Abstract
Context: Diabetes mellitus is characterized by oxidative stress, which in turn induces endothelial dysfunction. As a potent antioxidant compound, lycopene might rescue diabetic endothelial dysfunction by reducing oxidative stress.
Objective: The present study investigated whether lycopene could lower oxidative stress and attenuate endothelial dysfunction in diabetic rats.
Methods: Different doses of lycopene (10, 30, and 60 mg/kg/day, p.o.) were administered for 30 days to streptozotocin (STZ) (60 mg/kg)-induced diabetic rats. Biochemical parameters and aortic malondialdehyde (MDA) content, superoxidase dismutase (SOD) activity, nitric oxide (NO) levels, constitutive NOS (cNOS) activity, and inducible NOS (iNOS) activity were determined. Endothelium-dependent and endothelium-independent vasorelaxation were measured in aortas for estimating endothelial function.
Results: Compared with normal controls, endothelial function was significantly reduced in diabetic rats and the blunted endothelial function was dependently ameliorated with lycopene treatment. Compared with normal controls, the serum oxidized low-density lipoprotein (ox-LDL) levels, the aortic MDA levels, and iNOS activity in diabetic rats were increased by 113, 197, and 100%, respectively, whereas aortic SOD activity, NO levels, and cNOS activity were decreased by 73, 53, and 65%, respectively. Exogenous administration of lycopene to diabetic rats caused a dose-dependent decrease of serum glucose and ox-LDL levels, an increase of aortic SOD activity, NO levels, and cNOS activity, and a decrease of aortic MDA levels and iNOS activity.
Conclusion: Chronic lycopene treatment could attenuate endothelial dysfunction by reducing oxidative stress in STZ-induced diabetic rats. These results indicate that chronic lycopene treatment might be useful in preventing diabetic vascular complications associated with endothelial dysfunction.
Introduction
Cardiovascular complications continue to constitute major causes of morbidity and mortality in diabetic patients (CitationCoccheri, 2007). Endothelial dysfunction, which results from oxidative stress damage induced by the chronic hyperglycemia of diabetes mellitus (CitationCai & Harrison, 2000), plays an initiating and critical role in the development of these cardiovascular complications (CitationRodríguez-Mañas et al., 2003).
Various studies have demonstrated that dietary carotenoids could suppress oxidative stress and therefore delay or prevent oxidative damage in diabetes (CitationRao & Rao, 2007). Lycopene, a carotenoid mostly found in tomatoes and tomato products, is a potent antioxidant with a singlet-oxygen-quenching ability twice as high as that of β-carotene and 10 times higher than that of α-tocopherol (CitationRiccioni et al., 2008). Lycopene has demonstrated promising therapeutic effects on chronic diseases such as cancer and cardiovascular diseases (CitationGerster, 1997; CitationAli & Agha, 2009). However, there is a paucity of literature concerning a role of lycopene in ameliorating diabetes-induced endothelial dysfunction.
We hypothesized that dietary lycopene might be beneficial for suppressing oxidative stress and ameliorating endothelial dysfunction in diabetes mellitus. We tested this hypothesis in the present study using the streptozotocin (STZ)-induced diabetic rat as an animal model.
Materials and methods
Animals
Eight-week-old male Wistar rats (190–220 g) were purchased from the experimental animal center of Tianjin Medical University. All the rats were housed in an environmentally controlled breeding room (temperature: 20 ± 2°C, humidity: 60 ± 5%, 12-h light/dark cycle) and had free access to tap water. The present study was performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (CitationInstitute for Laboratory Animal Research, 1996) and was approved by Animal Care and Use Committee of our hospital.
Experimental protocol
The diabetic rat model was developed by a single intraperitoneal (i.p.) injection of STZ (60 mg/kg) freshly dissolved in sterile sodium citrate buffer (0.1 M, pH 4.5). Normal control rats received an equivalent volume of citrate buffer. Three days later, tail blood samples were obtained from each rat for fasting blood glucose measurements using the Abbott Optium Xceed system (Abbott Diabetes Care, Alameda, CA). Rats with 16.7 mM or greater fasting blood glucose levels were considered as diabetic rats.
Control and diabetic rats were then randomly divided into six groups: (1) control (CON) group (n = 6; control rats treated with corn oil in the same volume), (2) control high lycopene-treated [LYCO(60)] group (n = 6; control rats treated with 60 mg/kg lycopene), (3) diabetic mellitus (DM) group (n = 6; diabetic rats treated with corn oil in the same volume), (4) diabetic low lycopene-treated [DM + LYCO(10)] group (n = 6; diabetic rats treated with 10 mg/kg lycopene), (5) diabetic medium lycopene-treated [DM + LYCO(30)] group (n = 6; diabetic rats treated with 30 mg/kg lycopene), and (6) diabetic high lycopene-treated [DM + LYCO(60)] group (n = 6; diabetic rats treated with 60 mg/kg lycopene). Lycopene was dissolved in 0.5 mL corn oil and administered via intragastric intubation for 30 consecutive days.
Blood collection and biochemical analysis
After fasting 12 h, rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO). Blood samples were obtained from abdominal aorta and centrifuged (3000 g, 4°C, 10 min) immediately upon withdrawal. The supernatant was used for measurement of blood glucose and lipid profiles by standard laboratory techniques on a Hitachi 7170 Automatic Biochemical Analyzer (Hitachi Co., Tokyo, Japan). Serum oxidized low-density lipoprotein (ox-LDL) levels were determined by an enzyme-linked immunosorbent assay (ELISA) kit (Xinqidi, Wuhan, China) according to the manufacturer’s instructions.
Functional studies of isolated thoracic aortas
One segment of thoracic aorta was rapidly cut into rings of ~3 mm in length. The rings were suspended between the bases of two triangular steel wires and mounted in an organ bath (Hs-4; Chengdu Instrument Factory, Chengdu, China). The organ bath was filled with and oxygenated (95% O2 and 5% CO2) Krebs-Henseleit solution (composition in mM: NaCl 118.0, KCl 4.7, MgSO4•7H2O 1.2, NaHCO3 25.0, KH2PO4 1.2, CaCl2 2.5, and glucose 11.1; 37°C, pH 7.4). One of the ring ends was connected to the level of a force-displacement transducer (JZJ01; Chengdu Instrument Factory, Chengdu, China). After equilibration for 90 min under a resting tension of 2 g, all vessels were preconstricted with phenylephrine (PE) at a submaximal concentration of 10−6 M, which produced 70–80% of maximal contraction response. After reaching a plateau of contraction, endothelium-dependent vasorelaxation was assessed by a dose-dependent relaxation to acetylcholine (ACh; 10−9–10−5 M), and endothelium-independent vasorelaxation was assessed by a dose-dependent relaxation to sodium nitroprusside (SNP; 10−10–10−6 M). In all experiments, indomethacin (10−5 M) was added to prevent synthesis of prostaglandins.
Determination of MDA concentration in thoracic aortas
One segment of thoracic aorta was blotted dry and weighed, and then made into 5% tissue homogenate in ice-cold 0.9% NaCl solution. A supernatant was obtained from the tissue homogenate by centrifugation (1000 g, 4°C, 10 min). The levels of malondialdehyde (MDA) were determined spectrophotometrically by measuring the content of thiobarbituric acid reactive substance (TBARS) (CitationOhkawa et al., 1979) using a MDA-detection assay kit (Jiancheng Bioengineering, Nanjing, China) according to the manufacturer’s instructions. The levels of MDA were expressed as nmol/mg protein.
Measurement of superoxidase dismutase activity, NOS activity, and nitric oxide level in thoracic aortas
The supernatants of tissue homogenates of the thoracic aortas were obtained as described earlier. The superoxidase dismutase (SOD) activity in aorta homogenates was determined by monitoring the inhibition of the autoxidation of hydroxylamine (CitationLu et al., 2007) using a SOD-detection assay kit (Jiancheng Bioengineering) according to the manufacturer’s instructions. One unit was determined as the amount of enzyme that inhibited the autoxidation of hydroxylamine by 50%. The SOD activity was expressed as U/mg protein. Aortic total NOS (tNOS) and inducible NOS (iNOS) activity were assayed following commercial kits instruction, respectively. The tNOS activity minus the iNOS activity is the constitutive NOS (cNOS) activity, in rat aortas the main cNOS is endothelium-derived NOS (eNOS) (CitationQian et al., 2010). The nitric oxide (NO) levels were measured using a NO-detection assay kit (Jiancheng Bioengineering) according to the manufacturer’s instructions. This assay kit contains microtiter plate, nitrate reductase, nitrate reductase storage buffer, NADH, nitrate standard, nitrite standard, reaction buffer concentrate, Griess reagent I, and Griess reagent II.
Reagents
Lycopene (purity >90%) was purchased from North China Pharmaceutical Group Corporation (Shijiazhuang, China). SNP was purchased from Shuanghe Pharmacology Company (Beijing, China). STZ and other laboratory grade reagents were purchased from Sigma-Aldrich where not specified.
Statistical analysis
Statistical analyses were performed using SPSS 10.0 (SPSS Inc., Chicago, IL). All data were presented as mean ± standard error of the mean (SEM) and analyzed using one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Statistical significance was defined as P < 0.05.
Results
Effect of lycopene on body weight and biochemical parameters
The mean values of the body weight and the biochemical parameters from the control and diabetic rats are presented in . During the entire experiment period, diabetic rats presented significant hyperglycemia, hyperlipidemia, and emaciation compared with normal control rats. The administration of lycopene of 10, 30, 60 mg/kg to diabetic rats resulted in significantly increased body weight (by 105, 110, and 158%, respectively) and decreased fasting blood glucose concentrations (by 9, 31, and 55%, respectively). Chronic treatment with lycopene also significantly and dose-dependently reduced plasma ox-LDL levels. Moreover, although not significant, chronic treatment with lycopene showed a trend to decrease total and LDL cholesterol levels and increase HDL levels. Lycopene had no significant effects on the body weight and biochemical parameters in the control rats.
Table 1. Body weight and biochemical parameters in various groups.
Effect of lycopene on vasorelaxation of aortic rings
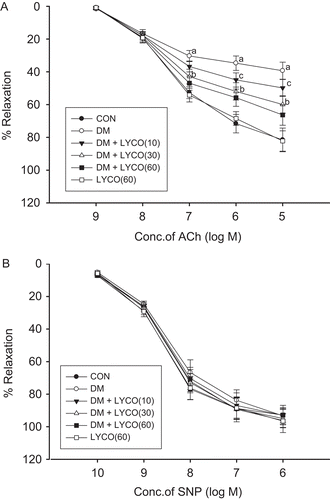
As shown in , endothelium-dependent vasorelaxation induced by ACh in the aortic rings from diabetic rats was significantly suppressed compared with normal controls (P < 0.01). In the presence of indomethacin, chronic treatment with lycopene (10, 30, 60 mg/kg) for 30 days significantly and dose-dependently improved the ACh-induced endothelium-dependent vasorelaxation in the aortic rings from diabetic rats (P < 0.05 and P < 0.01). Endothelium-independent vasorelaxation induced by SNP showed no difference among the six groups ().
Figure 1. Effect of different doses of lycopene ACh-induced endothelium-dependent vasorelaxation (A) and SNP-induced endothelium-independent vasorelaxation (B) in thoracic aortic rings in the presence of indomethacin. Relaxation responses to acetylcholine (ACh) and sodium nitroprusside (SNP) were expressed as a percentage of relative to phenylephrine (PE)-induced submaximal constriction. All data are mean ± SEM; n = 6 rats in all groups. aP < 0.01 versus control (CON) group, bP < 0.01 versus diabetic mellitus (DM) group, and cP < 0.05 versus DM group.
Effect of lycopene on MDA content and SOD activity in aorta
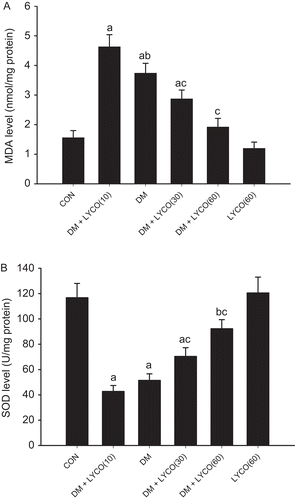
As shown in , diabetic rats had a significant elevation of MDA content (3.26 ± 0.37 nmol/mg protein vs. 2.08 ± 0.21 nmol/mg protein P < 0.01) and decreased SOD activity (3.26 ± 0.37 nmol/mg protein vs. 2.08 ± 0.21 nmol/mg protein P < 0.01) in aorta compared with the normal controls. Chronic treatment with lycopene significantly and dose-dependently attenuated the increased MDA content and reduced SOD activity in aorta (P < 0.01).
Figure 2. Effect of different doses of lycopene on malondialdehyde (MDA) content (A) and superoxidase dismutase (SOD) activity (B) in aorta. All data are mean ± SEM; n = 6 rats in all groups. (A) aP < 0.01 versus control (CON) group, bP < 0.05 versus diabetic mellitus (DM) group, and cP < 0.01 versus DM group. (B) aP < 0.01 versus CON group, bP < 0.05 versus CON group, and cP < 0.01 versus DM group.
Effect of lycopene on NO levels, cNOS activity, and iNOS activity in aorta
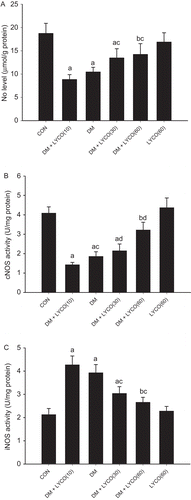
As shown in , compared with the normal controls, diabetic rats had a significant decrease of NO levels (8.83 ± 1.05 μmol/g protein vs. 18.72 ± 2.18 μmol/g protein P < 0.01) and cNOS activity (2.08 ± 0.21 nmol/mg protein vs. 3.26 ± 0.37 nmol/mg protein P < 0.01) in aorta, accompanied by an increase of iNOS activity (4.27 ± 0.38 nmol/mg protein vs. 2.13 ± 0.26 nmol/mg protein P < 0.01). Chronic treatment with lycopene significantly and dose-dependently attenuated the decrease of NO levels and cNOS activity and reduced the increased iNOS activity in diabetic rats (P < 0.01).
Figure 3. Effect of different doses of lycopene on nitric oxide (NO) levels (A), constitutive NOS (cNOS) activity (B), and inducible NOS (iNOS) activity (C) in aorta. All data are mean ± SEM; n = 6 rats in all groups. (A) aP < 0.01 versus control (CON) group, bP < 0.05 versus CON group, and cP < 0.01 versus diabetic mellitus (DM) group. (B) aP < 0.01 versus CON group, bP < 0.05 versus CON group, cP < 0.05 versus DM group, and dP < 0.01 versus DM group. (C) aP < 0.01 versus CON group, bP < 0.05 versus CON group, and cP < 0.01 versus DM group.
Discussion
The present study focused on the role of lycopene on the endothelial dysfunction of diabetic rats. Our results demonstrated that STZ-induced diabetes produced significant impairment on endothelial function, which was coupled with marked increase in oxidative stress and decrease in activity of antioxidant enzyme and NO levels in the aorta. Chronic treatment with lycopene significantly and dose-dependently ameliorated diabetes-related endothelial dysfunction.
Diabetes mellitus have been shown to trigger oxidative stress, with down-regulation of several key antioxides such as SOD in vascular tissues (CitationChen et al., 2008). In the present study, the higher MDA levels and the lower SOD levels in the aorta of the diabetic rats compared with the normal control animals demonstrated the presence oxidative damage and impaired antioxidant capacity. The increased oxidative stress in aorta played a central role in endothelial dysfunction, which has been recognized as a critical and initiating factor in the pathogenesis of diabetic cardiovascular complications (CitationRodríguez-Mañas et al., 2003). Endothelial dysfunction in experimental animal models is commonly demonstrated by impairment of endothelium-dependent vasorelaxation, which has been shown to be associated with a reduction in the release and/or production or abnormal oxidative metabolism of endothelium-derived NO in the vessel wall (CitationSmith et al., 2007; CitationJin et al., 2009; CitationWang et al., 2009). The present study demonstrated that aortic NO levels were significantly reduced in diabetic rats. This result could be attributed to at least three reasons. First, it is consistent with that of the reduced cNOS activity. In rat aortas the main cNOS is eNOS, which is a key enzyme that produces NO in vascular endothelial cells through the catalyzed transformation of l-arginine to l-citrulline (CitationWang et al., 2009). Second, it is consistent with the overproduction of reactive oxygen species (ROS) such as O2−, which could react with NO to form peroxynitrite (CitationMaritim et al., 2003). Third, it is also believed that the uncoupling of NOS by NO reaction with O2− produced O2− rather than NO (CitationQian et al., 2010). All these results might explain the low NO levels in aorta from diabetic rats in spite of the high iNOS activity and support the interaction between NO and endothelial dysfunction resulting from oxidative stress.
Lycopene is a naturally present carotenoid mostly found in tomatoes and tomato products. It is an acyclic isomer of β-carotene containing 11 conjugated and two non-conjugated double bonds arranged in a linear array (CitationBritton, 1995). Due to its high number of conjugated dienes, lycopene is one of the most potent antioxidants among the natural carotenoids, with a singlet-oxygen-quenching ability twice as high as that of β-carotene and 10 times higher than that of α-tocopherol (CitationRiccioni et al., 2008; CitationRiccioni, 2009). In the present study, we demonstrated that chronic lycopene treatment caused a dose-dependent decreased MDA levels as well as increased SOD levels in thoracic aortas. These results outlined the important role of lycopene in the reduction of aortic oxidative stress. Furthermore, owing to the lipophilic nature of lycopene, it is found to concentrate in serum LDL and very-low-density lipoprotein fractions (CitationClinton, 1998), thus protecting LDLs against oxidation. In the recent study, exogenous administration of different doses of lycopene to diabetic rats significantly reduced serum ox-LDL levels. These results indicated that lycopene reduced aortic oxidative stress in part by preventing the oxidization of LDL and thus inhibiting its uptake by the macrophages inside the arterial wall.
The present study revealed that lycopene dose-dependently attenuated impairment of ACh-induced endothelium-dependent vasorelaxation but did not affect SNP-induced endothelial-independent vasorelaxation in the aortas of diabetic rats. These results showed the ameliorative effect of lycopene on diabetes-related endothelial dysfunction. Endothelial dysfunction is a common feature in diabetes characterized by an imbalance between NO and ROS (CitationSchäfer et al., 2008). The underlying cellular and molecular mechanisms associated with diabetes-related endothelial dysfunction include increased breakdown of NO due to augmented production of O2− and an imbalance between reactive oxygen/nitrogen species production and disposal within the microenvironment of the vessels (CitationSena et al., 2008). Thus, antioxidants with singlet-oxygen-scavenging capacity might attenuate endothelial dysfunction through increasing NO production in aortic endothelial cells. In accordance with this hypothesis, we found that lycopene dose-dependently ameliorated diabetes-related endothelial dysfunction in parallel with an increased aortic NO levels. The present study also demonstrated lycopene could dose-dependently attenuate the overexpression of iNOS. iNOS is activated to produce NO and O2− (CitationBardell and MacLeod, 2001). The direct inactivation of NO by O2− could generate the highly reactive molecules and trigger a cascade of events responsible for the pathogenesis of endothelial dysfunction, including endothelium apoptosis and NOS uncoupling (CitationMaritim et al., 2003; CitationQian et al., 2010). Thus, lycopene treatment at varying doses attenuated endothelial dysfunction might also be due to its iNOS inhibitory potential. Moreover, we also demonstrated that this ameliorative effect was likely not affected by supplementation with indomethacin, a cyclooxygenase (COX) inhibitor. These results suggested that the ameliorative effect on endothelial dysfunction induced by lycopene mainly involved the NOS–NO pathway, but not involved the COX–prostaglandin I2 (PGI2) pathway.
Our study also demonstrated that chronic lycopene treatment significantly reduced blood glucose levels in STZ-induced diabetic rats, which is compatible with many previous studies (CitationKuhad et al., 2008; CitationAli & Agha, 2009). The hypoglycemic effect of lycopene might be attributed to several mechanisms, including stimulation of insulin secretion, increasing repair/proliferation of β-cells, enhancing the effect of insulin and adrenaline, and increasing the antioxidative capability (CitationEl-Missiry & El Gindy, 2000; CitationAli & Agha, 2009). CitationAli and Agha (2009) confirmed that lycopene could dose-dependently reduce blood glucose concentration in STZ-induced diabetic rats by promoting repair/proliferation of β-cells. As high glucose has many toxic effects on endothelial cells, such as decreasing NO bioactivity, generating free radicals, and increasing apoptosis (CitationCalles-Escandon & Cipolla, 2001), the major limitation of the present study is that lycopene decreased fasting blood glucose; thus, we cannot rule out that lycopene attenuates endothelial dysfunction due to reducing fasting glucose levels.
In conclusion, we demonstrated that chronic lycopene treatment can decrease oxidative stress and ameliorate endothelial dysfunction in STZ-induced diabetic rats. These results indicated that chronic lycopene treatment might be useful in preventing diabetic vascular complications associated with endothelial dysfunction.
Acknowledgement
We thank Dr. Xiao Zhang for their helpful suggestions.
Declaration of interest
All authors have no financial or other conflict of interests in connection with the submitted article.
References
- Ali MM, Agha FG. (2009). Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest, 69, 371–379.
- Bardell AL, MacLeod KM. (2001). Evidence for inducible nitric-oxide synthase expression and activity in vascular smooth muscle of streptozotocin-diabetic rats. J Pharmacol Exp Ther, 296, 252–259.
- Britton G. (1995). Structure and properties of carotenoids in relation to function. FASEB J, 9, 1551–1558.
- Cai H, Harrison DG. (2000). Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res, 87, 840–844.
- Calles-Escandon J, Cipolla M. (2001). Diabetes and endothelial dysfunction: A clinical perspective. Endocr Rev, 22, 36–52.
- Chen YS, Zhu XX, Zhao XY, Xing HY, Li YG. (2008). Hemin, a heme oxygenase-1 inducer, improves aortic endothelial dysfunction in insulin resistant rats. Chin Med J, 121, 241–247.
- Clinton SK. (1998). Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev, 56, 35–51.
- Coccheri S. (2007). Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs, 67, 997–1026.
- El-Missiry MA, El Gindy AM. (2000). Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann Nutr Metab, 44, 97–100.
- Gerster H. (1997). The potential role of lycopene for human health. J Am Coll Nutr, 16, 109–126.
- Institute for Laboratory Animal Research. (1996). Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press.
- Jin BH, Qian LB, Chen S, Li J, Wang HP, Bruce IC, Lin J, Xia Q. (2009). Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur J Pharmacol, 616, 200–205.
- Kuhad A, Sethi R, Chopra K. (2008). Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci, 83, 128–134.
- Lu J, Zhu SM, Zang WJ, Xu XL, Luo HL, Yu XJ, Wang SP, Kong SS, Wu J, Horie M, Sun L. (2007). Protective mechanism of adenosine to the rat arterial endothelial dysfunction induced by hydrogen peroxide. Biol Pharm Bull, 30, 1206–1211.
- Maritim AC, Sanders RA, Watkins JB 3rd. (2003). Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol, 17, 24–38.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Qian LB, Wang HP, Chen Y, Chen FX, Ma YY, Bruce IC, Xia Q. (2010). Luteolin reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol Res, 61, 281–287.
- Rao AV, Rao LG. (2007). Carotenoids and human health. Pharmacol Res, 55, 207–216.
- Riccioni G. (2009). Carotenoids and cardiovascular disease. Curr Atheroscler Rep, 11, 434–439.
- Riccioni G, Mancini B, Di Ilio E, Bucciarelli T, D’Orazio N. (2008). Protective effect of lycopene in cardiovascular disease. Eur Rev Med Pharmacol Sci, 12, 183–190.
- Rodríguez-Mañas L, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer A, Cercas E, López-Dóriga P, Sánchez-Ferrer CF. (2003). Early and intermediate Amadori glycosylation adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia, 46, 556–566.
- Schäfer A, Fraccarollo D, Pförtsch S, Flierl U, Vogt C, Pfrang J, Kobsar A, Renné T, Eigenthaler M, Ertl G, Bauersachs J. (2008). Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br J Pharmacol, 153, 886–893.
- Sena CM, Nunes E, Louro T, Proença T, Fernandes R, Boarder MR, Seiça RM. (2008). Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol, 153, 894–906.
- Smith JM, Sondgeroth KB, Wahler GM. (2007). Inhibition of nitric oxide synthase enhances contractile response of ventricular myocytes from streptozotocin-diabetic rats. Mol Cell Biochem, 300, 129–137.
- Wang C, Li J, Lv X, Zhang M, Song Y, Chen L, Liu Y. (2009). Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol, 620, 131–137.
