationWilliams et al., 2003), (2) dihydroquercetin (CitationTjukavkina et al., 1993), (3) apigenin 7-O-glucoside (CitationSvehliková et al., 2004), and (4) apigenin-7-neohesperidoside (CitationAbd-Alla et al., 2009), together with the new flavonoid glycoside (5) apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside.
Previous studies reported the hepatoprotective effect of flavonoids apigenin 7-O-glucoside (CitationZheng et al., 2005), which has protective effects against hepatic oxidative injury induced by chemicals possibly due to its antioxidant properties, acting as scavengers of reactive oxygen species. Dihydroquercetin (2) exhibits antioxidant and hepatoprotective properties, and inhibits the process of spontaneous lipid peroxidation in the liver (CitationSiegers & Vounes, 1981). So these two flavonoids were not tested on in this study.
The molecular formula for compound 5 was obtained by HREIMS, and DEPT analyses, showed one methyl, one methylene, 15 methane, and 10 quaternary carbons corresponding to molecular formula C27H30O16, as well as bands of hydroxyl groups (3420 cm−1), α,β-unsaturated ketone (1651 cm−1) and aromatic rings (1606, 1552 cm−1). Acid hydrolysis of the 5 resulted in the aglycon apigenin which was compared with authentic apigenin. Also, the NMR shifts were compatible with apigenin (CitationYan et al., 1996). 1H and 13C spectra showed the characteristic signal for δH 6.96 (s, H-8) which suggested the C-6 substituted. The missing signal of the proton at C-3 indicated the presence of substituent at C-3. A sharp singlet observed at δH 12.6 was assigned to the C-5 chelated hydroxyl proton. 13H NMR spectrum exhibited two dobletes at δH 7.14 and 6.92 characteristic of protons H-2′, H-6′ and H-3′, H-5′ in a monosubstituted B-ring. The substitution pattern of ring B was proposed with a hydroxyl group at the 4′-position. 13C NMR spectrum signals by hydrogen 7 and 4′ were not observed around δC 156 indicated the hydroxyl groups at C-7 and C-4′. The sugar moiety from acid hydrolysis of compound 5 indicated rhamnose and glucose. Two anomeric protons were easily identified in the 1H NMR spectrum. They resonated at δH 4.93 (d, J = 7.5 Hz, H-1″), and δH 5.18 (d, J = 7.0 Hz, H-1′″), and correlated, respectively, with 13C resonances at δC 101.1 and δC 103.2. The HMBC spectrum was utilized to identify position of sugar, a long correlation between H-1″′ (δH 5.18) to C-3 (δC 135.7) indicated the attachment of rhamnosyl group at C-3 with 3-O-α-linkage. The coupling constant of the anomeric proton (J = 7.0) confirm the linkage of the sugar (CitationSathiamoorthy et al., 2007). The absolute configuration of rhamnose was of l-type. Although the mid-field region of the 1H NMR spectrum contained several overlapping signals, analysis of homonuclear COSY experiments allowed us to assign all the proton resonances of the sugar moieties and this was very useful to elucidate the monosaccharide relative stereochemistry. Hence, when the anomeric proton at δH 4.93 (H-1″) was used as a starting point, a sequence of three oxymethines and one oxymethylene was identified from the above spectra. The large coupling constants observed for all the protons, in accordance with axial–axial relationships, indicated the β-glucopyranose nature of this sugar. In addition, the ROESY couplings of H-1″, with H-3″ and H-5″, and of H-2″ with H-4″ further supported the above conclusion. This C-glucose should be linked to the C-6 of the aglycone, as indicated by the key HMBC correlation peak between the anomeric proton H-1″ and C-6 (δC 108.8) and the cross peak between C5-OH-5, C10-OH-5, C6-OH-5, C7-H1″ and C5-H-1″ (). These results suggested that the structure apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside was therefore assigned to 5.
In the present investigation, flavonoids have been shown to be important constituents of P. michuacana so the antihepatotoxic activity of these compounds was investigated using CCl4-induced injury model in mice. It is well established from the earlier studies that the cleavage of carbon-chlorine bond (C-Cl bond) of CCl4 leads to the formation of trichloromethyl peroxy radical (CCl3.O2-), which is involved in the pathogenesis of liver injury (CitationDrotman & Lawhorn, 1978).
The abnormal higher levels of serum ALT, AST, ALP, and bilirubin; and decrease in TSP observed is the consequence of CCl4-induced liver dysfunction and denotes the damage to the hepatic cells. P. michuacana flavonoids seem to offer protection and maintain the functional integrity of hepatic cells. Decrease in TSP observed in the rats treated with CCl4 may be associated with the decrease in the number of hepatocytes, which in turn may result into the decreased hepatic capacity to synthesize protein (CitationRecknagel et al., 1989); but when the flavonoids were given along with the CCl4, increase in TSP indicating the antihepatotoxic activity.
The elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membrane in liver (CitationZimmerman & Seeff, 1970). Thus, lowering of enzyme content in serum is a definite indication of hepatoprotective action of a drug. High level of AST indicates liver damage such as due to viral hepatitis. ALT catalysis the conversion of alanine to pyruvate and glutamate and is released in a similar manner. Therefore, ALT is more specific to the liver and a better parameter for detecting liver damage (CitationWillianson et al., 1996). Flavonoids 1, 4, and 5, at the dose of 20 mg/kg, decreased the levels of both AST and ALT significantly.
Serum ALP and bilirubin levels are also related to the status and function of hepatic cells. Increase in serum ALP level is due to increased synthesis, in the presence of increasing biliary pressure (CitationMoss et al., 1974). In the present study, 1, 4 and 5 have been found to reduce both serum ALP and bilirubin in the treated groups compared with the untreated ones.
The reversal of increased serum enzymes in CCl4-induced liver damage by the compounds may be due to the prevention of the leakage of intracellular enzymes by its membrane stabilizing activity (CitationDrotman & Lawhorn, 1978). This is in agreement with the commonly accepted view that serum levels of transaminase return to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes (CitationThabrew & Joice, 1987). Effective control of ALP level, bilirubin and TSP levels points towards an early improvement in the secretary mechanism of the hepatic cells.
Flavonoids 1, 4, and 5 exhibited good protection against CCl4. Among the purified compounds, apigenin-7-neohesperidoside (4) was clearly the most active, scutellarein 6-methyl ether (1) also showed moderate activity and apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside (5) is less effective against CCl4-induced lived injury. The results of the biological study revealed that flavonoids 1, 4, and 5 showed moderate hepatoprotective activity as compared with silymarin.
Conclusion
Our results indicate that flavonoids isolated from bulbs of P. michuacana are able to protect mice from CCl4-induced liver injury, as evidenced by the biochemical parameters measured and compared with the respective values in control mice. This indicates a stabilization of plasma membrane as well as a protection of the hepatic tissue damaged by CCl4.
Declaration of interest
This study has been supported by the Instituto Politecnico Nacional Mexico D.F.
References
- Abd-Alla HI, Moharram FA, Gaara AH, El-Safty MM. (2009). Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z Naturforsch, C, J Biosci, 64, 495–501.
- Anonymous. (1985). Guide for the Care and Use of Laboratory Animals. NH Publication number 85-23. Bethesda MD: National Institutes of Health.
- Bessay OA, Lowry OH, Bross MJ. (1946). A method for rapid determination of alkaline phosphatase with five cubic milliliters of serum. J Biol Chem, 164, 321–329.
- Dianzani MU, Muzio G, Biocca ME, Canuto RA. (1991). Lipid peroxidation in fatty liver induced by caffeine in rats. Int J Tissue React, 13, 79–85.
- Drotman RB, Lawhorn GT. (1978). Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol, 1, 163–171.
- Fried B, Sherma J. (1982). Thin-layer Chromatography. Volume I. New York: Marcel Dekker, Inc., 254–256.
- Gornallet AG, Barddawill CJ, David MM. (1994). Protein determination with Biuret reagent. J Biochem, 117, 751–756.
- Handa SS, Sharma A, Chakraborti KK. (1986). Natural products and plants as liver protecting drugs. Fitoterapia, 57, 307–345.
- Henderson AR. (1986). Isoenzymes of lactate dehydrogenase. In: Bergmeyer HU ed. Methods of Enzymatic Analysis. Volume III. Third edition. USA and Canada: VCH, 138.
- Jendrassik L, Grof P. (1938). Simplified photometric method for determination method for determination of blood bilirubin. Biochem J, 297, 81–89.
- Lee HU, Bae EA, Kim DH. (2005). Hepatoprotective effects of irisolidone on tert-butyl hyperoxide-induced liver injury. Biol Pharm Bull, 28, 531–533.
- Moss DW, Butterworth PJ. (1974). Enzymology and medicine. London: Pitman Medical, p. 139.
- Ngounou FN, Meli AL, Lontsi D, Sondengam BL, Atta-Ur-Rahman Choudhary, MI et al. (2000). New isoflavones from Ceiba pentandra. Phytochemistry, 54, 107–110.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Perez-Gutierrez RM, Vargas-Solis R. (2009a). Anti-inflammatory and wound healing potential of Prosthechea michuacana in rats. Phcog Mag, 4, 219–225.
- Perez-Gutierrez RM, Vargas-Solis R. (2009b). Hepatoprotective and inhibition of oxidative in liver of Prosthechea michuacana. Nat Prod Records, 3, 46–51.
- Perez-Gutierrez RM, Neira-Gonzales MA. (2010). Studies on the constituents of bulbs of the orchid Prosthechea michuacana and antioxidant activity. Chem Nat Compounds, 46, 554–561.
- Poli G. (1993). Liver damage due to free radicals. Br Med Bull, 49, 604–620.
- Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther, 43, 139–154.
- Sathiamoorthy B, Gupta P, Kumar M, Chaturvedi AK, Shukla PK, Maurya R. (2007). New antifungal flavonoid glycoside from Vitex negundo. Bioorg Med Chem Lett, 17, 239–242.
- Siegers CP, Vounes M. (1981). Effects of bioflavonoids on lipid peroxidation induced by gluthathione depletion. Akademia Kiado Budapest: Proc Int Bioflavonoid Symp, 36, 403–409.
- Subramoniam A, Pushpangadan P. (1999). Development of phytomedicine for liver diseases. Indian J Pharmacol, 31, 166–175.
- Svehliková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA et al. (2004). Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (recutita [L.] Rauschert). Phytochemistry, 65, 2323–2332.
- Thabrew MI, Joice PD, Rajatissa W. (1987). A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med, 53, 239–241.
- Tjukavkina NA, Rulenko IA, Kolesnik VA. (1993). Confection of food stuffs with biologically active additives. Confection stuffs with dihydroquercetin as curative and prophylactic remedies. Biotech Managem, 3–4, 36–39.
- Vargas-Solis R, Perez-Gutierrez RM. (2009). Relaxant and antispasmodic effect in isolated guinea pig ileum treated with extracts of orchid Prosthechea michuacana. J Nat Med, 63, 65–68.
- Ward FM, Daly MJ. (1999). Hepatic disease. In: Walker R, Edwards C, eds. Clinical Pharmacy and Therapeutics. New York: Churchill Livingstone, pp. 195–212.
- Williams CA, Harborne JB, Greenham JR, Grayer RJ, Kite GC, Eagles J. (2003). Variations in lipophilic and vacuolar flavonoids among European Pulicaria species. Phytochemistry, 64, 275–283.
- Williamson EM, Okpako DT, Evans FJ, Selection. (1996). Preparation and pharmacologic evaluation of plant material. England: John Wiley, p. 1.
- Yan F, Li X, Wang H, Yang C. (1996). Flavonoid glycosides from Colebrookea oppositifolia. Phytochemistry, 3, 867–869.
- Zheng QS, Sun XL, Xu B, Li G, Song M. (2005). Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed Environ Sci, 18, 65–70.
- Zimmerman HJ, Seeff LB. (1970). Enzymes in hepatic disease. Diagnostic Enzymology. Philadelphia: Lea and Febiger, p. 1.