Abstract
Introduction: Boerhaavia diffusa Linn. (Nyctaginaceae) is widely used in traditional Indian medicines against renal afflictions including calcium oxalate (CaOx) urolithiasis and is known for antioxidant activity.
Objective: The present study was designed to investigate the ameliorating effect of aqueous extract of B. diffusa roots (BDE) in hyperoxaluric oxidative stress and renal cell injury.
Material and methods: In vitro antioxidant activity of BDE was estimated in terms of total phenolic content and 1,1-diphenyl-2-picryl hydrazyl free radical scavenging activity. Wistar albino rats were given 0.75% v/v ethylene glycol in drinking water to induce chronic hyperoxaluria and simultaneously BDE was given to nephrolithiasic treated rats at the dose of 100 and 200 mg/kg b.w. orally for 28 days. Urinary volume, oxalate, serum creatinine, blood urea nitrogen (BUN), malondialdehyde (MDA) and antioxidant enzyme (SOD, CAT, GST, GPx) were evaluated.
Results and discussion: BDE extract was found to posses a high total phenolic content and exhibited significant free radicals scavenging activity. Oxalate excretion significantly increased in hyperoxaluric animals as compared to control which was protected in BDE-treated animals. BDE treatment significantly reduced level of MDA and improved the activity of antioxidant enzymes followed by reduction in BUN and serum creatinine. In addition, BDE reduced the number of CaOx monohydrate crystals in the urine. Histological analysis depicted that BDE treatment inhibited deposition of CaOx crystal and renal cell damage.
Conclusion: The present study reveals that antioxidant activity of BDE significantly protects against hyperoxaluric oxidative stress and renal cell injury in urolithiasis.
Introduction
Hyperoxaluria is one of the major risk factors of human idiopathic calcium oxalate (CaOx) urolithiasis. Oxalate is a natural byproduct of metabolism and harmlessly excreted through urine in normal individuals. However, increased urinary excretion of oxalate (hyperoxaluria) can be highly toxic because of its propensity to crystallize at physiologic pH and form CaOx (CitationKhan, 2005). Exposure to oxalate generates toxic responses in renal epithelial cells, including altered membrane surface properties, mitochondrial dysfunction, formation of reactive oxygen species (ROS) and decreased cell viability (CitationJonassen et al., 2005).
Overproduction of ROS and reduction in cellular antioxidant capacities, leads to the development of oxidative stress (CitationKhan, 2005). Oxidative stress followed by renal cell and loss of membrane integrity, which subsequently facilitates the retention and growth of CaOx stones in renal tubules (CitationSelvam, 2002). Recent studies suggest that treatment with antioxidants reduced CaOx crystal induced renal injuries. Pretreatment with vitamin E along with mannitol abolished the deposition of CaOx crystals in the kidneys of rats injected with sodium oxalate (CitationThamilselvan & Selvam, 1997). Therefore, treatment with natural antioxidants seems to be the possible therapeutic strategy for ameliorating hyperoxaluria-induced oxidative stress and renal cell injury in urolithiasis. Diuretic and crystallization inhibition property of these herbs adds a synergistic effect to combat with hyperoxaluria induced urolithiasis.
Boerhaavia diffusa Linn., commonly known as Punarnava in Sanskrit, is a herbaceous plant of family Nyctaginaceae. Medicinal values of this plant in the treatment of a large number of human ailments including renal disorders are mentioned in Ayurveda, Charaka Samhita, and Sushrita Samhita. Root decoction of this plant is used to treat kidney stones by indigenous and tribal people of India (CitationKasana et al., 2009). Some clinical and preclinical studies evidenced the effectiveness of this herb in various renal disorders like nephritic syndrome (CitationSingh & Udupa, 1972), kidney regeneration (CitationMishra & Singh, 1980), diuresis (CitationGaitonde et al., 1974) and antiurolithiatic activity (Pareta et al., 2010). The herbal extract of B. diffusa also reported to inhibit the growth of struvite crystals in vitro (CitationChauhan et al., 2009). Further, B. diffusa is included as herbal diuretic agent in the Indian Pharmacopoeia (2006) and also a major constituent of polyherbal formulation Cystone (Himalaya Health Care Pvt. Ltd., India) is used to cure renal ailments such as urolithiasis. These studies incorporated multidisciplinary interests that antioxidant activity of plant may have a role in protecting the kidney from hyperoxaluric oxidative stress in urolithiasis. Therefore, in the present study, an effort has been made to establish the scientific validity for the ameliorating effect of B. diffusa root aqueous extract in ethylene glycol (EG)-induced hyperoxaluric oxidative stress and renal cell injury in rat kidney.
Materials and methods
Plant material: extraction and phytochemical screening
B. diffusa was collected from Birla Institute of Technology, Mesra campus, Ranchi, India, in month of August (2008). A sample herbarium of the plant was submitted to the herbarium of Botanical Survey of India (BSI) (A Central National Herbarium), Kolkata. The plant was authenticated by Dr. M.S. Mondal (Additional Director, BSI) as B. diffusa variety red (letter no. CHN/I-I/2009/Tech II/9).
The roots of plant were cleaned, dried and ground to powder using a commercial mill. Approximately, 500 g of the plant powder was soaked in 2.5 L water at room temperature for 24 h with occasional shaking. It was filtered through a single layer of muslin cloth and then finally filtrate was collected by passing it through a Whatman grade No. 1 filter paper in a Buchner funnel under vacuum. The filtrate was evaporated to dryness on a rotary evaporator under reduced pressure. About 48 g crude aqueous extract of B. diffusa roots (BDE) was obtained; the approximate yield was 9.6% (w/w).
Characterization of extract
Characterization of BDE was performed using the high performance thin layer chromatography (HPTLC) system (Camag, Muttanz, Switzerland) which consisted of (i) TLC scanner connected to PC running WinCATS software under MS DOS and (ii) Linomat IV sample applicator with 100 µL syringe and connected to a nitrogen tank. The plate was accommodated according to the following settings: band width 5 mm; distance between bands 10 mm; application volume 10 µL; gas flow 150 nL/s. The plate was developed to 7 cm in a twin trough glass chamber pre-saturated with the upper layer of a mixture ethyl acetate-hexane (5:5, v/v) and dried. The scanner was set for maximum light optimization and with the following settings: slit dimension 5.00 × 0.45 mm; scanning speed 20 mm/s; data resolution 100 µm/step; scanning wave length 254 nm in absorbance reflectance mode. All remaining measurement parameters were left at default settings.
In vitro antioxidant activity
Total phenolic content
Total phenolic content in BDE was determined according to Prussian blue method (CitationBudinin et al., 1980).
DPPH radicals scavenging
Free radical scavenging activity of BDE was estimated by using 1,1-diphenyl-2-picryl hydrazyl (DPPH). A series of different concentrations of extract and standard (ascorbic acid) in the same extraction solvent were prepared (25, 50, 100, 150, 200, 250, 500 and 1000 μg/mL). Then, 50 μL of extract at different concentrations were mixed with 1000 μL of 0.004% DPPH in methanol. The disappearance of DPPH was read spectrophotometrically at 517 nm after 30 min of incubation at room temperature in dark. A purple to yellow color change was observed. Methanol was used as blank and control was used without extract (CitationSevil et al., 2008). The measurements were performed in triplicate and the results were averaged. Free radical scavenging capacity was expressed as percentage inhibition of DPPH radical and was calculated as follows:
From the obtained values, IC50 was determined.
Animals
Twenty-four inbred male Wistar albino rats (180–200 g body weight) were used in this study. Animals were procured from the Institutional animal House (Reg no. 621/02/ac/CPCSEA) of Birla Institute of Technology, Mesra, India. All animals were kept in polyacrylic cages and maintained under standard housing conditions (room temperature 24–27°C and humidity 60–65% with 12:12 light:dark cycles). Food was provided in the form of dry pellets and water ad libitum. The animals were allowed to get acclimatized to the laboratory conditions for 7 days before the commencement of the experiment. All experiments involving animals comply with the ethical standards of animal handling and were approved by Institutional Animal Ethical Committee.
Induction of oxidative stress by oxalate
EG-induced hyperoxaluria model (CitationAtmani et al., 2003) was used to induce oxidative stress and renal cell injury in Wistar albino rats. Animals were divided into four groups comprising six animals in each. Group 1 was used as normal control and given water only and groups 2–4 were given 0.75% v/v EG for 28 days in drinking water to induce low chronic grade hyperoxaluria. Simultaneously, the following treatment was given once daily: Group 1 was given normal saline 10 mL/kg body weight p.o served as normal control group; Group 2 was given normal saline 10 mL/kg body weight p.o. served as untreated hyperoxaluric rats group; groups 3 and 4 were given BDE 100 and 200 mg/kg body weight p.o., respectively, served as treated hyperoxaluric rats group. The doses of BDE were selected on the basis of previous toxicity studies (one tenth of LD50) (CitationChandan et al., 1991; CitationDhar et al., 1968). During the study of 28 days, various biochemical parameter of urine and serum was estimated. At the end of the experimental period, animals were sacrificed and dissected to isolate kidneys for estimation of antioxidant markers and histopathological analysis.
Assessment of renal function and oxalate excretion
Animals were kept in metabolic cages individually for the collection of 24 h urine on 0, 7, 14, 21 and 28th day and volume was measured immediately after collection. Urinary oxalate level was estimated by the colorimetric method using commercial oxalate kit (Sigma). Aliquots of urine sample were taken and examined under microscope (Lieca EZ-4D) for crystalluria analysis. Blood was obtained by cardiac puncture under ether anesthesia at the end of experiment. Blood urea nitrogen (BUN), serum and urine creatinine were estimated using commercial kits (Bio in vitro). Creatinine clearance was calculated according to standard clearance formula C = U/S × V, where U is the urinary concentration of creatinine, S is the concentration of creatinine in the serum and V is the urine volume in mL/min.
Assessment of parameters for oxidative stress and antioxidant status in renal tissue
The isolated right kidneys were used for the preparation of kidney homogenate. Renal cortex was separated and subsequently homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4). The renal cortical homogenates were centrifuged at 1500×g for 10 min at 4°C (CitationBashir & Gilani, 2009). The resulting supernatant were used for the determination of malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), reduced glutathione (GSH) and glutathione peroxidase (GPx).
MDA assay
According to the method of CitationEsterbauer and Cheeseman (1990), MDA was estimated in terms of thiobarbituric acid reactive species (TBARS). Homogenized renal tissue (1 mL) in 2 mL of normal saline was mixed with 1 mL tri-chloro acetic acid (20%), 2 mL thiobarbituric acid (0.67%) and heated for 1 h at 100°C. After cooling, the precipitate was removed by centrifugation. The absorbance of the sample was measured at 535 nm using a blank containing all the reagents except the sample. As 99% TBARS are MDA, TBARS concentrations of the samples were calculated using the extinction coefficient of MDA (1.56 × 105 M−1cm−1) (CitationEl-Demerdash et al., 2009).
CAT assay
The CAT activity was measured using the method of CitationChance and Maehly (1955) by following the decomposition of hydrogen peroxide. The reaction mixture consisted of 2 mL of 100 mM phosphate buffer (pH 7.0), 0.90 mL of hydrogen peroxide (30 mM) and 0.1 mL of supernatant in a final volume of 3 mL. Absorbance was recorded at 240 nm at every 10 s interval for 1 min. One unit of CAT is defined as the amount of enzyme required to decompose 1 µM H2O2/min, at 25°C.
SOD assay
An indirect method of inhibiting auto-oxidation of epinephrine to its adrenochrome was used to assay SOD activities. Kidney homogenate (0.05 mL) was added to 2.0 mL of carbonate buffer and 0.5 mL of 0.01 mM EDTA solution. The reaction was initiated by addition of 0.5 mL of epinephrine (3 × 10−4 M) at pH 10.2 and the change in optical density every minute was measured at 480 nm against reagent blank for 5 min. A graph of absorbance against time was plotted for each sample and the rate of auto-oxidation calculated. One unit of SOD activity is defined as the concentration of the enzyme (mg protein/mL) in the plasma that caused 50% reduction in the auto-oxidation of epinephrine (CitationMisra & Fridovich, 1972; CitationSaggu et al., 1989).
GST assay
GST activity was estimated by the method of CitationHabig et al. (1974) from the rate of increase in conjugate formation between GSH and 1-chloro-2,4-dinitrobenzene (CDNB) by measuring the increase in absorbance at 340 nm. In a 3.00 mL reaction mix, the final concentrations were 97 mM potassium phosphate, 0.97 mM EDTA, 2.5 mM GSH, 1.0 mM CDNB, 3.2% (v/v) ethanol and 0.0075–0.015 unit GST. In the test, kidney homogenate added instead of enzyme. Immediately, absorbance was taken at 340 nm at 30 s interval for 5 min.
GPx assay
GPx activity was measured by using the method of CitationPaglia and Valentine (1967). Reaction mixture contained 2.5 mL of 0.1 M/l Tris-HCl buffer (pH 7.2), 75 µL of 0.04 M/l GSH, 100 µL of 0.1 M/l nicotinamide adenine dinucleotide phosphate (NADPH) and 100 µL of GSH (0.24 units). Homogenate (20 µL) was added to the reaction mixture. Reaction was initiated by adding 100 µL of 0.75 mM hydrogen peroxide. The decrease in absorbance was measured at 340 nm for 3 min at every 30 s interval. The activity was expressed as unit/mg protein using molar extinction coefficient of 6.22 × 103 (mM/L)−1cm−1.
Reduced glutathione
GSH was measured by using the method described by CitationDringen and Hamprecht (1996) with slight modifications. Tissue homogenate 50 µL was diluted with 50 µL of 100 mM phosphate buffer containing 1 mM EDTA. To this mixture, 100 µL of reaction buffer (295 µM 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) made in 10 mL of phosphate buffer) was added and change in absorbance was measured at 412 nm for 5 min at every 30 s interval. Reduced pure GSH was used to obtain a standard curve. Reduced GSH was expressed as µM GSH/mg tissue.
Protein estimation
Protein estimation was done by using standard protocol of CitationLowry et al. (1951). Bovine serum albumin was used as standard, and the color developed was read at 660 nm.
Histopathological studies
The left kidney excised from animal was immediately fixed in 10% buffered formalin (pH 7.0). The tissues were dehydrated with ascending grade of alcohol and embedded with paraffin wax (M.P. 55°C). Paraffin kidney sections (6 µm thick) were cut, mounted on slides with Mayer’s albumin solution, deparaffinized, rehydrated with descending grade of alcohol and finally stained with hemotoxylin and eosin (CitationAtmani et al., 2009). The kidney sections were examined under light microscope (Lieca EZ-4D) to evaluate pathological changes and photomicrographs were taken. A semiquantitative scoring method was used to analyze the renal tissue damage and crystal deposition.
Statistical calculations
All the data were expressed as mean ± standard error of mean (SEM). All statistical comparisons between the groups were made by means of one way analysis of variance (ANOVA) with post hoc Tukey-Kramer’s multiple comparison test. The concentration-response curves were analyzed by nonlinear regression and IC50 was calculated by “log (inhibitor) vs. response—Variable slope” using Graphpad Prism 5 software.
Results
HPTLC characterization, total polyphenolics and DPPH scavenging of BDE
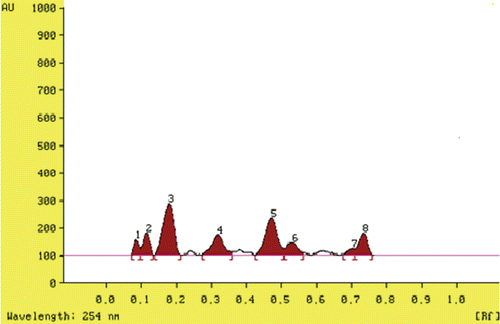
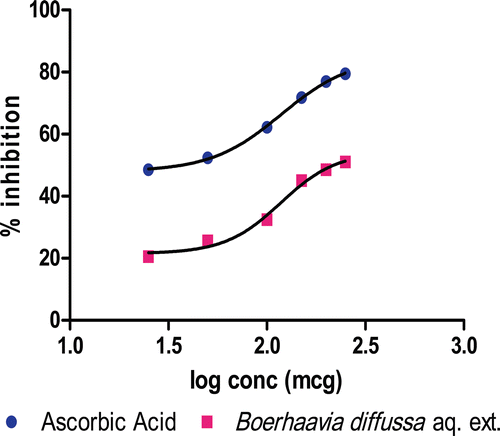
The HPTLC fingerprints of the BDE is shown in , prominent peaks were observed at Rf values of 0.09, 0.12, 0.18, 0.31, 0.47, 0.51, 0.69 and 0.72 with area under the curve 726.8, 1428.6, 5369.0, 2355.0, 4293.3, 1355.0, 444.7 and 1815.7 unit, respectively. The total polyphenolic content was found to be 5.2 mg/g of extract, expressed as gallic acid equivalent. BDE shows dose-dependent scavenging of DPPH, with IC50 value of 119.9, which is comparable with ascorbic acid used as standard (IC50, 122.0) ().
Evaluation of renal functioning
The urine output was increased significantly (p < 0.001) in untreated as well as BDE-treated hyperoxaluric rats. The urine output in control group was 6.77 ± 0.48 mL/24 h/rat, which was increased to about 79.47% in hyperoxaluric rats (Group II) and 136.93 and 254.95% in BDE treated rats, which still remains significantly (p < 0.01; p < 0.001) higher in BDE treated rats in dose-dependent manner as compared to untreated hyperoxaluric group. Hyperoxaluric treatment (EG) induced hyperoxaluria in rats and urinary excretion of oxalate in untreated hyperoxaluric rats (Group II) increased tremendously (p < 0.001), as compared to control rats (Group I). In BDE treated rats (group III and IV) again the oxalate excretion is high as compared to control rats but there was a significant (p < 0.001) reduction as compared to untreated hyperoxaluric rats ().
Table 1. Effect of BDE treatment on oxalate excretion and renal function in rats.
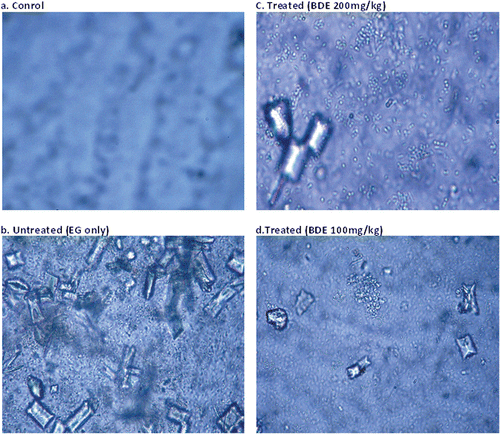
Microscopic observation revealed that urine of control rats was devoid of any crystal (). revealed the presence of numerous aggregated CaOx monohydrate (COM) crystals (dumbbell-shaped) and CaOx dihydrate (COD) crystals (bipyramidal shaped) in untreated hyperoxaluric rat’s urine. Only few COD crystals were observed in BDE-treated animals as shown in and . BDE visibly reduced the size and number of the crystals in dose-dependent manner.
Figure 3. CaOx crystals in the urine of different treatment groups (Leica EZ-4D) at 40 × 10× magnification. (A) Control rats showed very few or no crystals; (B) Untreated rats excreted numerous oval COM and pyramidal shaped COD crystals; (C and D) Treated rats excreted a significantly reduced number of crystals.
Hyperoxaluric treatment caused impairment of renal functions in untreated hyperoxaluric rats as evident from the markers of glomerular and tubular damage, viz, raised BUN (p < 0.001) and serum creatinine (p < 0.01), and reduced creatinine clearance (p < 0.001), which were dose-dependently prevented in the animals receiving a simultaneous treatment with BDE.
Renal oxidative stress and antioxidant status
Hyperoxaluria inducing treatment enhanced MDA and total protein content (p < 0.001), decreased GSH level (p < 0.001) and activities of the antioxidant enzymes including SOD (p < 0.001), GPx (p < 0.001) and CAT (p < 0.001) in kidneys of the untreated rats as compared to the control animals. A simultaneous treatment with BDE protected against the oxidative changes induced by hyperoxaluric treatment in a dose-dependent manner (data shown in ).
Table 2. Effect of BDE treatment on renal enzymes and other antioxidant markers in rat kidney homogenate analysis.
Histological analysis
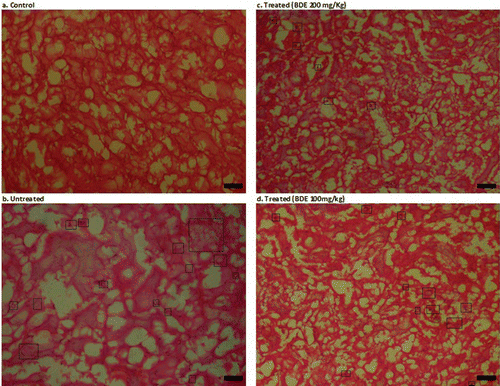
Renal histology of control animals showed no crystal deposits with normal glomeruli and tubular architecture (), whereas animals exposed to EG only showed shrinkage of glomeruli and severe tubular damage (). Flat epithelial lining and glomerular capsule was also broken at few instances. Numerous crystal depositions were seen in renal tubule lumen and interstitial sites. Grossly, kidneys of untreated hyperoxaluric animal were damaged severely (). However, simultaneous treatment with BDE ( and ) reduced these renal injuries and normalized the renal architecture in dose-dependent manner. Crystal deposits were also visibly small and less abundant compared to those in the untreated kidneys ().
Table 3. Histopathological analysis of hyperoxaluria-induced renal damage and crystal deposits.
Figure 4. Histopathological examination of kidney slides of different treatment groups (Leica EZ-4D) at 10 × 10× magnification. (A) Control rats showing normal renal architecture; (B) Kidney of untreated rats showing polymorphic irregular crystal deposits tubular damage (in figure, black spots inside the rectangular box are crystal deposits); (C) BDE-treated (100 mg/kg) showed few crystal deposits and little dilation of tubules; (D) BDE-treated (200 mg/kg) showed a very few or no crystal deposits and nearly normal renal architecture.
Discussion
It has been postulated that oxalate-induced damage to renal cells may contribute to a number of renal pathologies, including the deposition of CaOx stone in kidney (CitationHackett et al., 1995; CitationScheid et al., 1995). High concentration of oxalate is toxic for renal epithelial cells, producing injury, alteration in membrane integrity and death of renal cells through the generation of ROS and increased oxidative stress (CitationKurien & Selvam, 1989; CitationMiller et al., 2000). Compounds with antioxidant potential have been found to be fairly useful in combating these disorders. Therefore, a huge body of research is focused on exploring safe and effective antioxidant compounds. Plant extracts or plant-based antioxidants are not only efficient, but also relatively safer than the synthetic ones.
B. diffusa contains a large number of phenolic constituents, viz, 3,4-dihydroxy-5-methoxycinnamoyl-rhamnoside, eupalitin 3-O-galactosyl(12) glucoside, caffeoyltartaric acid, kaempferol 3-O-robinobioside, eupalitin 3-O-galactoside, quercetin and kaempferol (CitationFerreres et al., 2005). These phenolic constituents are responsible for antioxidant activity of B. diffusa. Total phenolic content and DPPH radical scavenging activity of BDE shows its potent antioxidant potential. BDE efficiently quenches hyperoxaluria generated ROS such as superoxide, peroxide, and hydroxyl radicals.
The most harmful consequence of hyperoxaluria is deposition of the CaOx crystal in kidney. Primary or secondary hyperoxaluria is the major risk factor for the urolithiasis (CitationSellaturay & Fry, 2008). In the present study, low chronic grade hyperoxaluria in animals was induced by adding the 0.75% EG in drinking water to mimic the idiopathic hyperoxaluric condition in human (CitationAtmani et al., 2009). Oxlate excretion significantly increased in hyperoxaluric animals as compared to control and protected in BDE-treated animals. EG increase oxalate production by way of increase substrate availability that induce the activity of oxalate synthesizing enzyme. Glycolic acid oxidase (GAO) and lactate dehydrogenase (LDH) catalyses the oxidation and reduction of glyoxalate results in formation of glycolate and oxalate (CitationSoundararajan et al., 2006). In a recent study, it was reported that pretreatment with aqueous and ethanolic extracts of B. diffusa decreased the activities of LDH (CitationOlaleye et al., 2010). Probably, BDE inhibits the oxalate synthesizing enzyme and prevent the increased urinary excretion of oxalate. BDE-treated animals showed increased urine volume with respect to control and hyperoxaluric animals. Increase of palatability due to sweetness of EG increased the water intake in untreated rats followed by increased urine volume, but it still remains significantly higher in BDE-treated group. This is due to well-established diuretic activity of B. diffusa (CitationGaitonde et al., 1974). Increased urine volume decreases the saturation of the oxalate and prevents the precipitation of the CaOx at physiological pH. Diuresis also flushes out the renal system and helps in mechanical expulsion of the stone. Diuretic action of the drug is due to Punarnavine in conjunction with potassium nitrate and other potassium salts (CitationChopra et al., 1923).
Various physiological inhibitors of urolithiasis found in urine including inorganic (e.g. magnesium) and organic (e.g. Glycosaminoglycans, citrate and other macromolecule) substances were known to inhibit stone formation. Organic inhibitory compounds adsorb to the surface of the crystal, thereby inhibiting crystal nucleation, growth and aggregation (CitationBasavaraj et al., 2007). Macromolecule of higher molecular weight of plant extract exhibits their action similar to natural urinary inhibitors and inhibits crystal aggregation and growth. It is reported that B. diffusa has some macromolecular constituent (>20 KD) which may be responsible for crystallization inhibitory effect of plant (CitationAwasthi & Verma, 2006). Decreased excretion of COM crystal in BDE-treated animals () has several positive virtues. First, it shows that substances from the plants excerpt their action directly or indirectly on crystals morphology. Second, the apparition of more COD than COM particles is advantageous since COM crystals have high adhesion affinity to renal epithelial cells when compared to COD particles (CitationAtmani et al., 2009).
CaOx crystal agglomerate tends to retain in kidney by trapping in renal tubules and develop into renal stones, which damage the renal tissue and deteriorate the renal function. Impairment of renal functions of untreated rats is evident from the markers of glomerular and tubular damage: raised BUN serum creatinine and reduced creatinine clearance (CitationKaradi et al., 2006). Renal dysfunction diminishes the ability to filter urea and creatinine clearance, so the BUN and creatinine level rises in blood (CitationCao et al., 2003). The normalization of BUN and creatinine clearance in BDE-treated animals as compared to hyperoxaluric animals shows that it protects the deterioration of the renal function by minimizing tubular damage and crystal deposition.
Further, we assessed the effect of oxalate exposure on the rat kidneys by estimating oxidative stress markers. Hyperoxaluric treatment caused extensive CaOx crystal deposition in kidneys of untreated rats accompanied by oxidative damage as reflected from increased levels of markers of oxidative injury such as MDA and protein carbonyl content and decreased activities of antioxidant enzymes along with GSH level. MDA is a major end product of lipid peroxidation in membrane fatty acids representing oxidative tissue damage caused by ROS resulting in structural alteration of membrane with release of cell and organelle contents, loss of essential fatty acids with formation of cytosolic aldehyde and peroxide products (CitationKato et al., 2007). In the present investigation, the level of MDA was found to be significantly elevated with oxalate exposure in hyperoxaluric rats. Exposure to oxalate generates toxic responses in renal epithelial cells, including altered membrane surface properties, changes in gene expression (NF-κB), disruption of mitochondrial function and formation of ROS (CitationJonassen et al., 2005). Mitochondria are a major site of ROS formation and oxalate-induced activation of NADPH oxidase is another source of ROS in renal cells. Animal model studies have provided evidence for the hyperoxaluria-induced activation of the renin-angiotensin system (RAS) and angiotensin II; implicated in causing oxidative stress by activating membrane associated NADPH oxidase, which leads to the production of ROS (CitationAntus et al., 2001; CitationKhan, 2004, 2005). Reduction of angiotensin II production by inhibiting ACE or blocking angiotensin receptors has been shown to significantly reduce renal CaOx crystal deposition as well as the development of interstitial inflammation (CitationToblli et al., 2002).
It was reported that B. diffusa have ACE inhibitory activity (CitationHansen et al., 1995). ROS also culminate in phospholipase A2 activation through transcription factor NF-κB (CitationLappas et al., 2004), as NF-κB can be activated by the stress of oxidants (CitationSiebenlist et al., 1994) and oxalate exposure promotes rapid degradation of IκBα, an endogenous inhibitor of the NF-κB transcription factor (CitationJonassen et al., 2005). The inhibition of the lipid peroxidation (decreased MDA level) after post-treatment of BDE can be attributed to scavenging the ROS and indirect inhibition of phospholipase A2 through inactivation of NF-κB. Eupalitin a important constituent of the B. diffusa is accredited with NF-κB inactivation activity (CitationPandey et al., 2005). Free radical scavenging enzymes such as CAT, SOD and GPx are the cellular defense enzymes against oxidative injury, decomposing superoxide and peroxide before their interaction to form the more reactive hydroxyl radical. Under oxidative stress conditions, ROS are reduced by conjugation with GSH directly or by means of GSH-related enzymes, which decrease GSH levels (CitationLi et al., 2010). GST also plays a key role in cellular detoxification by catalyzing the reaction of glutathione with toxicants to form an S-substituted glutathione (CitationEl-Demerdash et al., 2009).
Several in vivo and in vitro studies have demonstrated that exposure to high level of oxalate results in greater production of superoxide and peroxide free radicals, leading to redox imbalance and have been manifested as antioxidant depletion, peroxidation of lipid and oxidation of protein (CitationHackett et al., 1995; CitationThamilselvan & Selvam et al., 1997). Recent studies have provided evidence that CaOx kidney stone patients excrete significantly higher amounts of GST and MDA in their urine, indicating ROS in kidneys of CaOx stone patients (CitationHuang et al., 2003; CitationPuntel et al., 2007). The accumulation of these products was concomitant with the decrease in the antioxidant enzymes SOD, CAT, and GPx as well as GSH and protein thiol. All the above parameters were reported to decrease in hyperoxaluria induced urolithiasis (CitationRodrigo & Bosco, 2006; CitationPuntel et al., 2007). Recent studies evidenced that vitamin E therapy prevents CaOx deposition in the rat kidney and reduced renal cell injury by restoring these enzymes (CitationThamilselvan & Menon, 2005).
Similarly, antioxidant constituent (flavanoids) of B. diffusa effectively scavenge the superoxide and peroxide radicals and protect the renal cell from oxidative stress induced injuries, which is evident from restoration of SOD, CAT, GPx, GST and GSH level in BDE-treated animal as compared to hyperoxaluric animals.
Renal histopathology also supports the above results as evident from CaOx crystal deposition, shrinkage of glomeruli and tubular damage in kidneys of untreated rats. Tissue injury, loss of membrane integrity and inflammation in kidney of these animals are due to hyperoxaluria-induced lipid peroxidation and depletion of antioxidant enzymes (CitationSanthosh Kumar & Selvam, 2003; CitationThamilselvan & Menon, 2005; CitationItoh et al., 2005). Renal epithelial injury promotes crystal retention, as epithelial injury exposes a variety of crystal adhesion molecules on epithelial surfaces (CitationBijarnia et al., 2008). These changes facilitate CaOx crystal adherence and retention in renal tubules (CitationKhan, 2005). However, treatment with BDE ( and ) inhibited crystal deposition and ameliorates renal injury through free radical scavenging, inhibition of lipid peroxidation and restoration of antioxidant enzyme.
Conclusion
Results of this study demonstrate the ameliorating effect of B. diffusa in hyperoxaluria-induced oxidative stress. BDE potently scavenge the ROS, inhibit the lipid peroxidation, restore the antioxidant enzyme activity, prevent the CaOx crystal deposition and maintain the renal function. Thus, various phytoconstituents of B. diffusa synergistically combats with hyperoxaluria-induced oxidative stress and renal cell injury, possibly mediated through antioxidant activity of plant.
Acknowledgment
The authors are thankful to the Department of Pharmaceutical Sciences, BIT, Mesra, for providing the necessary facilities to carry out the research work.
Declaration of interest
The authors report no conflicts of interest.
References
- Antus B, Exton MS, Rosivall L. (2001). Angiotensin II: A regulator of inflammation during renal disease? Int J Immunopathol Pharmacol, 14, 25–30.
- Atmani F, Sadki C, Aziz M, Mimouni M, Hacht B. (2009). Cynodon dactylon extract as a preventive and curative agent in experimentally induced nephrolithiasis. Urol Res, 37, 75–82.
- Atmani F, Slimani Y, Mimouni M, Hacht B. (2003). Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int, 92, 137–140.
- Awasthi LP, Verma HN. (2006). Boerhaavia diffusa—A wild herb with potent biological and antimicrobial properties. Asian Agri-History, 10, 55–68.
- Basavaraj DR, Biyani CS, Browning AJ, Cartledge JJ. (2007). The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Series, 5, 26–136.
- Bashir S, Gilani AH. (2009). Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J Ethnopharmacol, 122, 106–116.
- Bijarnia RK, Kaur T, Aggarwal K, Singla SK, Tandon C. (2008). Modulatory effects of N-acetylcysteine on hyperoxaluric manifestations in rat kidney. Food Chem Toxicol, 46, 2274–2278.
- Budinin R, Tonelli D, Girotti. (1980). Analysis of total phenols using the Prussian blue method. J Agric Food Chem, 28, 1236–1238.
- Cao ZG, Liu JH, Radman AM, Wu JZ, Ying CP, Zhou SW. (2003). An experimental study of effect of different extracts of Alisma orientalis on urinary calcium oxalate stones formation in rats. Zhongguo Zhong Yao Za Zhi, 28, 1072–1075.
- Chance B, Maehly C. (1955). Assay of catalase and peroxidase. Methods Enzymol, 11, 764–775.
- Chauhan CK, Joshi MJ, Vaidya ADB. (2009). Growth inhibition of struvite crystals in the presence of herbal extract Boerhaavia diffusa Linn. Am J Infect Dis, 5, 177–186.
- Chopra RN, Ghosh S, Dey P, Ghosh BN. (1923). Pharmacology and therapeutics of Boerhaavia diffusa (Punarnava). Ind Med Gaz, 68, 203–208.
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. (1968). Screening of Indian plants for biological activity: I. Indian J Exp Biol, 6, 232–247.
- Dringen R, Hamprecht B. (1996). Glutathione content as an indicator for the presence of metabolic pathways of amino acids in astroglial cultures. J Neurochem, 67, 1375–1382.
- El-Demerdash FM, Yousef MI, Radwan FM. (2009). Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol, 47, 249–254.
- Esterbauer H, Cheeseman KH. (1990). Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Meth Enzymol, 186, 407–421.
- Ferreres F, Sousa C, Justin M, Valentão P, Andrade PB, Llorach R, Rodrigues A, Seabra RM, Leitão A. (2005). Characterisation of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem Anal, 16, 451–458.
- Gaitonde BB, Kulharni HJ, Nabar SD, Joglekar SN. (1974). Diuretic activity of Punarnava (Boerhavia diffusa). Bull Haffkine Inst, 2, 24.
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem, 249, 7130–7139.
- Hackett RL, Shevock PN, Khan SR. (1995). Alterations in MDCK and LLC-PK1 cells exposed to oxalate and calcium oxalate monohydrate crystals. Scanning Microsc, 9, 587–596.
- Hansen K, Nyman U, Smitt UW, Adsersen A, Gudiksen L, Rajasekharan S, Pushpangadan P. (1995). In vitro screening of traditional medicines for anti-hypertensive effect based on inhibition of the angiotensin converting enzyme (ACE). J Ethnopharmacol, 48, 43–51.
- Huang HS, Ma MC, Chen CF, Chen J. (2003). Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology, 62, 1123–1128.
- Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. (2005). Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urol Res, 33, 261–266.
- Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M. (2005). Oxalate toxicity in renal cells. Urol Res, 33, 329–339.
- Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. (2006). Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol, 105, 306–311.
- Kasana MS, Chauhan N, Prachi. (2009). Plants of Muzaffarnagar district used in treatment of urinary tract and kidney stones. Indian J Traditional Knowledge, 8, 191–195.
- Kato J, Ruram AA, Singh SS, Devi SB, Devi TI, Singh WG. (2007). Lipid peroxidation and antioxidant vitamins in urolithiasis. Indian J Clin Biochem, 22, 128–130.
- Khan SR. (2004). Crystal-induced inflammation of the kidneys: Results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol, 8, 75–88.
- Khan SR. (2005). Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res, 33, 349–357.
- Santhosh Kumar M, Selvam R. (2003). Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem, 14, 306–313.
- Kurien TB, Selvam R. (1989). Induction of lipid peroxidation in calcium oxalate stone formation. Indian J Exp Biol, 27, 450–453.
- Lappas M, Permezel M, Georgiou HM, Rice GE. (2004). Regulation of phospholipase isozymes by nuclear factor-κB in human gestational tissues in vitro. J Clin Endocrinol Metab, 89, 2365–2372.
- Li ZH, Velisek J, Zlabek V, Grabic R, Machova J, Kolarova J, Randak T. (2010). Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem Biol Interact, 183, 98–104.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Miller C, Kennington L, Cooney R, Kohjimoto Y, Cao LC, Honeyman T, Pullman J, Jonassen J, Scheid C. (2000). Oxalate toxicity in renal epithelial cells: Characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol, 162, 132–141.
- Mishra J, Singh R. (1980). The effect of indigenous drug Boerhaavia diffusa on kidney regeneration. Indian J Pharmacol, 12, 59–64.
- Misra HP, Fridovich I. (1972). The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem, 247, 6960–6962.
- Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA. (2010). Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol, 48, 2200–2205.
- Paglia DE, Valentine WN. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. j Lab Clin Med, 70, 158–169.
- Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S. (2005). Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol, 5, 541–553.
- Puntel RL, Roos DH, Paixão MW, Braga AL, Zeni G, Nogueira CW, Rocha JB. (2007). Oxalate modulates thiobarbituric acid reactive species (TBARS) production in supernatants of homogenates from rat brain, liver and kidney: Effect of diphenyl diselenide and diphenyl ditelluride. Chem Biol Interact, 165, 87–98.
- Rodrigo R, Bosco C. (2006). Oxidative stress and protective effects of polyphenols: Comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol, 142, 317–327.
- Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, Marsden CD. (1989). A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem, 53, 692–697.
- Scheid CR, Koul HK, Kennington L, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Menon M. (1995). Oxalate-induced damage to renal tubular cells. Scanning Microsc, 9, 1097–105; discussion 1105.
- Sellaturay S, Fry C. (2008). The metabolic basis for urolithiasis. Surgery, 26, 136–140.
- Selvam R. (2002). Calcium oxalate stone disease: Role of lipid peroxidation and antioxidants. Urol Res, 30, 35–47.
- Sevil A, Ahmet A, Ergin H. (2008). Determination of antimicrobial and antioxidant activities of Turkish endemic Salvia halophila Hedge. Turk J Biol, 32, 265–270.
- Chandan BK, Sharma AK, Anand KK. (1991). Boerhaavia diffusa: A study of its hepatoprotective activity. J Ethnopharmacol, 31, 299–307.
- Siebenlist U, Franzoso G, Brown K. (1994). Structure, regulation and function of NF-κB. Annu Rev Cell Biol, 10, 405–455.
- Singh RH, Udupa KN. (1972). Studies on the Indian indigenous drug Punarnava (Boerhaavia diffusa Linn) Part IV: Preliminary controlled clinical trial in nephrotic syndrome. J Res Indian Med, 7, 28–33.
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. (2006). Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol, 44, 981–986.
- Thamilselvan S, Menon M. (2005). Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int, 96, 117–126.
- Thamilselvan S, Selvam R. (1997). Effect of vitamin E and mannitol on renal calcium oxalate retention in experimental nephrolithiasis. Indian J Biochem Biophys, 34, 319–323.
- Toblli JE, Ferder L, Stella I, De Cavanaugh EM, Angerosa M, Inserra F. (2002). Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol, 168, 1550–1555.