Abstract
Context: The genus Veronica L. (Plantaginaceae) is represented by 79 species, 26 of which are endemic in Turkey. Some Veronica species are used for the treatment of different inflammatory diseases and cancer in traditional medicine. In addition, chemotaxonomy of the genus is important for the reclassification of the family Plantaginaceae after different phylogenetic studies.
Objective: Veronica cuneifolia subsp. cuneifolia D. Don and V. cymbalaria Bodard were studied from the view point of iridoid glucosides which are known as chemotaxonomical markers for this genus. Radical scavenging and cytotoxic activities of the extracts were also determined in this study.
Material and methods: Major compounds, isolated from iridoid fractions of V. cuneifolia subsp. cuneifolia were used as the standard compounds for HPLC after determination of their structures, and investigated for their presence in iridoid fractions of V. cymbalaria. Additionally, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and SO radical scavenging and cytotoxic activities against three cancer and a noncancerous cell lines of both extract were also tested using the MTT method.
Results: While aucubin, catalpol, verproside, amphicoside, verminoside, and veronicoside were obtained from V. cuneifolia subsp. cuneifolia, two more iridoid glucosides, 6-O-veratroylcatalposide and 6-O-isovanilloylcatalpol, were isolated from V. cymbalaria. Comparing both species, V. cuneifolia subsp. cuneifolia showed stronger radical scavenging and cytotoxic activities than V. cymbalaria.
Discussion: Our results demonstrated that the iridoid contents of both species were very close to each other confirming to the chemotaxonomic studies on Veronica species and their different bioactivity range make the plants interesting from the view point of natural drug discovery research.
Introduction
The genus Veronica L. has traditionally been considered a member of Scrophulariaceae family. However, recent extensive molecular investigations of this and related families have demonstrated that traditional Scrophulariaceae is polyphyletic (CitationOlmstead & Reeves, 1995; CitationOlmstead et al., 2001; CitationOxelman et al., 2005). As a consequence of these results, the genus Veronica has been transferred to the Plantaginaceae (CitationAlbach et al., 2005; CitationOlmstead, 2005). Recently, it has been shown that the iridoid glucosides aucubin, catalpol, and 6-O-catalpol esters are characteristic for this genus and chemotaxonomic research concerning iridoid content of the genus have great importance for the reclassification of the close genera (Harput et al., Citation2002a, Citation2002b; CitationTaskova et al., 2004; CitationJensen et al., 2005).
The genus Veronica is represented by 79 species, 26 of which are endemic in Turkish flora (CitationFisher, 1978). Some Veronica species are used as diuretics, for wound healing and against rheumatic pains in traditional medicine in Turkey (CitationBaytop, 1984; CitationFujita et al., 1995). In addition, several Veronica species are used to treat cancer, influenza, hemoptysis, laryngopharyngitis, hernia, cough, and respiratory diseases, and are also used as an expectorant and antiscorbutic (CitationTomassini et al., 1995; CitationSu et al., 1999; Grahamet et al., 2000). Earlier investigations performed on Veronica species resulted in the isolation mainly of iridoid glucosides, especially benzoic and cinnamic acid esters of catalpol, some phenylethanoid, and flavonoid glycosides (CitationChari et al., 1981; CitationTaskova et al., 1998; Harput et al., Citation2002a, Citation2002b; CitationSaracoglu et al., 2002; CitationJensen et al., 2005). In this study, Veronica cuneifolia subsp. cuneifolia D. Don and V. cymbalaria Bodard were investigated from the view point of iridoid glucosides together with their radical scavenging and cytotoxic activities.
Material and methods
Plant material
Veronica cuneifolia subsp. cuneifolia D. Don from Antalya, Akseki, and V. cymbalaria Bodard was collected from Şanlıurfa, Turkey in June, 1999 and 2006, respectively. Both plants were identified by Prof. Dr. H. Duman from Gazi University, Ankara, Turkey. A voucher specimen has been deposited in the Herbarium of the Faculty of Pharmacy, Hacettepe University, Ankara, Turkey (HUEF 06006; HUEF 99131).
General
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide (MTT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitro blue tetrazolium (NBT), ascorbic acid were obtained from Sigma-Aldrich Chem Co (St. Louis, MO). 3-t-butyl-4-Hydroxyanizole (BHA) was purchased from Nacalai Tesque Co. (Kyoto, Japan). Fetal bovine serum (FBS) and minimal essential medium with Earl’s salts (MEM-EARLE) with nonessential amino acids and antibiotics (penicillin and streptomycin) were purchased from Biochrom AG (Berlin, Germany). Trypsin (1:250) was obtained from Biochrom KG (Berlin, Germany). Media were supplemented with 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. Hep-2 (human larynx epidermoid carcinoma), RD (human rhabdomyosarcoma), L-20B (transgenic murine cells) and VERO (African Green Monkey kidney cells) were provided by Refik Saydam Hygiene Center, Virology Laboratory, Ankara, Turkey.
Preparation of the aqueous extract and isolation of the compounds from V. cuneifolia subsp. cuneifolia
The air-dried aerial parts of the plant (30 g) were extracted with methanol (MeOH) at 40°C for 12 h (3 × 300 mL). The MeOH phases were evaporated under vacuum to give MeOH extract (6.31 g). MeOH extracts was dissolved in water and partitioned with petroleum ether to remove chlorophylls. The water fraction was lyophilized to yield 5.97 g dry weight. Water extract was tested for cytotoxic and radical scavenger activities. Remaining extract was subjected to polyamide column chromatography eluted with H2O, followed by increasing concentrations of MeOH to give five main fractions: MeOH 0% A; MeOH 25% B; MeOH 50% C; MeOH 75% D; MeOH 100% E. Fr. A consisted of 6 subfractions. VLC of Fr. A2 using Lichroprep C18, eluting with increasing concentration of MeOH (0% → 100%), gave compounds 1 and 2 in pure form. Similarly, VLC of Fr. A5 in same conditions was resulted to the isolation of 3, 4 and 5, purely. Fr. C consisted of three subfractions and Sephadex column chromatography of Fr. C1 using MeOH gave compound 6 in pure form. Compound 7 was determined from the HPLC chromatograms comparing its retention time with that of authentic sample.
Preparation of the aqueous extract and the isolation of compounds from V. cymbalaria
Dried aerial parts of V. cymbalaria (10 g) were extracted with MeOH. Five main polyamide fractions were obtained from the aqueous extract (3.75 g) of the plant as the same conditions of V. cuneifolia subsp. cuneifolia. Iridoid fraction of V. cymbalaria was subjected to analytical RP-HPLC analysis to determine iridoid composition of the plant using compounds 1-7 as standard compounds. As a result of silica gel column chromatography of Fr. A3 which was determined to contain different structures, compounds 8 and 9 were isolated using CHCl3:MeOH (100:0→85:15) as solvent system.
DPPH radical scavenging effect
The DPPH radical scavenging effect of the aqueous extract of V. cuneifolia subsp. cuneifolia and V. cymbalaria were assessed by the decoloration of MeOH solution of DPPH spectroscopically; butyl-4-hydroxyanisole (BHA) and ascorbic acid were used as reference compounds. MeOH solution (100 µL) of the samples at various concentrations were added to DPPH/MeOH (1 mM) solution. The reaction mixture was shaken vigorously and the absorbance of remaining DPPH was measured at 520 nm after 30 min. The radical scavenging activity was determined by comparing the absorbance with that of blank (100%) containing only DPPH and solvent. All the analyses were done in 3 replicates (CitationHatano et al., 1989; Harput et al., Citation2002a; CitationKostadinova et al., 2007; CitationJensen et al., 2010).
Superoxide radical scavenging effect by alkaline DMSO method
The method of CitationElizabeth and Rao (1990) was used for the detection of superoxide radical scavenging activity of the extract with slight modification. Briefly, a superoxide radical was generated in a nonenzymatic system. The reaction mixture containing 10 µL of NBT (1 mg/mL solution in DMSO) and 30 µL of the extract or reference compounds were dissolved in DMSO. Alkaline DMSO (100 µL of a1 mL DMSO containing, 5 mM NaOH in 0.1 mL water) was added to give a final volume of 140 µL and the absorbance was measured at 560 nm using microplate reader (CitationElizabeth & Rao, 1990; CitationSrinivasan et al., 2007; CitationJensen et al., 2010).
Cytotoxic activity
Cell suspensions (0.1 mL) of cancer cells were seeded into 96-multiwell plates at the concentration of 2 × 105 cells/mL for Hep-2, 1 × 105 cells/mL for RD, L-20B and VERO cells and cultured for 24 h in cell culture media in a humidified 5% CO2 incubator at 37°C. Cells were incubated with various concentrations of the test samples for 48 h (800, 400, 200, 100, 50, 20, 10 µg/mL). After incubation, the cells were washed and replaced by fresh medium. MTT solution (10 µL of 5 mg/mL in PBS) was added and incubated for 4 h. Following the incubation, 100 µL of 10% SDS (sodium dodecyl sulfate) was added to each well to dissolve formed formazan crystals. The absorbance was measured at 577/655 nm using microplate reader (CitationMossman 1983; Harput et al., Citation2002a).
Results
The MeOH extract of V. cuneifolia subsp. cuneifolia was suspended in water and partitioned with petroleum ether. The water fraction of the MeOH extract was subjected to polyamide column chromatography to afford five main fractions. Repeated column chromatography (RP, silica gel, Sephadex LH-20) of the fractions which eluted with water from the polyamide column, resulted in the isolation of 6 compounds (1-6) in pure form and a minor iridoid glucoside (7) was determined by HPLC chromatograms comparing its retention time and UV spectrum with that of authentic sample. After structure determination of the isolated compounds, they were used as standard compounds for iridoid fractions of V. cymbalaria. Two more compounds (8 and 9) found in addition to 7 compounds from the HPLC analysis of V. cymbalaria and isolated from V. cymbalaria using different chromatographic techniques ().
Figure 1. Structures of compounds 1–9 isolated from V. cuneifolia subsp. cuneifolia and V. cymbalaria.
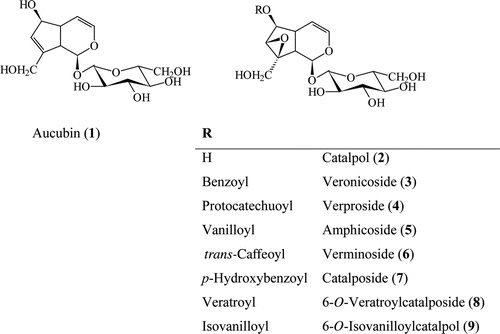
Compounds 1-6 were isolated as colorless amorphous compounds. Their TLC characteristics, UV and IR spectra were suggested iridoid structures for these compounds. Absorption band at 202 nm in their UV spectra was characteristic for their enol-ether systems and indicated to the presence of nonsubstituted C-4 position for the compounds. The HR ESI-MS of 1 exhibited a pseudomolecular ion peak [M+Na]+ and [2M+Na]+ at m/z 369.1168 and 715.2454 suggesting the molecular formula C15H22O9 which was confirmed by the observation of two methylene (CH2), 12 methyn (CH) and a quaternary carbon resonances in its 13C and DEPT spectra.
Anomeric proton and carbon signal at δH 4.67 (d, J = 7.9 Hz, H-1′) and δC 99.98 (CH, C-1′) together with other signals in the region of δH 3.21-3.85 indicated the presence of glucose unit (-2). β-Anomeric configuration of the glucose was judged based on the large 3JH-1,H-2 coupling constant of the anomeric proton (J = 7.9 Hz). When 6 glucose signals removed from total C signals in 13C NMR spectra, remaining 9 C signals suggested to the cyclopentan-pyran iridoid skeleton for the aglycon. H-1 acetal signal at δH 4.95 (1H, d, J = 7.3 Hz) correlated to δH 2.89 (1H, t, J = 7.5 Hz, H-9) and H-9 proton signal at δH 2.89 correlated to δH 2.65 (1H, m, H-5) in COSY spectrum. COSY correlations between H-5 (δH 2.65) to δH 5.09 (1H, dd, J = 6.1/4.0 Hz, H-4) and 4.43 (1H, m, H-6); H-4 (δH 5.09) to δH 6.30 (1H, dd, J = 6.2/2.0 Hz, H-3) confirmed the presence of double bond between C-3 and C-4. Additional COSY correlations from H-6 (δH 4.43) proton signal to δH 5.76 (1H, t, J = 1.5 Hz, H-7) and triplet H-9 signal indicated the quaternary C-8 signal and second double bound between C-7 and C-8. The position of hydroxymethyl group at C-8 was confirmed from the HMBC correlation of H2-10/C-8, H2-10/C-7 and H1-10/C-9. In addition, H-1′′/C-1 correlation in HMBC spectrum showed us to a β-glucose unit at C-1 position. From these results structure of compound 1 was determined as aucubin and confirmed with the comparison of its spectral data with the previously published data for aucubin (CitationAkdemir & Calis, 1991). Similar UV, IR, 1H and 13C NMR spectra of compound 2 with 1 suggested to 4 nonsubstituted iridoid glucoside structure for 2. Its pseudomolecular ion peaks at 385.1110 [M + Na]+ and 747.2344 [2M + Na]+ in HR ESI-MS spectrum and C signals in 13C NMR and DEPT spectra suggested to molecular formula of 2 as C15H22O10. 1H NMR and 13C NMR spectra of compound 1 and 2 were found to be similar to each other. COSY correlations of δH 3.90 (1H, H-6) to oxymethyn at δH 3.44 (1H, d, J = 0.9 Hz, H-7), and no other correlations of H-7 make the main difference of two compounds. Two oxymethyn carbon signals at δC 79.57 and 62.52 for C-6 and C-7 position in 13C-NMR spectrum and comparison of these data with catalpol and catalpol derivatives confirmed the presence of epoxide function between C-7 and C-8 (CitationBianco et al., 1983). As no difference was observed other than epoxide function, the structure of compound 2 was determined as catalpol with the comparison of its spectral data with previously published articles (CitationChaudhuri & Sticher, 1981; CitationAkdemir & Calis, 1991; CitationOzipek et al., 2000).
Table 1. 13C NMR data of isolated compounds (CD3OD; 125 MHz).
The UV and IR spectra of compounds 3-6 were similar to those of compound 1 and 2. However, the signals at 233 and 274 nm in their UV spectra for aromatic ring and IR absorption bands at 3410 cm−1 for hydroxyl, 1640 cm−1 for olephinic double bounds, ester carbonyl at 1705 cm−1 and aromatic signals at 1515–1600 cm−1 indicated to aromatic esterification for these compounds. C-1 monoglucosidic catalpol structure for compounds 3-6 was confirmed from the comparison of their spectral data with compound 2, catalpol. The difference of compounds 3-6 to catalpol was found from the aromatic rings and ester carbonyl signals. Of particular interest were downfield-shifted resonances of C-6 and H-6 (δH 5.03–5.17, H-6 and δC 81.37–82.12, C-6) suggested that esterification was placed at C-6. The position of ester moiety was unambiguously determined by HMBC experiment from the correlations between H-6 and carbonyl (C=O) resonances. Detailed examination of aromatic resonances of compounds 3-6 from COSY and HMQC experiments led to the finding that esterified acids were benzoic, protocatechuic, vanillic, and trans-caffeic acids, HMBC correlations and comparison of previously published data for compounds 3-6 were confirmed their structures as benzoylcatalpol (veronicoside [3]), protocatechuoylcatalpol (verproside [4]), vanilloylcatalpol (amphicoside [5]), trans-caffeoylcatalpol (verminoside [6]; CitationSticher & Afifi-Yazar, 1979a, Citation1979b; CitationAfifi-Yazar & Sticher, 1980; CitationLahloub, 1983). In addition to these compounds, one more compound was observed in the HPLC chromatogram of the polyamide fractions. It was determined as p-hydroxybenzoylcatalpol (catalposide [7]) from its HPLC chromatograms comparing time and UV spectra with that of authentic sample which was previously isolated from Veronica species ().
After structure determination of compounds 1-7, they were used as standard compounds for HPLC analysis of iridoid fractions of V. cymbalaria. Two more compounds were detected in addition to 7 compounds from the HPLC analysis of V. cymbalaria in 40% MeOH:(H2O + 1% H3PO4) system. These two compounds (8 and 9) were isolated from V. cymbalaria using different chromatographic techniques. As a result of detailed examination of UV, IR, and NMR spectra of these compounds ( and ) and comparison of their spectral data with those of compounds 1-6, their structures were determined as 6-O-veratroylcatalposide (8; CitationOzipek et al., 2000) and 6-O-isovanilloylcatalpol (9; ; CitationSticher & Afifi-Yazar, 1979b). Although catalpol derivatives are well known compounds for genus Veronica, their NMR data has not been available in recent publications. Detailed 1H and 13C NMR data of aucubin and catalpol derivatives in and given by us could be considered to be helpful for the researcher working with these iridoid glucosides.
Table 2. 1H NMR data of isolated compounds (CD3OD; 500 MHz).
In addition to the phytochemical studies, the aqueous extracts of V. cuneifolia subsp. cuneifolia and V. cymbalaria were evaluated for their radical scavenging and cytotoxic activities. Cytotoxic activity of the aqueous extracts was tested against three different cancer cells and one non-cancerous cell line. 2 × 105 cells/mL cells for Hep-2, 1 × 105 cells/mL for L-20B, RD and VERO cell lines were used for the assay and incubated for 48 h with various concentrations of the extracts. After incubation, viability was determined by the MTT method. Both extracts showed cytotoxic activity against Hep-2, RD, L-20B and non-cancerous VERO cell lines. Maximum effect was observed in 800 µg/mL concentration of the extracts for three of the cancer cell lines (). While V. cuneifolia subsp. cuneifolia showed higher cytotoxicity than V. cymbalaria, their cytotoxicity against VERO cell line found to be lower than tested cancer cells (). Although, both extracts showed moderate cytotoxic activity against Hep-2, RD and L-20B cell lines, different activity range against different cells indicated the selective effect of the extracts.
Table 3. Cytototoxic and radical scavenging activities of V. cuneifolia subsp. cuneifolia (Vcc) and V. cymbalaria (Vc).
Figure 2. Cytotoxic activity of V. cuneifolia subsp. cuneifolia (Vcc) and V. cymbalaria (Vc)aqueous extract (800 µg/mL) against different cancer cell lines using the MTT assay.
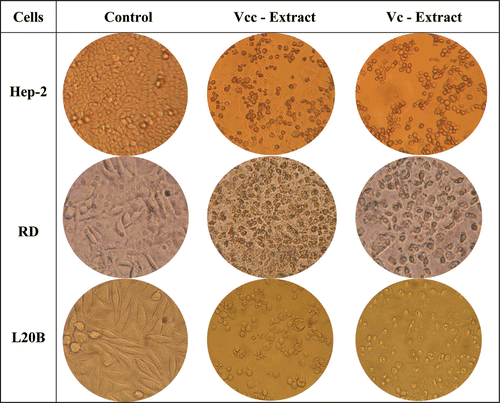
Similarly, DPPH and SO radical scavenging activities of the aqueous extracts and the reference compounds were tested and V. cuneifolia subsp. cuneifolia was found to show higher scavenging activity than V. cymbalaria against tested radicals. However, both extracts showed very low scavenging activity comparing to reference compounds, BHA and AA (IC50 < 10 µg/mL; ). Different cytotoxic activity against cancer cell lines and scavenging activity of V. cuneifolia subsp. cuneifolia led us to focus on phytochemical and biological studies on V. cuneifolia subsp. cuneifolia. In our previous research, V. cymbalaria, V. hederifolia, V. pectinata var. glandulosa, V. persica, and V. polita were found to be cytotoxic against KB epidermoid carcinoma and B16 melanoma cell lines. Similar cytototoxic activity of V. cymbalaria against KB, B16, Hep-2, RD, and L20B cell lines indicated a wide cytotoxic activity spectrum for Veronica species.
Discussion
In our continuous research on the genus Veronica, we present here radical scavenging and cytotoxic activities of the aqueous extracts of V. cuneifolia subsp. cuneifolia and V. cymbalaria together with their iridoid contents for the first time in this study. While aucubin, catalpol, verminoside, verproside, amphicoside, and catalposide were obtained from V. cuneifolia subsp. cuneifolia, two more iridoid glucosides, 6-O-veratroylcatalposide and 6-O-isovanilloylcatalpol were isolated from V. cymbalaria. Comparing both species, V. cuneifolia subsp. cuneifolia showed stronger radical scavenging and cytotoxic activities than V. cymbalaria for all tested concentrations. Cytotoxic activities of aucubin, catalpol, and catalpol derivatives isolated from Veronica species were also determined against Hep-2, RD, and L-20B cell lines, previously (CitationSaracoglu et al., 2008; CitationSaracoglu & Harput-Hudaverdi, 2009). Verminoside (6), the trans-caffeic acid ester of catalpol, showed very strong cytotoxicity against tested cell lines. While cytotoxic activity of amphicoside (5) and veronicoside (3) found lower than that of verminoside, 6-O-veratroylcatalposide and verproside were found to have cytostatic activity. Catalpol and aucubin did not show any cytotoxic activity against three of the tested cell lines (CitationSaracoglu et al., 2008; CitationSaracoglu & Harput-Hudaverdi, 2009). Our previous results are in good agreement with present results and could be good explanation of the cytototoxic activity of V. cuneifolia subsp. cuneifolia and V. cymbalaria extracts. However, our results were also evaluated for the chemotaxonomic research on the genus Veronica and iridoid glucosides were aimed to research in this study because of their chemotaxonomic importance for the reclassification of the Plantaginaceae family (CitationJensen et al., 2005). According to CitationTaskova et al. (1998, Citation2004) the genus Veronica consists of four subsections: Chamaedrys, Alsinebe, Beccabunga, and Veronicastrum and 4-substituted iridoid glucosides were not isolated from the subsections Chamaedrys (A1-2) and Alsinebe (F-G) up to now. Our results are in good correlation with these results with the absence of 4-substituted iridoid glucosides in V. cuneifolia subsp. cuneifolia (subsection Chamaedrys) and V. cymbalaria (subsection Alsinebe).
Conclusion
The results of this study give an insight to the chemotaxonomic and bioactivity research on the genus Veronica which is widely distributed and used for the treatment of different diseases in Turkey and the world. These species together with other Veronica species from different subsections should be investigated in detail both for their chemical constituents and different bioactivities for the future studies.
Acknowledgments
The authors are grateful to Prof. Dr. H. Duman of the Faculty of Sciences, Gazi University, Ankara, Turkey, for the authentication of the plant specimen.
Declaration of interest
This study was financially assisted by Hacettepe University Research Foundation (No: 0202301007; 0302301010) and TUBITAK (No: 108T518).
References
- Afifi-Yazar FU, Sticher O. (1980). Verproside, a new iridoid glucoside from Veronica officinalis L. (Scrophulariaceae). Helv Chim Acta, 63, 1905–1907.
- Akdemir Z, Calis I. (1991). Iridoid and phenylpropanoid glycosides from Pedicularis pontica. DOĞA-Tr. J Pharmacy, 1, 65–75.
- Albach DC, Meudt HM, Oxelman B. (2005). Piecing together the “new” Plantaginaceae. Am J Bot, 92, 297–315.
- Baytop T. (1984). Therapy with Medicinal Plants in Turkey (Past and Present) No: 3255. Publications of Istanbul University, Istanbul, p. 423.
- Bianco A, Passacantilli P, Polidori G. (1983). NMR spectroscopy of epimeric pairs of glucosidic iridoids. Gaz Chim Ital, 113, 829–834.
- Chari VM, Grayer-Barkmerjier RJ, Harborne JB, Osterdahl BG. (1981). An acylated allose-containin 8-hydroxyflavone glycoside from Veronica filiformis. Phytochemistry, 20, 1977–1979.
- Chaudhuri RK, Sticher O. (1981). New iridoid glucosides and a lignan diglucoside from Globularia alypum L. Helv Chim Acta, 64, 3–15.
- Fisher MA. (1978). Veronica L. In: Davis PH, ed. Flora of Turkey and East Aegean Islands, Vol. 6. University Press, Edinburgh, pp. 689–753.
- Elizabeth K, Rao MNA. (1990) Oxygen radical scavenging activity of curcumin. Int J Pharmacol, 58, 237–240.
- Fujita T, Sezik E, Tabata M, Yesilada E, Honda G, Takeda Y, Tanaka T, Takaishi Y. (1995). Traditional medicine in Turkey VII. Folk medicine in Middle and West Black Sea regions. Econ Bot, 49, 406–422.
- Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. (2000). Plants used against cancer - an extension of the work of Jonathan Hartwell. j Ethnopharmacol, 73, 347–377.
- Harput US, Saracoglu I, Inoue M, Ogihara Y. (2002a). Anti-inflammatory and cytotoxic activities of five Veronica species. Biol Pharm Bull, 25, 483–486.
- Harput US, Saracoglu I, Inoue M, Ogihara Y. (2002b). Phenylethanoid and iridoid glycosides from Veronica persica. Chem Pharm Bull, 50, 869–871.
- Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T (1989) Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull, 37, 2016–2021.
- Jensen SR, Albach DC, Ohno T, Grayer RJ. (2005). Veronica: Iridoids and cornoside as chemosystematic markers. Biochem Syst Ecol, 33, 1031–1047.
- Jensen SR, Gotfredsen CH, Harput US, Saracoglu I. (2010). Chlorinated iridoid glucosides from Veronica longifolia and their antioxidant activity. J Nat Prod, 73, 1593–1596.
- Kostadinova EP, Alipieva KI, Kokubun T, Taskova RM, Handjieva NV. (2007). Phenylethanoids, iridoids and a spirostanol saponin from Veronica turrilliana. Phytochemistry, 68, 1321–1326.
- Lahloub MF. (1983). Isolierung, Charakterisierung und Strukturaufklarung von Glykosiden Einiger Veronica-Arten (Scrophulariaceae). (Doctoral Dissertation). ETH Nr. 7340, Zürich.
- Mossman T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immun Met, 65, 55–63.
- Olmstead RG, Reeves PA. (1995). Polyphyletic origin of the Scrophulariaceae: Evidence from rbcL and ndhF sequences. Ann Mo Bot Gard, 82, 176–193.
- Olmstead RG. (2005). Asynoptical classification of the Lamiales e-version 2.0. http://depts.washington.edu/phylo/classifications/Lamiales.html
- Olmstead RG, dePamphilis CW, Wolfe AD, Young ND, Elisons WJ, Reeves PA. (2001). Disintegration of the Scrophulariaceae. Am j Bot, 88, 348–361.
- Oxelman B, Kornhall P, Olmstead RG, Bremer B. (2005). Further disintegration of Scrophulariaceae. Taxon, 54, 411–426.
- Ozipek M, Saracoglu I, Calis I, Kojima K, Ogihara Y. (2000). Catalpol derivative iridoids from the roots of Veronica multifida. Hacettepe University, Journal of Faculty of Pharmacy, 20, 1–6.
- Saracoglu I, Harput-Hudaverdi US. (2009). In vitro Anticancer Activity and Structure-Activity Relationships of Iridoid Glucosides, 9th International Symposium on Pharmaceutical Sciences (ISOPS-9), Ankara, June 23–26.
- Saracoglu I, Harput US, Inoue M, Ogihara Y. (2002). New phenylethanoid glycosides from Veronica pectinata var. glandulosa and their free radical scavenging activities. Chem Pharm Bull, 50, 665–668.
- Saracoglu I, Harput-Hudaverdi US, Nagatsu A, Varel M. (2008). Cytotoxic Activity and Structural Aspects of Iridoids Isolated from Veronica thymoides subsp. pseudocinerea, Natural Products with Pharmaceutical, Nutraceutical, Cosmetic, and Agrochemical Interest, 7th Joint Meeting of AFERP, ASP, GA, and PSE (49th Annual Meeting of the American Society of Pharmacognosy), Athens (August 2008). Planta Med, 74, 1046.
- Srinivasan R, Chandrasekar MJ, Nanjan MJ, Suresh B. (2007). Antioxidant activity of Caesalpinia digyna root. J Ethnopharmacol, 113, 284–291.
- Sticher O, Afifi-Yazar FU. (1979a). Minecoside and verminoside, two new iridoid glucosides from Veronica officinalis L. (Scrophulariaceae). Helv Chim Acta, 62, 535–539.
- Sticher O, Afifi-Yazar FU. (1979b). Veronicoside, a new iridoid glucoside from Veronica officinalis L. (Scrophulariacea). Helv Chim Acta, 62, 530–534.
- Su B, Zhu Q, Jia Z. (1999). Aquaticol, a novel bis-sesquiterpene from Veronica anagallis-aquatica. Tetrahedron Lett, 40, 357–358.
- Taskova R, Handjieva N, Peev D, Popov S. (1998). Iridoid glucosides from three Veronica species. Phytochemistry, 49, 1323–1327.
- Taskova RM, Albach DC, Grayer RJ. (2004). Phylogeny of Veronica–a combination of molecular and chemical evidence. Plant Biol (Stuttg), 6, 673–682.
- Tomassini L, Brkic D, Serafini M, Nicoletti M. (1995). Constituents of Veronica hederifolia and Veronica polita. Fitoterapia 66, 382.
