Abstract
Objective: Atriplex lentiformis (Torr.) S.Wats (Chenopodiaceae) is a wild plant which is in use by Bedouin in treatment of general fatigue, therefore, there is a need to explore the potential antioxidant activity of the extracts and isolated compounds of this plant.
Methods: Column chromatography and spectroscopic analysis were used for isolation and identification of the compounds. The antioxidant activity was evaluated in vitro using the ABTS•+ (2,2′-azino-bis-3-ethyl-bezthiazoine-6-sulphuric acid) radical scavenging model. Liver and kidney functions were investigated after oral administration of total alcohol, successive extracts, and isolated compounds.
Results: Two new flavonoids, quercetin-6,4′-dimethoxy-3-fructo-rhamnoside 1 and quercetin-4′-methoxy-3-fructo-rhamnoside 2 in addition to five known compounds (kaempferol-4′-methoxy-3-rutinoside 3, kaempferol-7-O-rhamnoside 4, kaempferol-3,7-O,O-dirhamnoside 5, quercetin 6, and kaempferol 7) were isolated. Oral administration of total ethanol, diethyl ether, chloroform, ethyl acetate and n-butanol extracts showed no signs of toxicity up to (5 g/kg. b.wt.). All extracts and isolated compounds showed varied antioxidant activity ranged from 129 to 952 µmol Trolox equivalent/gram dry weight with maximum level for the two new isolated flavonoids (985 and 895 µmol Trolox equivalent/gram dry weight). Animals received both total ethanol and n-butanol extracts showed a significant increase in ALT, AST, blood urea, and serum creatinine levels.
Introduction
Chenopodiaceae (goosefoot family) is one of the plant families which have an interesting distribution throughout the world especially in arid and saline regions, it contains 104 genera and more than 1400 species, most of them are growing naturally in saline soils. Atriplex is one of Chenopodious members (Boulos, 2000; Maharan, 1967) that contains high levels of oxalates and low percentage of quaternary alkaloids (CitationOsmond, 1963; CitationSandberg et al., 1967) in addition to HCN, saponins, selenium, nitrites, tannin, alkaloids, essential oils, flavonoids, and triterpenoids (CitationDuke, 1977). The aerial parts of Atriplex spp contain 1-O-ferulyl β-d-glucose (CitationDuke, 1977). Atriplex spp. also contains sterols, saponins (CitationAbu-Zanzta et al., 2003), in addition to a number of flavonoids such as rhamnetin, isorhamntein, quercetin, kaempferol, kaempferol-3-O-sulfate-7-O-α-arabinopyranoside, quercetin-3-O-sulfate-7-O-α-arabinopyranoside, isorhamnetin-3-O-rhmnosyl(1→6)β-d-glucopyranoside and isorhamnetin 7-O-glucopyranoside (CitationBylkaa, 2001; CitationHayley et. al., 2004). Atriplex spp. were characterized by their high content of sodium chloride (CitationWatt et al., 1962).
Atriplex lentiformis (Torr.) S.Wats. was cultivated successfully under saline conditions in South Sinai (CitationSabry et al., 2003). However, there is no previous reported data on its chemical contents or related pharmacological use, therefore this study was carried out to investigate the different chemical components, pharmacological, and toxicological activates of this plant.
Material and methods
Plant material
The aerial parts of Atriplex lentiformis (Quail saltbush) were collected from the Najed desert in the summer of 2008. The sample was kindly identified by Dr. M. Gebali, botanist, and by comparison with the published plant description (CitationEl-Gohary, 2004). A voucher specimen of the titled plant has been deposited in the Chemistry Department, King Saud University. Plant material was air dried in shade, reduced to fine powder, packed in tightly closed containers and stored at room temperature for phytochemical and biological studies.
Apparatus
UV spectra were measured on Shimadzu 1201 spectrophotometer. Melting points were determined on a Kofler hot-stage apparatus; mass spectra samples were dissolved in acetonitrile and injected into a Micromass Quattro spectrophotometer. 1H and 13C NMR spectra, using external electronic referencing through the deuterium resonance frequency of the solvent and determined at 600.17 or 150.91 MHz, respectively, with a JEOL ECA600 NMR spectrophotometer fitted with an auto-tune 5 mm X/H probe. Carbon atom types were established in the 13C NMR spectrum by employing a combination of broad-band proton-decoupled and DEPT (90 and 135) experiments. [1JC-H] and 2JC-H and 3JC-H] 1H-13C correlations were established by using HMQC and HMBC pulse sequences respectively. 1H-1H correlations were determined by double quantum filtered COSY. Shimadzu HLPC system, system controls SCL− 10 Agvp, system control SCL-10 Avp, pumps (binary pumps) LC-10 ADvp, degasser: DGU- 14A, refractive index detector: RID-10A, column: phenomenex NH2 column (250 mm length × 4.6 mm diameter, 5 um particle size), mobile phase: 0.1 N sulphuric acid, Flow rate: 0.5 mL/min and temperature.
Extraction and isolation
The air-dried powder (3 kg) of Atriplex lentiformis (aerial parts) was extracted by percolation in ethanol (5 L) at the room temperature untill exhaustion for two days and filtered off. The mark lifted was re-percolated again (this process was repeated four times). The combined ethanol extracts were concentrated under reduced pressure at a temperature not exceeding 35°C. The obtained total dry extract (720 g) was diluted with water (1000 mL) and successively extracted with diethyl ether, chloroform, ethyl acetate, and n-butanol, respectively. Each extract was dried over anhydrous sodium sulphate and concentrated as before to yield 16.55, 13.46, 23.28, and 94.9 g dry extracts, respectively.
Using silica gel G for TLC (precoated plates)
The extracts were examined using solvent systems, ethyl acetate-methanol-water (30:5:4) (a); chloroform-methanol (95:10) (b); ethyl acetate-methanol-acetic acid-water (65:15:10:10) (c); butanol-acetic acid-water (4:1:5) (d) and methanol-water (1:1) (e). Visualization of chromatograms was achieved under UV before and after exposure to ammonia vapor or by spraying with aluminum chloride (CitationStahl, 1969).
TLC examination of the different extracts using solvent systems (a and c) and visualizing reagent revealed presence of same two spots in ethyl acetate and chloroform extracts while ethyl acetate and n-butanol extracts were found to have four similar spots.
From the pharmacological results, only ethyl acetate and butanol extracts showed high antioxidant activity. Both extracts (100 g) were subjected to column chromatography backed with silica gel (1500 g) and eluted with system (a), 200 fractions (100 mL each) were collected. Fractions of similar spots and Rf values were combined together to obtained 6 sub-groups with yields of (6.9, 8.5, 9.6, 7.8, 10.4 and 9.5 g, respectively). Each group was reapplied on silica gel column again (210, 255, 285, 240, 300, and 280 g, respectively) and each eluted gradually with ethyl acetate-methanol, eight collective fractions were obtained from all columns and reapplied on preparative paper chromatography using systems (b) and (c) followed by preparative thin layer chromatography using system (a). Bands corresponding to each compound were separately eluted with methanol and concentrated using reduced pressure. For final purification each bands was applied on the top of column backed with Sephadex LH-20 eluted with system (e). Compounds 1–7 were isolated and crystallized from methanol.
Determination of LD50
Using the method of CitationLorke, (1983) LD50 of all tested extracts was estimated in mice. In a preliminary test, animals in groups of three, received one of 10, 100, or 1000 mg kg−1 of the tested extract suspended in the vehicle (3% v/v Tween 80). Animals were observed for 24 h for signs of toxicity and number of deaths. Depending on the results of the preliminary test, doses of (500–4000 mg kg−1) of the tested extracts were administered to fresh groups, each of 6 mice. Control animals were received the vehicle and kept under the same conditions. Signs of toxicity and number of deaths per dose were recorded within 24 h and the LD50 was calculated.
Antioxidant
The antioxidant evaluation was carried out in vitro via scavenging of the ABTS•+ (2,2′-azino-bis-3-ethyl-bezthiazoine-6-sulphuric acid) radical, generated by a metmyoglobin/hydrogen peroxide system, as described by (CitationRice-Evans & Miller, 1994). The plant extracts (total ethanol, ether, chloroform ethyl acetate and n-butanol) were centrifuged (2000 × g, 10 min) to remove insoluble matter, and the supernatants retained were tested for their antioxidant activity in addition to the isolated compounds 1–7. Each plant extract (10 µl) was added separately to a 1 cm path length spectrophotometer cuvette (1 mL capacity) containing 20 mM phosphate buffered saline pH 7.4, 2.5 µM metmyoglobin and 150 µM ABTS. The reaction was initiated by addition of 75 µM hydrogen peroxide, and the absorbance change at 734 nm monitored at 30°C. A quantitative relationship exists between the absorbance at 734 nm measured after 6 min, and the antioxidant status of the plant extract.
The antioxidant assay kit was purchased from Randox Laboratories (Diamond Road, Crumlin, Co. Antrim, United Kingdom) and the assay was performed using a Boehringer Mannheim Hitachi 717 automatic analyzer. A two-point calibration was used. In the first stage of the assay, metmyoglobin was reacted with hydrogen peroxide to produce the ferrimyoglobin free radical which was then incubated with a chromagen, 2,2-amino-di-(3-ethylbenzthiazole sophonate) to produce ATBSR + (a radical cation with a blue green color measured at 600 nm). Antioxidants in the added plant aqueous extracts suppressed the blue–green color to a degree that was proportional to their concentration. The absorbance of the resulting oxidized solution was compared to that of the calibrated standard, Trolox (6-hydroxy-2,5,7-tetramethylchroman-2-carboxylic acid) (a water soluble vitamin E analogue). Results were expressed as µmol Trolox equivalents per gram dry weight of plant.
Effect on liver and kidney functions
Sixty male Sprague-Dawley rats (120–150 g b.wt.) were used. Animals were kept under standard laboratory conditions with commercial pellet diet and water. These rats were randomly divided into 6 equal groups. First group (G1) was received the vehicle (water) and served as control. The second group (G2) was received ether extract. The third group (G3) was received chloroform extract. The fourth group (G4) was received ethyl acetate extract. The fifth (G5) group received n-butanol extract. The sixth group (G6) was received total ethanol extract. Groups (2–6) received oral dose of (400 mg/kg b. wt.) daily for 45 days. On the other hand, two groups of rats (5 animals each) were used, where compounds 1 and 2 were orally administrated to animals at dose (60 mg/kg b. wt.) daily for 5 days. Blood samples were collected after 15, 30, and 45 days for the first six groups and after 5 days for the two other groups by puncturing of retro-orbital plexus. Blood sample was placed in a plain centrifuge tube for serum separation and determination of the activities of aspertate aminotransferase (AST) and alanine aminotransferase (ALT), serum creatinine and blood urea (CitationHenary, 1974).
Total acid hydrolysis
The isolated compound 1 and 2 (10 mg each) were subjected separately to total and mild acid hydrolysis using 0.1N HCl (CitationEL-Sayed et al., 1998) for 1 h. The aqueous extracts were neutralized using barium carbonate and filtered off. The filtrates were extracted with ethyl acetate to separate the aglycone moiety from the glycone. Ethyl acetate extracts were concentrated and subjected to UV shift reagents, 1H-NMR and 13C-NMR.
The aqueous layers (contain glycone) were tested on TLC alongside with sugars using the system ethyl acetate-methanol-acetic acid-water (65:15:10:10 v/v/v/v) and sprayed with naphthoresocinol-sulphuric acid, these results were confirmed by HPLC
Quercetin-6,4′-dimethoxy-3-fructo-rhamnoside (1)
Yellow crystals (320 mg), m.p. 231°C, soluble in methanol. It gave positive result with Molisch’s test, its Rf = 0.59 in system (a). UV: λmax (MeOH): (nm) 256, 348, 352, (NaOMe) 272, 334, 385, (AlCl3) 269, 381, (AlCl3/HCl) 268, 379, (NaOAc) 254, 360, (NaOAc/H3BO3) 257, 361. 1H NMR(DMSO-d6), δ; 7.81(1H, dd, J = 8.5 Hz, J = 2.5 Hz, H-2′), 7.45 (1H, dd, J = 8.5 Hz, J = 2.5 Hz, H-6′), 6.87 (1H, d, J = 8.5 Hz, H-5′), 6.43 (1H, d, J = 2.5 Hz, H-8), 5.39 (1H, d, J = 2 Hz, H1′’ rhamnose), 4.37 (1H, d, J = 2, H1′′′ glucose), 3.79, 3.70 protons of two OCH3 groups, 3.28–3.36 (m, remaining sugar protons) and 0.94 (3H, d, J = 6, CH3 rhamnose. 13C NMR (DMSO-d6): 177.7 (C-4), 156.7 (C-7), 152.69 (C-5), 152.42 (C-2), 149.86 (C-9), 147.38 (C-4′), 133.13 (C-3′), 132.1 (C-3), 122.69 (C-6′), 121.65 (C-1′), 113.74 (C-2′), 115.75 (C-5′), 104.27(C-10), 101.43 (C-1′′), 101.80 (C-1′′′), 98.8 (C-6), 93.7 (C-8), 56.14,and 60.37 (two-OCH3 groups), The remaining sugar carbons appeared at 63.6–76.9, 18.24 C-6′′. EIMS m/z, (M-1) 655, 345 (100%), 330 (70%), 329 (20%), 302 (10%), 287 (10%).
Quercetin-4′-methoxy-3-fructo-rhamnoside (2)
Yellow crystals (340 mg), m.p. 229–31°C, soluble in methanol. It gave positive result with Molisch’s test, Rf = 0.60 in system (a). UV: λmax (MeOH): (nm) 254, 354, (NaOMe) 272, 401, 406, (AlCl3) 269, 381, (AlCl3/HCl) 268, 379, (NaOAc) 273, 321, 369, (NaOAc/H3BO3) 254,266,355. 1H NMR (DMSO-d6), δ: 7.81 (1H, d, J = 8–5, H-2′), 7.47 (1H, dd, J = 8.5, J = 2.5 Hz, H-6′), 6.88 (1H, d, J = 8.5 Hz, H-5′), 6.37 (1H, d, J = 2.5 Hz, H-8), δ 6.15 (1H, d, J = 2.5 Hz,H-6), 5.4 (1H, d, J = 2 Hz, H1′′ rhamnose), 4.37 (1H, d, J = 2 Hz, H1′′′ fructose), 3.79, (3H, OCH3), 3.20–3.7 (m, remaining sugar protons) and 0.94 (3H, d, J = 6 Hz, CH3 rhamnose). 13C NMR (DMSO-d6): 177.7 (C-4), 164 (C-7), 161.69 (C-5), 157.01 (C-2), 156.92 (C-9), 149.93 (C-4′), 147.39 (C-3′), 133.49 (C-3), 122.76 (C-6′), 121.54 (C-1′), 113.74 (C-2′), 115.76 (C-5′), 104.27(C-10), 101.43 (C-1′′′), 101.72 (C-1′′′), 99.36 (C-6), 94.38 (C-8), 63.6–76.89 rest of sugar carbons, 56.29 (-OCH3), 18.24 (C-6′′′).
Results and discussion
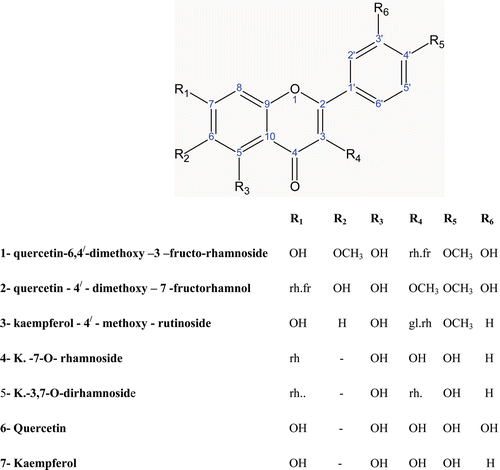
Seven compounds () were isolated and identified using UV spectrum with different shift reagents, EI-MS, 1H-NMR and 13C-NMR spectra. Five of the isolated compounds them (3–7) were found to be known and identified as; kaempferol-4′-methoxy-3-rutinoside 3, kaempferol-7-O-rhamnoside 4, kaempferol-3,7-O,O-dirhamnoside 5, quercetin 6, and kaempferol 7. The other two compounds were found to be new and identified as quercetin-6,4′-dimethoxy-3-fructo-rhamnoside 1 and quercetin-4′-methoxy-3-fructo-rhamnoside 2; they were identified as follows.
Compound 1 showed in UV spectrophotometer two main bands. Band I in methanol appears at 352 nm, indicating that it is a flavonol type with substitution at position 3. The intensity of the band was decreased from methanol to sodium methoxide indicated that position 4′ is blocked. The bathochromic shift occurred in band II (+12 nm) on addition of NaOAc indicates the presence of free OH group at position 7. No shift were observed with H3BO3 indicating the absence of o-dihydroxy groups. The bathochromic shift of band I with AlCl3/HCl (31 nm) indicated the presence of OH group at position 5. 1H-NMR showed Signals at δ 7.81 integrated for one proton J = 8.5 Hz for the most downfield signals, its position in the aromatic ring indicates the highest deshielded proton at position 2′, δ 7.45 (1H, dd, J = 8.5 Hz, J = 2.5 Hz, H-6′), 6.87 (1H, d, J = 8.5 Hz, H-5′) indicates aromatic ring with two substitution meta and para, 6.43 (1H, d, J = 2.5 Hz, H-8). 5.39 (1H, d, J = 2 Hz, H1′′ rhamnose), 4.37 (1H, d, J = 2, H1′′′ fructose), 3.79, 3.70 protons of two OCH3 groups, 3.28–3.36 (m, remaining sugar protons) and 0.94 (3H, d, J = 6, CH3 rhamnose). In 13C-NMR peaks were observed at 177.7. The highest oxygenated carbon for ketonic carbon at C-4, at 156.7 for C-7, at 152.69 for C-5 followed by 152.42 for C-2, 149.86 for C-9, 147.38 for C-4′, 133.13 for C-3′, 132.1 For C-3 appeared more upfield indicating the presence of substitution at this position, followed by 122.69 for C-6′, 121.65 for C-1′, at 113.74 for C-2′, 115.75 for C-5′ and C-10 at 104.27. Two anomeric carbons appeared at 101.43 and 1.80 for C-1′′ rhamnose and C-1′′′ fructose. The lower shielded aromatic carbons C-6 and C-8 appeared at 98.8 and 93.7, respectively. The remaining sugar carbons appeared at 63.6–76.9. Two signals were recorded at 56.14 and 60.37 (-OCH3 groups). Dept-135 showed eleven quaternary carbons, two methoxy groups at 56.14 and 60.37 and two secondary carbons at 63.59 and 67.35 indicating the presence of−CH2 groups in the sugar other than the rhamnose. Mass spectrum revealed peak at 655(M-1) in addition to other peaks at M/e 345 (100%), 330 (70%), 329 (20%), 302 (10%), 287 (10%). HMQC showed correlations between the following δ; 7.81 for H2′ and δ 113.74 for C-2′, 7.45 H6′ and δ 122.4 for C-6′, 7.45 H-6′ and δ 122.4 for C-6′, 6.43 for H-8 and δ 93.7 for C-8, 5.39 for H-1′′ of rhamnose and δ 101.43 for C-1′′ of rhamnose. In HMBC showed correlations spectrum at δ 5.4 (H-1′′ for rhamnose) and δ 132.1 (C-3 for quercetin); δ 7.8 (H-2′, for quercetin) and 113.74 (C-2′ for quercetin) and 147.38 (C-4′ for quercetin); δ 0.94 (CH3 of rhamnose) and 18.24 (CH3 of rhamnose); δ 3.7 (OCH3) and 60.37 (OCH3); δ 3.79 (OCH3) and 56.14 (OCH3).
Total acid hydrolysis revealed the presence of rhamnose and fructose. These results were confirmed by HPLC. Free aglycone spectral data were matched with 6, 4′-dimethoxyquercetin by comparing its data with the published one (CitationKhamis et al., 1999).
The second new compound gave absorbance in UV as follows, Band I in methanol appears at 354 nm, indicates that it is a flavonol type with substitution at position 3. The bathochromic shift in band I appeared on addition of AlCl3 indicating the presence of free OH group at position 5. 1H-NMR revealed signals at: δ 7.81 integrated for one proton J = 8.5 Hz for the most downfield signals, its position in the aromatic ring indicating the highest deshielded protons at 2′, δ 7.47 (1H, dd, J = 8.5, J = 2.5 Hz, H-6′), δ 6.88 (1H, d, J = 8.5 Hz, H-5′), δ 6.37 (1H, d, J = 2.5 Hz, H-8), δ 6.15 (1H, d,J 2.5 Hz,H-6), δ 5.4 (1H, d, J = 2 Hz, H-1′′ rhamnose), δ 4.37 (1H, d, J = 2 Hz, H1′’’ fructose), δ 3.79, (3H, OCH3), δ 3.20–3.7 (m, remaining sugar protons) and δ 0.94 (3H, d, J = 6 Hz, CH3 rhamnose). 13C-NMR showed peaks at 177.7 for ketonic carbon at C-4, at 164 for C-7, at 161.69 for C-5 followed by 157.01 for C-2, 156.92 for C-9, 149.93 for C-4′, and 147.39 for C-3′; 133.49 for C-3 appeared more up filed indicating the presence of substitution at this position, followed by 122.76 for C-6′, 121.54 for C-1′, at 113.74 for C-2′, 115.76 for C-5′ and C-10 at 104.27; Anomeric carbons appeared at 101.43 for C-1′′′ rhamnose and 10 1.72 for C-1′′′ fructose indicating the presence of two sugar moieties. The lower affected aromatic carbon C-6 and C-8 appeared at 99.36 and 94.38, respectively. The remaining sugar carbons appeared at 63.6–76.89.The -OCH3 carbon appeared at 56.29. C-6′′′ (CH3 of rhamnose) at 18.24. In addition the dept-135 showed ten quaternary carbon atoms and two−CH2 groups at 63.59 and 67.35 indicating the presence of−CH2 groups in the sugar rather than the rhamnose sugar which was corresponding to fructose. HMQC revealed correlation between δ 7.81 for H-2′ and δ 113.74 for C-2′; δ 7.47 H-6′ and δ 122.76 for C-6′; δ 6.88 for H-5′ and δ 115.76 for C-5′; δ 6.37 for H-8 and δ 94.7 for C-8; δ 5.4 for H-1′′ of rhamnose and δ 101.43 for C-1′′ of rhamnose. HMBC showed correlations at; δ 5.4 (H-1′′ for rhamnose) and δ 133.49 (C-3 for quercetin); δ 7.8 (H-2′, for quercetin) and 113.74 (C-2′ for quercetin) and 147.39 (C-4′ for quercetin); δ 0.94 (CH3 of rhamnose), 18.24 (CH3 of rhamnose), δ 3.79 (OCH3) and 56.14 (OCH3).
Total acid hydrolysis revealed the presence of rhamnose and fructose were obtained. These results were confirmed by HPLC. Free aglycone spectral data were matched with 4′-methoxyquercetin by comparing its data with the published one (CitationMabry et al., 1970).
The values of LD50
Animals received the total ethanol extract up to 4000 mg kg−1 did not showed any behavioral changes and mortality. Accordingly, it suggested that oral LD50 of the total ethanol extract was higher than 4000 mg kg−1. So this plant can be categorized as highly safe since substances possessing LD50 higher than 50 mg kg−1 are nontoxic (CitationBuck et al., 1976).
Antioxidant
This study has revealed that the antioxidant capacities of the tested extracts () to scavenge ABTS•+ radical were ranged from 129 to 952 µmol Trolox equivalent/gram dry weight. It was 952 µmol/g for the total alcohol extract, 132 and 129 µmol/g for both ether and chloroform extracts, respectively, with the lowest activity, while n-butanol and ethyl acetate extracts showed the highest activity with values 843 and 810 µmol/g for respectively. In addition, all isolated compounds have closer activity to butanol ().
Table 1. Antioxidant activity of different extracts and isolated compounds of Atriplex lentiformis (Torm.) S. Wats.
Table 2. Effect of Atriplex lentiformis (Torm.) S. Wats extracts on liver functions of rats (M ± SE, n = 5).
Effect on liver and kidney functions
From the data obtained in , , and it is clear that n-butanol (G5) and total ethanol extract (G6) significantly increased the levels of ALT, AST, blood urea, and serum creatinine. ALT attained its highest level 10.2 and 11.0 U/l for (G5) and (G6) respectively after 45 days of administration, also AST in (G5) and (G6) attained a significant elevation in all periods in comparing to control group, blood urea and serum creatinine have the same trend of AST. However, the results of the isolated compounds (Compound 1 and Compound 2) revealed that these compounds have no significant adverse effects on liver and kidney functions.
Table 3. Effect of Atriplex lentiformis (Torm.) S. Wats, extracts on kidney functions of rats (M ± SE, n = 5).
Table 4. Effect of isolated compounds on liver and kidney functions of rats (M ± SE, n = 5).
Conclusions
In the present study, seven flavonoids were isolated from the total ethanol extracts of Atriplex lentiformis, two of them were novel compounds. All extracts and isolated compounds showed antioxidant activates with no side effects on liver and kidney functions.
Declaration of interest
The authors are very grateful to research group program at KSU NO.2345 (group of natural products in remediation) for funding this work. The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abu-Zanat MW, Al-hassanat FM, Alawi M, Ruyle, GB. (2003). Oxalate and tannins assessment in Atriplex halimus L and Atriplex nummularia L. J Range Management, 56, 370–374.
- Buck WB, Osweiter GD, Van Gelder AG. (1976). Clinical and Diagnostic Veterinary Toxicology, 2nd Edn. Iowa: Kendall/hunt Publishing Co., 5211.
- Bylkaa WL, Stobieckib MF, Frańskic RN. (2001). Sulfated flavonoids glycosides from leaves of Atriplex hortensis. Acta Physio Plantrum, 23, 285–295.
- Duke JA. (1977). Phytotoxin tables. crc Crit Rev Toxicol, 5, 189–237.
- El-Gohary HM. (2004). Study of essential oils of the tubers of Cyperus rotundus L. and Cyperus alopecuroides Rottb. Bull Fac Pharm Cairo Univ, 42, 157–164.
- EL-Sayed NH, Amani S., Awaad Mabry, TJ. (1998). A flavonol triglucoside from Chenopodium murale. Phytochemistry, 51, 591–593.
- Hayley CN, Colby F, David GM, Allan JR, Robyn AD, Ian HW. (2004). Variation within and between two saltbush species in plant composition and subsequent selection by sheep. Australian J Agric Res, 55, 999–1007.
- Henary RJ, Cannon DC, Winkleman JW. (1974). Clinical Chemistry Principles and Techniques, 2nd Edn. New York: Harper and Row, pp. 543–550.
- Khamis NH, Mabry TJ, Hifnawy MS,Awaad SA. (1999). A Flavonol Triglucoside from Chenopodium murale, Phytochemistry, 5, 591–593
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol, 54, 275–287.
- Mabry TJ, Markham KR, Thomas MB. (1970). The Systematic Identification of Flavonoids. Berlin, Springer-Verlag, p. 345.
- Mahran GH. (1967). Medicinal Plants. Cairo: Anglo Egyptian Bookshop, p. 203.
- Osmond B. (1963). Oxalate and ionic equilibria in Australian salt bushes (Atriplex). Nature, 198, 503–504.
- Rice-Evans C, Miller NJ. (1994). Total antioxidant status in plasma and body fluids. Methods Enzymol, 34, 279–293.
- Sabry GM, Fahmy AA, Donia AMA. (2003). The nutritive value of Atriplex lentiformis as affected by drought and its impact on sheep performance. J. Agric Sci Mansoura Univ, 28, 6631–6644.
- Sandberg F, Michel KH, Staf B, Tjernberg Nelson M. (1967). Screening of plants of family chenopodiaceae for alkaloids. Acta Pharm Succica, 4, 51.
- Stahl E. (1969). Thin Layer Chromatography a Laboratory Handbook. 2nd Edn. London: George Allen and Unwinlid, p. 880.
- Watt JM, Breyer-Brandwijk MG. (1962). Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd Edn. Edinburgh and London: Livingstone E.&S. LTD., pp. 445–449.