Abstract
Context: The galls of Terminalia chebula Retz. (Combretaceae) frequently appear in many Thai Lanna medicinal plant recipes for promotion of longevity.
Objective: The objective of this study was to evaluate the skin anti-aging of gel containing niosomes loaded with a semi-purified fraction containing gallic acid from T. chebula galls.
Method: The semi-purified fraction containing phenolic compounds including gallic acid isolated from T. chebula galls loaded in non-elastic or elastic niosomes, and its developed gel, were evaluated for rabbit skin irritation by the closed patch test and skin anti-aging in human volunteers by measuring skin elasticity and roughness.
Results: Gel containing the fraction unloaded (SS) or loaded in non-elastic (SN) or elastic (SE) niosomes and gallic acid loaded in non-elastic (GN) or elastic (GE) niosomes showed no skin irritation, whereas the unloaded gallic acid (GS) gave the irritation in rabbit’s skin by the closed patch test. The % parameter changes of skin elastic recovery and skin elastic extension when applied with SN and SE gels were +28.73 and +32.57; −21.25 and −22.63%, respectively. SN and SE gel also showed a significant decrease of the maximum and average roughness values with the parameter changes of −29.43 and −32.38; −39.47 and −35.28%, respectively.
Conclusion: The semi-purified fraction loaded in niosomes indicated not only higher chemical stability of gallic acid containing in the fraction, but also more in vivo anti-aging activities than the unloaded fraction when incorporated in gel.
Introduction
As aging occurs, the skin shows many changes including more winkles and pigmentation with less moisture and becomes sagged because the skin loses elasticity (CitationTakema et al., 1994; CitationTsukahara et al., 2000). Elastic fibers play an important role in maintaining the elasticity of the skin. Free radicals and solar ultraviolet (UV) exposure induces the expression of elastase enzyme that can degenerate elastic fibers. The degeneration of elastic fibers leads to the decrease of skin elasticity, which in turn results in wrinkle formation (CitationTsuji et al., 2001; CitationTsukahara et al., 2001). In addition, free radicals which are highly reactive molecules with the unpaired electrons can cause damage to cell membranes, lipids, proteins, and DNA resulting in collagen breakdown. UV radiation and progressive age impair the synthesis of collagen and induce the expression of matrix metalloproteinases (MMPs) (CitationBerneburg et al., 2000; CitationBrenneisen et al., 2002; CitationBrennan et al., 2003). MMPs are a large family of zinc-dependent endoproteases with a broad range of substrate specificities that are capable of degrading all extracellular matrix proteins (CitationKim et al., 2008). MMP-2 (gelatinase A) digests native collagen types I, II and III in a similar manner to the collagenases (CitationAimes & Quigley, 1995; CitationPatterson et al., 2001). An increase of MMP-2 expression has been reported to involve with collagen degradation in aged human skin (CitationSteinbrenner et al., 2003). Thus, antioxidants and MMP-2 inhibitors that sustain skin elasticity by delaying skin collagen degradation can be used in anti-aging products.
In the recent years, Terminalia chebula gall extract containing phenolic compounds including gallic acid, punicalagin, isoterchebulin, 1,3,6-tri-O-galloyl-β-d-glucopyranose, chebulagic acid and chebulinic acid have been demonstrated to have in vitro anti-aging ability (Manosroi et al., Citation2010a, Citation2010b). The extract and its phenolic compounds have shown superior DPPH radical scavenging, Fe2+ chelating, mushroom tyrosinase enzyme inhibition as well as anti-melanogenesis on B16 murine melanoma activities indicating their potential for anti-aging application (Manosroi et al., Citation2010a, Citation2010b, Citation2011). However, the extracts or phenolic compounds from T. chebula galls have never been investigated for in vivo anti-aging activities, such as skin roughness, hydration, elastic and whitening effect. One of the major problems of phenolic compounds is the deterioration of their activities owing to their chemical instability during long exposure to air, light and temperature (CitationBoles et al., 1988). As known, nanovesicles including liposomes and niosomes have been applied to improve stability of many degradable active agents (CitationSahin, 2007). Niosomes that are surfactant-based nanovesicles and were first reported in the 1970s have more advantages than liposomes to be used as carriers in cosmetics (CitationPardakhty et al., 2007). They are bilayer vesicles formed from self assembly of non-ionic surfactants such as Tween 61 and Span 60 in an aqueous media. They can entrap both water and oil soluble substances similar to liposomes, but with lower cost and more chemical stability than liposomes. The enhancement of physical and chemical stability of many antioxidants and drug such as diclofenac diethylammonium (DCFD) (CitationManosroi et al., 2008), protein such as gallidermin (Manosroi et al., Citation2010c) as well as DNA such as luciferase plasmid (CitationManosroi et al., 2009, Citation2010d) when entrapped in niosomes has been demonstrated.
Several researchers have developed novel elastic vesicles in order to deeply and easily penetrate through the skin. The elastic niosomes are the most recent novel elastic nanovesicles which have been developed (CitationManosroi et al., 2008). Ethanol that is known as an efficient permeation enhancer has been added at 20–45% as well as several edge activators (sodium cholate, Span 80, Tween 80 and oleic acid) at 10–25% in the niosomal formulation to prepare the elastic niosomes. These edge activators are a single-chain molecule with a high radius of curvature that destabilize the lipid bilayers of the vesicles and increase the deformability of the vesicles, whereas ethanol can interact with the polar head group region of the lipid molecules resulting in the reduction of the melting point of the stratum corneum lipid, thereby increasing lipid fluidity, and cell membrane permeability. The high flexibility of the vesicular membranes from adding ethanol or edge activators permits the niosomes to squeeze themselves through the pores which are much smaller than their diameters. Thus, elastic niosomes could overcome the limitation of low penetration ability of the non-elastic niosomes across the skin. The enhancements of transdermal absorption through rat skin of DCFD, luciferase (pLuc) and tyrosinase plasmid (pMEL34) when entrapped in non-ionic and cationic elastic niosomes were observed (CitationManosroi et al., 2008, Citation2009, Citation2010d).
In this present study, in vitro DPPH radical scavenging and MMP-2 inhibition activities as well as the in vivo performance test in human volunteers of 8-week application including skin roughness, hydration, elasticity and whitening effect of the developed gel formulations incorporated with the semi-purified fraction containing phenolic compounds isolated from T. chebula galls loaded in non-elastic and elastic niosomes were investigated for anti-aging application.
Materials and methods
Materials
l-(+)-Ascorbic acid, 2,2-diphenyl-1-picryhydrazyl radical (DPPH), α-tocopherol, butylated hydroxytoluene (BHT), Tween 61, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), acrylamide, Tris (hydroxymethyl)-methlamine, calcium chloride (CaCl2), sodium azide (NaN3), sodium lauryl sulfate (SLS) and standard gallic acid were purchased from Sigma Chemical Co. (St. Louis, MO). Sodium dodecyl sulfate (SDS) and Coomassie® Brilliant Blue G-250 from Bio-Rad Laboratories (Hercules, CA), and Carbopol® 980 and cholesterol from Fluka (Buchs, Switzerland) were used. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin and streptomycin were obtained from GIBCO (Grand Island, NY). All other analytical grade chemicals and reagents were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The commercial gel containing 6% magnesium ascorbyl phosphate and 1% sodium hyaluronate solution was purchased from the local department store in Chiang Mai, Thailand.
Plant materials and preparation of the semi-purified fraction
T. chebula galls were collected from Chiang Mai Province in Thailand, during January to February in 2008. The specimen was authenticated by a botanist at Faculty of Pharmacy, Chiang Mai University, Thailand, and deposited in the medicinal plant herbarium, Faculty of Pharmacy, Chiang Mai University in Thailand for authenticated specimen (voucher specimen no. 012102). The crude extract and the semi-purified fraction containing gallic acid from T. chebula galls were isolated and purified as previously described (CitationManosroi et al., 2011).
Entrapment of the semi-purified fraction and pure gallic acid in elastic niosomes
Tween 61 mixed with cholesterol (1:1 molar ratios) at 20 mM was placed in a clean and dry round bottom flask. The mixture was dissolved in chloroform. The organic solvent was removed by a rotary evaporator under vacuum (R-124 Buchi, Flawil, Switzerland). The resulting film was dried overnight under vacuum at room temperature (27 ± 2°C). The film was rehydrated with 5 mM phosphate buffer (PB) at pH 7.0 for non-elastic niosomes or 25% ethanol (95% v/v) in PB for elastic niosomes. The niosomal dispersion was put in an ice bath (4°C) while sonicating by a microtip probe sonicator (Vibra Cell TM, Sonics & Materials, Inc., Newtown, CT) at pulse on 3.0 and pulse off 1.0, 33% amplitude for 10 min. The resulting niosomal dispersions were kept in vials tightly covered with aluminum caps. For the preparation of the loaded non-elastic and elastic niosomes, the pure gallic acid or the semi-purified fraction dissolved in PB with or without 25% ethanol was used to hydrate the film.
In vitro biological activities
Free radical scavenging assay
Free radical scavenging activity of gallic acid and semi-purified fraction loaded in non-elastic and elastic niosomes was determined by DPPH of the method previously described with slight modification (CitationTachibana et al., 2001). Briefly, an amount of 5 µL of five serial concentrations of the samples (1–100 µg/mL), 195 µL of ethanol (95% v/v) and 50 µL of 500 µM DPPH in ethanol were mixed in each well of the 96-well microplate (Nalge Nunc International, NY). The reaction mixture was allowed to stand for 30 min at 27 ± 2°C, and the absorbance was measured at 515 nm by a well reader (Bio-Rad, model 680 microplate reader, CA) against a blank (phosphate buffer pH 7.4). Ascorbic acid, BHT and α-tocopherol were used as positive controls. The experiments were done in triplicate. The free radical scavenging activity was calculated according to the equation: % scavenging activity = [(Acontrol − Asample)/Acontrl] × 100, where Acontrl was the absorbance of the control and Asample was the absorbance of the sample. The SC50 value which was the concentration of the sample that scavenged 50% of the DPPH radical was determined by the method of Probit-graphic interpolation of five concentration levels.
Cytotoxicity on normal human skin fibroblasts
The normal human skin fibroblasts were provided by Dr. Natthanej Luplertlop at the Department of Tropical Hygine, Faculty of Tropical Medicine, Mahidol University in Bangkok, Thailand. The cells at the 25th passage were cultured under the standard conditions in DMEM culture medium supplemented with 10% (v/v) FBS, penicillin (100 Unit/mL) and streptomycin (100 mg/mL), at 5% CO2, 37°C in a temperature-controlled, humidified incubator (Shel Lab, model 2123TC, Cornelius, OR). Cytotoxicity of gallic acid and the semi-purified fraction loaded in non-elastic and elastic niosomes were determined by MTT based colorimetric assay by the method previously described (CitationTabata et al., 2005) with slight modification. Briefly, the fibroblasts (1 × 104 cells/well) were seeded onto each well in a 96-well culture plate with medium and maintained for 24 h. Then, the test samples were added to the cells to give the final concentrations at 1–100 µg/mL. After 2 days of incubation, MTT solution (10 µL, 5 mg/mL in phosphate-buffered saline) was added and further incubated for 3 h. Isopropanol containing 0.04 M HCl was then added to dissolve the produced formazan. The absorbance of the mixture in each well was then read at 570 nm by a microplate reader (Bio-Rad, model 680 microplate reader). The absorbance of the system with no treatment was used as a negative control. Ascorbic acid was used as a positive control. The assays were done in three independent separate experiments. The percentages of cell proliferation in comparing to the control were calculated using the following equation: Cell viability (%) = (Asample − Ablank/Acontrol − Ablank) × 100, where Acontrol was the absorbance of the control, Asample was the absorbance of the sample and Ablank was the absorbance of the medium.
MMP-2 inhibition activity by gelatinolytic zymography
Gallic acid and the semi-purified fraction loaded in non-elastic and elastic niosomes were assayed for gelatinolytic activity of MMP-2 inhibition in comparing to ascorbic acid. Monolayer of 2.5 × 105 cells/well of the aged human skin fibroblasts (25th passage) were seeded onto a 6-well culture plate and maintained in the culture medium without FBS for 24 h. The cells were treated with the samples and ascorbic acid at the concentrations of 1–50 µg/mL and incubated for 48 h. Then, the culture supernatants were collected to assess the gelatinolytic activities of MMP-2 by SDS-PAGE zymography using gelatin as a substrate. Briefly, 20 µL of the supernatant were suspended in the loading buffer (0.125 M Tris (pH 6.8), 4% SDS and 0.04% bromophenol blue) and run on 10% SDS-polyacrylamide gel containing 1 mg/mL of gelatin. After electrophoresis, gels were washed to remove SDS and incubated for 20 min in renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 2.5% Triton X-100). The gels were then incubated for 24 h at 37°C in developing buffer (50 mM Tris (pH 7.5), 5 mM CaCl2, 0.02% NaN3 and 1% Triton X-100), and subsequently stained with 0.5% Coomassie® Brilliant Blue G-250 and destained in 30% methanol and 10% acetic acid (v/v) to detect gelatinolytic activity (CitationKim et al., 2008). The gel was documented by a gel documentation system (Bio-Rad Laboratories, UK) and analyzed by Quantity 1-D analysis software. The relative MMP-2 expression was calculated by multiplication of the area with the intensity of each band on the gel. The percentages of MMP-2 expression in comparing to the control (the untreated system) were calculated using the following equation: MMP-2 expression (%) = (MMP-2 content of the sample/MMP-2 content of the control) × 100. The assays were done in three independent separate experiments.
Physicochemical stability of the gel containing the semi-purified fraction loaded in elastic niosomes
Preparation of the gel formulations
The elastic and non-elastic niosomes loaded with the pure gallic acid or the semi-purified fraction containing gallic acid were incorporated into the gel base composed of Carbopol® 980. Briefly, 0.6% of Carbopol® 980 gel was dispersed in the niosomal dispersion with gentle stirring, resulting in the gel containing 0.5% w/w of the pure gallic acid or the gel containing the semi-purified fraction with 0.08% w/w of gallic acid.
Physical stability
The gel containing non-elastic and elastic niosomes loaded with the pure gallic acid or the semi-purified fraction was put in tight containers and stored at room temperature (27 ± 2), 4 ± 2 and 45 ± 2°C for 3 months. The physical characteristics including color, phase separation and particle size determined by dynamic light scattering (DLS) and transmission electron microscope (TEM) were evaluated.
Chemical stability
The percentages remaining of gallic acid contents in various gel formulations at 0, 1, 2, 3 months were determined by HPLC. The HPLC conditions of gallic acid were LC1200 UV/VIS detector and LC1100 HPLC pump (AS 1000, Thermo Finigan, San Jose, CA) using Luna® C18, 10 µm i.d., 250 × 4.0 nm, Phenomenex USA column and a mobile phase containing MeCN/H2O/AcOH (10:90:1, v/v) at the flow rate of 1 mL/min. The gel samples were diluted to 30 times with distilled water before filtered through a 0.45 µm membrane filter to obtain clear solution, prior to injection onto the HPLC column. An amount of 20 µL of the samples was injected into the column and monitored at 254 nm UV detector. The retention time of gallic acid was 3.8 min. The gallic acid contents were determined from the standard curve of the standard gallic acid, which demonstrated linear with high correlation (r2 = 0.9993). The following regression equation was obtained: y = (9 × 107)x + 510586, where y was the peak area and x was the quantity of gallic acid (µg). The experiment was done in triplicate.
In vivo anti-aging evaluation
Rabbit skin irritation test by the closed patch test
Three male rabbits (New Zealand White, 1.5–2.5 kg) were kept carefully following an acclimation period of 7 days to ensure their suitability for the study within a limited-access rodent facility with environmental conditions set to 25 ± 2°C, 60–90% RH and 12 h light/12 h dark cycle. Animals were provided ad labium access to a commercial rabbit-diet and the drinking water was supplied to each cage. Back of the animals was shaved to be free of fur with an electric clipper 24 h before sample application. The shaved areas were divided into 10 sites of 2.5 × 2.5 cm each. An amount of 0.5 g of each sample and 5% SLS solution (positive control) was placed on each site. The untreated site was used as a negative control. The treated sites were covered with gauze and the wrapped with a non-occlusive bandage. After 24 h, the bandage and the test samples were removed and the treated sites were washed 2 times with distilled water and air dried. One hour later, the sites were examined by optical visualization and measured by a Mexameter® (Courage & Khazaka, Cologne, Germany) for skin edema and erythema. Scoring of erythema and edema was preformed at 24, 48 and 72 h according to CitationDraize et al. (1944) (and adopted by OECD Test Guideline 404). The Primary Irritation Index (PII) was calculated using the following equation: PII = [(∑ erythema grade at 24/48/72 h + ∑ edema grade at 24/48/72 h)/3 × number of animals]. The irritation degree was categorized based on the PII values as negligible (PII = 0–0.4), slight (PII = 0.5–1.9), moderate (PII = 2–4.9) or severe (PII = 5–8) irritation. This study protocol has been reviewed and approved by the ethical committee of Faculty of Medicine, Chiang Mai University in Thailand (Protocol Number: 7/2553).
Anti-aging evaluation in human volunteers
Subjects and study protocol
A total of 31 Thai volunteers (13 men, 18 women, average age 33.00 ± 6.76 years, range 27–47 years, average BMI 23.11 ± 3.73) which were recruited from Faculty of Pharmacy, Chiang Mai University in Chiang Mai, Thailand were enrolled in the study. They have no pathological symptoms on their arms and were not using any topical agents on the test areas. The samples were applied on the 4 × 4 cm2 area on the volar forearm. Each forearm was virtually divided into four areas. Seven areas were treated twice daily for 8 weeks with 0.2 mL of the 7 gel formulations listed in . The area without the treatment was used as a negative control. The areas were measured for anti-aging activity before sample application and after application at 2, 4, 6 and 8 weeks. Before each measurement, the volunteers were accommodated in a controlled room at 25 ± 2°C and 75 ± 2% RH for 20 min. All volunteers finished the study without any drop-outs. This study protocol was reviewed and approved by the ethical committee of Faculty of Pharmacy, Chiang Mai University in Thailand (Protocol Number: 4/2554).
Table 1. Descriptions and compositions of the gel samples for rabbit skin irritation test and human skin anti-aging evaluation.
In vivo anti-aging measurements
Skin elasticity
Skin elasticity of the treated areas was determined using a non-invasive in vivo suction skin elasticity meter, Cutometer® MPA580 (Courage & Khazaka, Cologne, Germany) which was equipped with a 2-mm measuring probe. The time/strain was used with a 5 s application of a constant negative pressure of 450 mbar, followed by a 3 s relaxation period and repeated for 3 times for each area. The immediate distention (Ue), the final distention (Uf), and the immediate retraction (Ur) were measured. The delayed distention (Uv) was obtained from the difference between Uf and Ue. The elasticity ratio Uv/Ue or skin elastic extension, as well as the ability of the skin to return to its initial position after deformation (Ur/Uf) or skin elastic recovery were calculated from the above parameters by Win-Cutometer MPA Version 2.12.15.9 software (Courage & Khazaka, Electronic GmbH; Cologne, Germany) which have been reported to be independent of skin thickness (CitationElsner et al., 1990; CitationTakema et al., 1994). Therefore, the Ur/Uf and Uv/Ue values were used as parameters of skin elasticity. For the relative inspection of the data, the significant differences were calculated as parameter changes (%) by the following equation: Percentages parameter changes (%) = (∑Qti/∑Q0i − 1) × 100 where Qti was the quotient after the time of application (t) of 8 weeks for each volunteer (i), while Q0i was the quotient before the time of application (0) for each volunteer (i).
Skin surface microstructure
Skin negative replicas were taken from the volar forearm surface area by applying a silicon-based gum material (SILFLO, Flexico Developments Ltd., England), according to the manufacturer’s instructions. In order to obtain the replicas from the same skin area, the skin was marked using an oil-based marking pen. The skin surface microstructure was assessed using the image analysis software. Briefly, light was directed at a 208 angle and the images were observed from the replica using a CCD camera (Visioscan® VC98, Courage & Khazaka, Cologne, Germany). The parameters of the replica image to evaluate skin surface microstructure including the level of wrinkle, the maximum roughness value (Rm) and the average roughness (Ra) were analyzed by CK Visiometer® SV600 FW; image analyzing program version 1.6.6.1 software (Courage & Khazaka, Electronic GmbH; Cologne, Germany), which can scan the microrelief from the replicas using a light transmission method.
The parameter changes (%) were calculated by the same equation as the skin elasticity measurement.
Skin hydration
Skin hydration was investigated using a Corneometer® CM825 (Courage & Khazaka, Cologne, Germany) which was mounted on a Multi Probe Adapter® MPA5 (Courage & Khazaka, Cologne, Germany). Capacitance changes depending almost solely upon the water content in the SC were detected and evaluated by CK MPA Multiprobe Adaptor version 1.4.2.2 (Courage & Khazaka, Electronic GmbH; Cologne, Germany). Five measurements were performed on each tested skin area.
Skin erythema and pigmentation
The degree of skin erythema (redness) and pigmentation (melanin) was measured using a Mexameter® MX18 (Courage & Khazaka, Cologne, Germany). Five measurements were performed on each tested skin area.
Statistical analysis
All data were presented as mean ± SD. Student’s paired t-test and the Kruskall–Wallis test were used to evaluate the significance of differences at the significant level of p-value < 0.05. Statistical analysis was performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL).
Results and discussion
In vitro biological activities
Free radical scavenging assay
showed the SC50 values of the DPPH radical scavenging assay of gallic acid and the semi-purified of T. chebula gall fraction loaded in non-elastic and elastic niosomes and the standard antioxidants (ascorbic acid, α-tocopherol and BHT). Gallic acid and the semi-purified fraction in PB solution gave the SC50 values of 8.84 and 6.29 µg/mL, respectively, which were slightly higher than ascorbic acid, α-tocopherol and BHT (4.31, 5.15 and 3.90 µg/mL, respectively) of about 2 times. Generally, the phenolic compounds have more hydroxyl groups and easy to be oxidized. The fraction containing gallic acid (15.3%), 1,3,6-trigalloylglucopyranoside (17.8%) and punicalagin (38.4%) (CitationManosroi et al., 2011) gave higher scavenging activity than the pure gallic acid. These phenolic compounds may have potential synergistic effect of this activity. Gallic acid or the semi-purified fraction loaded in elastic or non-elastic niosomes showed no DPPH radical scavenging activity with the SC50 value of more than 100 mg/mL the same as the blank non-elastic and elastic niosomes. Both gallic acid and the semi-purified fraction loaded in niosomes gave less activity than the unloaded compounds. This may be due to the shielding effect of niosomes. The phenolic compounds may not be released from the niosomes. Nevertheless, the enzymes such as esterase, hydrolase and monoxygenase existing in human skin may be able to break down the vesicles and release the phenolic compounds to in vivo.
Table 2. DPPH radical scavenging activity of gallic acid and the semi-purified fraction of T. chebula gall extract loaded in non-elastic and elastic niosomes.
Cytotoxicity on normal human skin fibroblasts
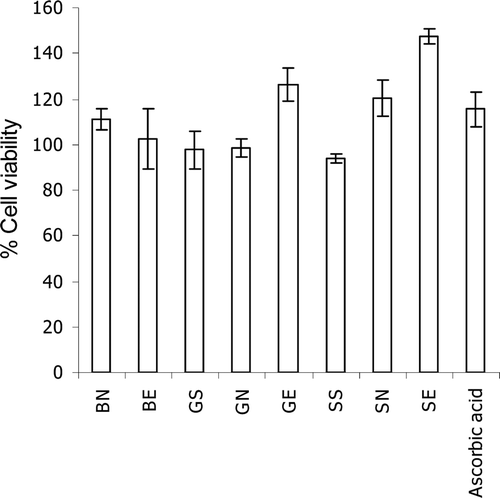
showed the effects of gallic acid and the semi-purified T. chebula gall fraction loaded in non-elastic and elastic niosomes on viability of human skin fibroblasts at 48 h incubation. Gallic acid or semi-purified fraction loaded in elastic niosomes (GE or SE) gave cell viability of 126.31 and 147.44% which was more than the unloaded gallic acid (GS at 97.57%) and semi-purified fraction (SS at 95.15%) of about 1.29 and 1.55 times, respectively. The toxicity of gallic acid and the semi-purified fraction on human skin fibroblasts has never been investigated. In the present study, the slight increase of % cell viability of gallic acid and the semi-purified fraction when loaded in niosomes has been observed, but not significant. This study has indicated not only that the unloaded gallic acid and semi-purified fraction exhibited no toxicity on human skin fibroblasts, but also even gallic acid or the semi-purified fraction loaded in elastic or non-elastic niosomes still gave no cytotoxicity. This may be due to the protection of these compounds by niosomes from the direct contact to the cells. However, when loaded in elastic niosomes, gallic acid (GE) and the semi-purified fraction (SE) showed higher cell viability than those loaded in non-elastic niosomes of about 1.28 and 1.23 times, respectively. The higher entrapment efficiencies of pure gallic acid and gallic acid containing in the semi-purified fraction in elastic niosomes (55.18 ± 3.87 and 24.34 ± 1.53%) than in non-elastic niosomes (29.72 ± 1.68 and 20.10 ± 3.91%) of about 1.86 and 1.21 times, respectively, have been demonstrated (CitationManosroi et al., 2011). The higher solubility of gallic acid in ethanol than in water of about 7 times (CitationAli et al., 2008) may facilitate the entrapment of gallic acid in the elastic niosomal membrane which contained 25% of ethanol. The less release of gallic acid from the elastic than the non-elastic niosomes can be anticipated, thereby reducing the cytotoxicity.
Figure 1. Effects of gallic acid at 50 µg/mL and the semi-purified fraction (at 50 µg/mL of gallic acid) of T. chebula gall extract loaded in non-elastic and elastic niosomes on human skin fibroblast viability at 48 h incubation. (BN, blank non-elastic niosomes; BE, blank elastic niosomes; GS, gallic acid solution; GN, non-elastic niosomes loaded with gallic acid; GE, elastic niosomes loaded with gallic acid; SS, the semi-purified fraction; SN, non-elastic niosomes loaded with the semi-purified fraction; SE, elastic niosomes loaded with the semi-purified fraction.)
MMP-2 inhibition activity by gelatinolytic zymography
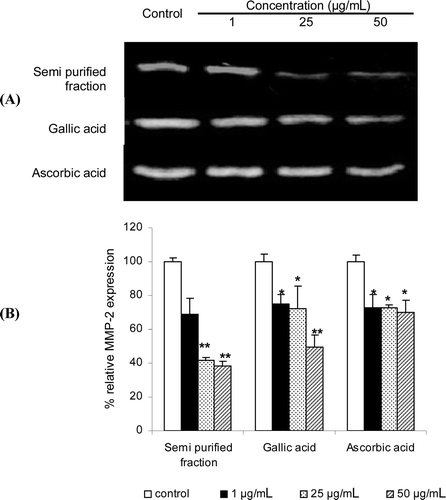
The inhibition effects of the gelatinolytic activity on MMP-2 expression of the semi-purified fraction and the pure gallic acid and the standard ascorbic acid at 1–50 µg/mL were shown in . Since all samples were not cytotoxic to human skin fibroblasts by the MTT assay, the MMP-2 inhibition were not due to the cytotoxicity. The semi-purified fraction at 50 µg/mL gave the lowest relative MMP-2 expression of 38.61% and indicated higher inhibition activity than gallic (49.63%) and ascorbic acid (69.87%) of 1.29 and 1.81 times, respectively. The reduction of the expression levels of MMP-2 and -9 in gelatin zymography has been reported due to high free radical scavenging abilities (CitationKim et al., 2010). However, the semi-purified fraction showed the highest MMP-2 inhibition, but lower DPPH radical scavenging activity than ascorbic acid. Hence, other pathways besides the free radical scavenging activity, such as the activation of the tissue inhibitors of matrix metalloproteases (TIMPs) and the inhibition of the inflammatory of cytokines may also affect the MMP-2 inhibition activity (CitationMillis et al., 1992; CitationStetler-Stevenson et al., 1989). All niosomal formulations both non-elastic and elastic niosomes loaded with gallic acid or the semi-purified fraction showed no MMP-2 inhibition activity (data not shown). This may be due to the stability of gallic acid and the other phenolic compounds containing in the semi-purified fraction loaded in niosomes which were not released, thereby showing no activities.
Figure 2. Effects of the gelatinolytic activity on MMP-2 expression of the semi-purified fraction, gallic acid and the standard ascorbic acid at 1–50 µg/mL: (A) zymograms; (B) % relative MMP-2 expression calculated from the following equation: relative MMP-2 expression (%) = (% MMP-2 expression of the sample / % MMP-2 expression of the control) × 100. Each value is expressed as mean ± SD. Kruskall–Wallis test was used to calculate the significant differences. *p < 0.05 and **p < 0.01 compared to each control (the untreated system).
Physicochemical stability of the gel formulations containing the semi-purified fraction loaded in elastic niosomes
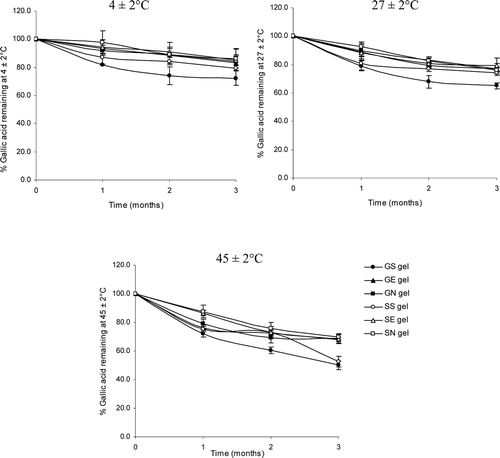
The gel formulations incorporated with the elastic and non-elastic niosomes loaded with gallic acid or semi-purified fraction from T. chebula galls extract gave good physical stability with no sedimentation, no layer separation and no color change at all temperatures (4, 27 and 45°C) for 3 months. showed the percentage remaining of gallic acid in various gel formulations at different storage temperatures for 3 months. At all temperatures, gallic acid or the semi-purified fraction containing gallic acid loaded in elastic and non-elastic niosomes and incorporated in gel formulations (GE and SE; GN and SN, respectively) showed higher percentages of gallic acid than those not loaded in niosomes and incorporated in gel (GS and SS). The percentages of gallic acid remaining in GE, SE, GN and SN were 83.00, 85.29, 84.32 and 85.99; 76.80, 75.99, 77.09 and 79.54; 67.74, 68.54, 68.89 and 69.98% stored for 3 months at 4 ± 2, 27 ± 2 and 45 ± 2°C, respectively. The percentages of gallic acid remaining after 3 months of all gel formulations at higher storage temperatures were less than at lower temperature owing to the decarboxylation of gallic acid to pyrogallol at high temperature (CitationJennifer et al., 1988). However, the percentages of gallic acid remaining in elastic niosomes were slightly lower than those in the non-elastic niosomes but not significant. This may be due to the evaporation of ethanol at elevated temperature during storage which may fluidize the vesicular membrane and facilitate the leakage of gallic acid from the elastic vesicles. When incorporation in gel, gallic acid and the semi-purified fraction loaded in niosomes were more stable against thermal degradation than those loaded in niosomes but not incorporated in gel of about 1.31 times (CitationManosroi et al., 2011). In addition, the gel structure may retard the leakage of gallic acid from the vesicles, thereby preventing its degradation from the thermal effects (CitationRuel-Gariepy et al., 1994; CitationBochot et al., 1998; CitationGlavas-Dodov et al., 2002).
Figure 3. The percentages of gallic acid remaining in various gel formulations at different storage temperatures (27 ± 2, 4 ± 2 and 45 ± 2°C) for 3 months. (GS gel, gel containing the unloaded gallic acid; GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
Skin irritation evaluation
Rabbit skin irritation by the closed patch test
The calculated PIIs of all gel formulations in rabbit skin irritation by the closed patch test at 72 h were in the range of 0.00–0.33 except the gallic acid gel (PII = 0.44–0.56, slight irritation), the gel base (PII = 0.11, negligible) and the positive control (5% SLS, PII = 0.78–1.22, slight irritant) (). Thus, all developed gel formulations gave no irritation, except the gallic acid gel. The irritation was from gallic acid which is a small molecule (MW = 170.12). Since the gel base gave no skin irritation, in the material safety data sheet, gallic acid may cause irritation to the skin with redness or minor inflammation (Mallinckrodt Chemical, J.T. Baker, NJ). This may be also due to its concentrations in the gel which was about 6 times (2500 µg of gallic acid) more than that in the semi-purified fraction (400 µg of gallic acid). In addition, gallic acid containing in the semi-purified fraction may be highly polymerized or associated with other polyphenolic compounds by hydrogen bonding (CitationHaslam, 1996), thereby retarding its skin permeation from this large complex molecular structure. Moreover, the higher in vitro rat skin permeation by Franz diffusion cells of pure gallic acid than gallic acid containing in the semi-purified fraction has also supported this skin irritation results (CitationKanikkannan & Singh, 2002; CitationManosroi et al., 2011). However, gallic acid loaded in non-elastic or elastic niosomes showed no irritation in rabbit skin, because of the reduction of the direct contact between gallic acid and the skin. Thus, all gel formulations except the gel containing the pure gallic acid was not used for in vivo anti-aging evaluation in human volunteers.
Table 3. Primary irritation index (PII) and category of irritation based on PII of various gel formulations.
Human skin erythema measured by Mexameter®
The erythema measurements of all gel samples except the gel containing pure gallic acid performed after 2, 4, 6 and 8 weeks of application revealed no statistically significant differences (p > 0.05, Student’s paired t-test) in comparing to the initial values on day 0 and the untreated area (). Therefore, all gels including the commercial product did not induce any human skin irritation and were well tolerated.
Human skin anti-aging evaluation
Skin elasticity
Parameters obtained from Cutometer® explain various skin mechanical properties, such as skin elastic extension, elastic recovery and deformation. During aging, change of skin elastic recovery (Ur/Uf) has been reported to decrease, whereas change of skin elastic extension (Uv/Ue) has been shown to increase progressively (CitationEscoffier et al., 1989; CitationTakema et al., 1994; CitationFujimura et al., 2007). These skin mechanical properties are related to the decrease in interstitial fluid viscosity as a result from low amount of glycosaminoglycans and soluble collagen, as well as the decrease in elastic properties of collagen and elastin fibres due to the damage, disintegration, fragmentation, or changes of the protein fiber structure (CitationDobrev 1998, 2002). Thus, an increase in Ur/Uf value and a decrease in Uv/Ue value will indicate the improvement of skin elastic properties. In this study, the values of Ur/Uf and Uv/Ue elastic parameters of various topical gel formulations after 8 weeks of application in 31 volunteers measured by Cutometer® were shown in . All Ur/Uf values increased while all Uv/Ue values decreased with times in all treated areas. However, the gel containing gallic acid loaded in elastic niosomes, the unloaded semi-purified fraction, gel containing the semi-purified fraction loaded in niosomes and elastic niosomes (GE, SS, SN and SE gel) showed the significant increase of Ur/Uf at 8 weeks of application. Moreover, SN and SE gel also gave the significant decrease of Uv/Ue at 6 and 8 weeks of application. For the parameter changes (%), which were determined from the values after and before application time, the application of GE, SS, SN and SE gels for 8 weeks gave the parameter changes of the Ur/Uf value at +53.92, +30.32, +28.73 and +32.57%, respectively. The parameter changes of the Uv/Ue values were −21.25 and −22.63% when applied with SN and SE gel, respectively (). The phenolic compounds, especially gallic acid, which were the constituents in the semi-purified fraction, may improve skin elasticity in the dermis by scavenging the free radical and inhibiting the MMP-2 activity. This has been evidenced that some phenolic compounds, such as tannins have been reported as a direct human neutrophil elastase (HNE) inhibitor (CitationMelzig et al., 2001). The decrease in skin elasticity was also accompanied with neutrophils, which supposed to participate in the aging process of human skin and the release of enzymatically active HNE (CitationTsuji et al., 2001). In addition, some hydrolysable tannins from root of Sanguisorba officinalis L. (Rosaceae) applied on the rat hind limb skin following UVB exposure have been reported to inhibit wrinkle formation, maintain skin elasticity and prevent the decrease of dermal elastic fiber linearity in a dose dependent manner (CitationMimaki et al., 2001; CitationTsukahara et al., 2001).
Table 4. Percentage changes of skin parameter (%) after 8 weeks of applications of various gel formulations and the negative control (the untreated area).
Skin surface microstructure
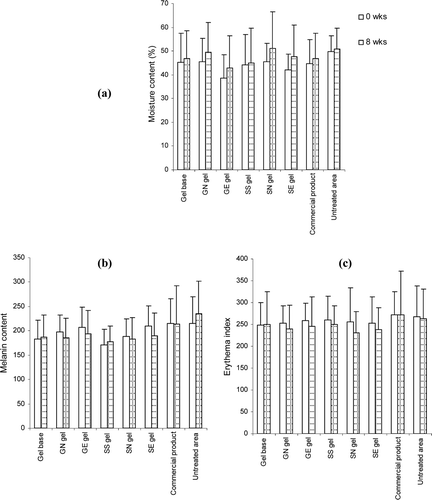
Skin roughness measurement was based on the principle of measuring the depth of furrows according to the shadow and brightness size due to the inflection under illumination. The maximum roughness (Rm) and average roughness (Ra) values can be used to indicate an increase of progressive aging. The comparison of skin surface roughness before and after 8 weeks of application of the gels containing non-elastic or elastic niosomes loaded with gallic acid or the semi-purified fraction measured by skin replica and Visiometer®, was shown in and . Rm and Ra values decreased with times in all treated groups. All samples, including gel base, GN, GE, SS, SN, SE and the commercial gel, showed a significant different decrease of the Ra values between before and after 8 weeks of application with the parameter changes of −19.05, −28.28, −30.95, −31.55, −39.47, −35.28 and −22.43%, respectively. In addition, for the Rm values, gel base, GN, GE, SS, SN and SE gels demonstrated a significant decrease with the parameter changes of −24.62, −26.08, −28.66, −30.87, −29.43 and −32.38%, respectively (). For the untreated area after 8 weeks of application, the Ra and Rm values showed only a slight increase. There was no significant difference of the roughness parameter values between the gels containing non-elastic and elastic niosomes loaded with gallic acid or semi-purified fraction (GE, GN, SE and SN). As known, the degeneration of the elastic fibers in the skin leads to a decrease of skin elasticity and wrinkle formation. The phenolic compounds which were the constituents in the semi-purified fraction may improve skin elastic resulting in the reduction of skin roughness.
Figure 4. Changes of the maximum roughness or Rm (A) and the arithmetic average roughness or Ra (B) of various topical gel formulations in 31 human volunteers after topical application for 8 weeks. Student’s paired t-test was used to calculate the significant differences. *p < 0.05 compared to before application (0 wks). (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
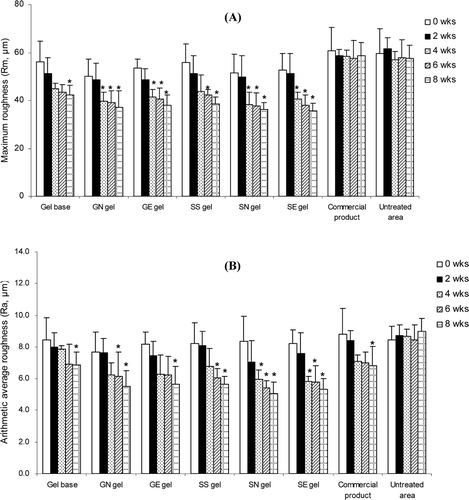
Figure 5. Comparison of the skin roughness before (left) and after application for 8 weeks (right) and % changes of the arithmetic average roughness (Ra) values of various topical formulations. (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
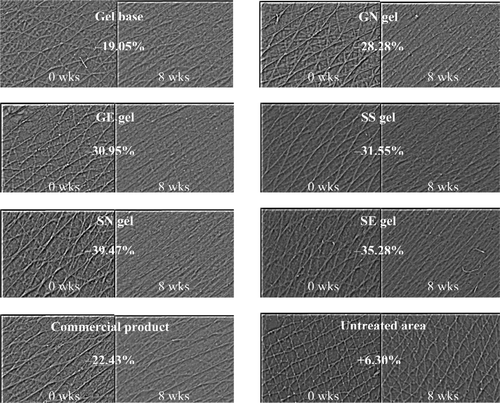
Figure 6. Changes of the skin elastic recovery or Ur/Uf (A) and changes of the skin elastic extension or Uv/Ue (B) of various topical gel formulations in 31 human volunteers after application for 8 weeks. Student’s paired t-test was used to calculate the significant differences. *p < 0.05 compared to before application (0 wks). (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
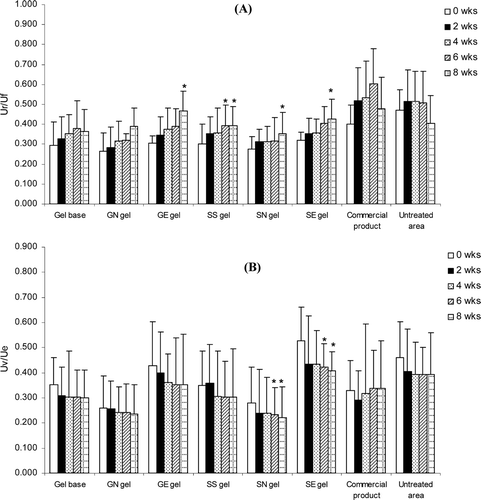
Figure 7. Comparison of moisture (A), melanin content (B) and erythema index (C) on skin surface of various topical formulations at initial and after 8 weeks of application. Student’s paired t-test was used to calculate significant differences. No significant difference was observed between before and after 8 weeks of application (p > 0.05).
Skin hydration and pigmentation
The skin hydration and pigmentation values measured by Corneometer® and Mexameter®, respectively before and after 8 weeks of topical application of all samples were not significantly different (p > 0.05, Student’s paired t-test). This indicated that all developed gel formulations and the commercial product did not improve any epidermal moisture content and pigmentation in human skin after 8 weeks of application. In order to improve skin hydration, it is necessary to maintain the normal conditions of the skin and to prevent the dryness of the skin by reducing the transepidermal water loss via the occlusive effect. In fact, the moisturizing effects of several cosmetic formulations can be influenced by many factors, including the base compositions and the concentration of the active ingredients (CitationLi et al., 2001; CitationSavica et al., 2004). Generally, gel formulations have only slight occlusive properties in comparing to other cosmetic formulation such as cream. Thus, all developed gels were expected not to improve skin hydration.
For skin pigmentation, UV is a major environmental factor that can cause aged-skin hyperpigmentation. The formulations which can reduce skin pigmentation should have the active compounds that can limit the extent of UV penetration through the epidermal layers by reflecting, scattering or absorbing the UV rays and scavenging the reactive oxygen radicals that may lead to oxidative DNA damage (CitationStanojevic et al., 2004). In addition, the active compounds should inhibit tyrosinase enzyme. In this present study, all developed gel formulations showed no significant different of skin pigmentation between before and after 8 weeks of application. This agreed with our previous study that T. chebula gall extracts gave only slight in vitro mushroom tyrosinase inhibition activity (Manosroi et al., Citation2010a).
Conclusion
The semi-purified fraction of T. chebula galls which contained the phenolic compounds including gallic acid showed high in vitro DPPH radical scavenging and MMP-2 inhibition activities. However, when the semi-purified fraction or gallic acid was loaded in non-elastic or elastic niosomes, they showed no activities because of the shielding effect from niosomes. All gel formulations showed no rabbit skin irritation by the closed patch test. For in vivo human skin test, the gel containing gallic acid and the semi-purified fraction loaded in elastic or non-elastic niosomes demonstrated significantly higher improvement of skin elastic and roughness than the gel containing the unloaded semi-purified fraction. However, there was no significant difference of these effects between the gel containing gallic acid or semi-purified fraction loaded in elastic and non-elastic niosomes. Thus, the decrease of skin roughness and the increase of skin elasticity of the gel containing semi-purified fraction were mainly from the antioxidative and MMP-2 inhibition activities of the phenolic compounds especially gallic acid containing in the semi-purified fraction. Moreover, the chemical degradation as well as skin irritation of gallic acid was reduced when loaded in non-elastic and elastic niosomes. The developed gel formulation containing the semi-purified fraction of T. chebula galls extract can be applied as a novel topical product for skin aging due to their superior in vitro and in vivo anti-wrinkle effects.
Declaration of interest
This work was supported by the Thailand Research Fund (TRF) under the RGJ-PhD program, Natural Products Research and Development Center (NPRDC), Institute for Science and Technology Research and Development (STRI), Nanoscience and Nanotechnology Research Center Project, Faculty of Science, Chiang Mai University, Thailand.
References
- Aimes RT, Quigley JP. (1995). Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific ¾- and ¼-length fragments. J Biol Chem, 270, 5872–5876.
- Ali D, Hassan SG, Nasrolah H. (2008). Solubility of gallic acid in methanol, ethanol, water and ethyl acetate. J Chem Eng Data, 53, 776–778.
- Berneburg M, Plettenberg H, Krutmann J. (2000). Photoaging of human skin. Photodermatol Photoimmunol Photomed, 16, 239–244.
- Bochot A, Fattal E, Gulik A, Couarraze G, Couvreur P. (1998). Liposomes dispersed within a thermosensitive gel: A new dosage form for ocular delivery of oligonucleotides. Pharm Res, 15, 1364–1369.
- Boles JS, Crerar DA, Grissom G, Key TC. (1988). Aqueous thermal degradation of gallic acid. Geochimica et Cosmochimica Acta, 52, 341–344.
- Brennan M, Bhatti H, Nerusu KC, Bhagavathula N, Kang S, Fisher GJ, Varani J, Voorhees JJ. (2003). Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol, 78, 43–48.
- Brenneisen P, Wlaschek M, Schwamborn E, Schneider LA, Ma W, Sies H, Scharffetter-Kochanek K. (2002). Activation of protein kinase CK2 is an early step in the ultraviolet B-mediated increase in interstitial collagenase (metrix metalloproteinase-1; MMP-1) and stromelysin-1 (MMP-3) protein levels in human dermal fibroblasts. Biochem J, 365, 31–40.
- Dobrev HP. (1998). Use of Cutometer to assess dermal oedema in erysipelas of the lower legs. Skin Res Technol, 4, 155–159.
- Dobrev HP. (2002). A study of human skin mechanical properties by means of Cutometer. Folia Med (Plovdiv), 44, 5–10.
- Draize JH, Woodard G, Calvery H. (1944). Methods for the study of irritation and toxicity of substances applied to the skin and mucous membranes. J Pharmacol, 82, 377.
- Elsner P, Wilhelm D, Maibach HI. (1990). Mechanical properties of human forearm and vulvar skin. Br J Dermatol, 122, 607–614.
- Escoffier C, de Rigal J, Rochefort A, Vasselet R, Lévêque JL, Agache PG. (1989). Age-related mechanical properties of human skin: An in vivo study. J Invest Dermatol, 93, 353–357.
- Fujimura T, Haketa K, Hotta M, Kitahara T. (2007). Loss of skin elasticity precedes to rapid increase of wrinkle levels. J Dermatol Sci, 47, 233–239.
- Glavas-Dodov M, Goracinova K, Mladenovska K, Fredro-Kumbaradzi E. (2002). Release profile of lidocaine HCl from topical liposomal gel formulation. Int J Pharm, 242, 381–384.
- Haslam E. (1996). Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J Nat Prod, 59, 205–215.
- Jennifer SB, David AC, Grady G, Tonalee CK. (1988). Aqueous thermal degradation of gallic acid. Geochim Cosmochim Acta, 52, 341–344.
- Kanikkannan N, Singh M. (2002). Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int J Pharm, 248, 219–228.
- Kim S, Kim Y, Kim JE, Cho KH, Chung JH. (2008). Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine, 15, 340–347.
- Kim JA, Kong CS, Seo YW, Kim SK. (2010). Sargassum thunbergii extract inhibits MMP-2 and -9 expressions related with ROS scavenging in HT1080 cells. Food Chem, 120, 418–425.
- Li F, Conroy E, Visscher M, Wickett RR. (2001). The ability of electrical measurements to predict skin moisturization. II. Correlation between one-hour measurements and long-term results. J Cosmet Sci, 52, 23–33.
- Manosroi A, Jantrawut P, Manosroi J. (2008). Anti-inflammatory activity of gel containing novel elastic niosomes entrapped with diclofenac diethylammonium. Int J Pharm, 360, 156–163.
- Manosroi A, Khositsuntiwong N, Götz F, Werner RG, Manosroi J. (2009). Transdermal enhancement through rat skin of luciferase plasmid DNA loaded in elastic nanovesicles. J Liposome Res, 19, 91–98.
- Manosroi A, Jantrawut P, Akihisa T, Manosroi W, Manosroi J. (2010a). In vitro anti-aging activities of Terminalia chebula gall extract. Pharm Biol, 48, 469–481.
- Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi J. (2010b). Biological activities of phenolic compounds isolated from galls of Terminalia chebula Retz. (Combretaceae). Nat Prod Res, 24, 1915–1926.
- Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, Manosroi J. (2010c). Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm, 392, 304–310.
- Manosroi J, Khositsuntiwong N, Manosroi W, Götz F, Werner RG, Manosroi A. (2010d). Enhancement of transdermal absorption, gene expression and stability of tyrosinase plasmid (pMEL34)-loaded elastic cationic niosomes: Potential application in vitiligo treatment. J Pharm Sci, 99, 3533–3541.
- Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi W, Manosroi J. (2011). Transdermal absorption enhancement of gel containing elastic niosomes loaded with gallic acid from Terminalia chebula galls. Pharm Biol, <Volume>.
- Melzig MF, Löser B, Ciesielski S. (2001). Inhibition of neutrophil elastase activity by phenolic compounds from plants. Pharmazie, 56, 967–970.
- Millis AJ, Hoyle M, McCue HM, Martini H. (1992). Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res, 201, 373–379.
- Mimaki Y, Fukushima M, Yokosuka A, Sashida Y, Furuya S, Sakagami H. (2001). Triterpene glycosides from the roots of Sanguisorba officinalis. Phytochemistry, 57, 773–779.
- Pardakhty A, Varshosaz J, Rouholamini A. (2007). In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm, 328, 130–141.
- Patterson ML, Atkinson SJ, Knäuper V, Murphy G. (2001). Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett, 503, 158–162.
- Ruel-Gariepy E, Leclair G, Hildgen P. (1994). Thermosensitive chitosan-based on hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release, 30, 1–15.
- Sahin NO. (2007). Chapter 4. Niosomes as nanocarrier systems. Nanomaterials and Nanosystems for Biomedical Applications. Mozafari MR, Ed., Springer Netherlands, pp. 67–81.
- Savica S, Tamburic S, Savic M, Cekic N, Milic J, Vuleta G. (2004). Vehicle-controlled effect of urea on normal and SLS-irritated skin. Int J Pharm, 271, 269–280.
- Stanojevic M, Stanojevic Z, Jovanovic D, Stojiljkovic M. (2004). Ultraviolet radiation and melanogenesis. Arch Oncol, 12, 203–205.
- Steinbrenner H, Ramos MC, Stuhlmann D, Sies H, Brenneisen P. (2003). UVA-mediated downregulation of MMP-2 and MMP-9 in human epidermal keratinocytes. Biochem Biophys Res Commun, 308, 486–491.
- Stetler-Stevenson WG, Krutzsch HC, Liotta LA. (1989). Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem, 264, 17374–17378.
- Tabata K, Motani K, Takayanagi N, Nishimura R, Asami S, Kimura Y, Ukiya M, Hasegawa D, Akihisa T, Suzuki T. (2005). Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol Pharm Bull, 28, 1404–1407.
- Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N. (2001). Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem, 49, 5589–5594.
- Takema Y, Yorimoto Y, Kawai M, Imokawa G. (1994). Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol, 131, 641–648.
- Tsuji N, Moriwaki S, Suzuki Y, Takema Y, Imokawa G. (2001). The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem Photobiol, 74, 283–290.
- Tsukahara K, Takema Y, Fujimura T, Moriwaki S, Kitahara T, Imokawa G. (2000). Determination of age-related changes in the morphological structure (sagging) of the human cheek using a photonumeric scale and three-dimensional surface parameters. Int J Cosmet Sci, 22, 247–258.
- Tsukahara K, Moriwaki S, Fujimura T, Takema Y. (2001). Inhibitory effect of an extract of Sanguisorba officinalis L. on ultraviolet-B-induced photodamage of rat skin. Biol Pharm Bull, 24, 998–1003.
