Abstract
Context: In the search for new sources of safe and inexpensive antioxidants, the leaves of eight species of seagrasses were screened for antioxidant properties.
Objective: Most of the seagrasses were evaluated for the first time for their antioxidant activities.
Materials and methods: Total phenolic content and in vitro antioxidant activity using, 2,2-diphenyl-1-picrylhydrozyl (DPPH) radical scavenging capacity and FRAP assay.
Results: The leaves of Halophila stipulacea (Forssk.) Aschers showed high levels of phenols (1.398 ± 0.055 mg GAE/g) and high reducing power (46.289 ± 1.002) in terms of mg GAE/g. Similarly, H. pinifolia exhibited high total antioxidant activity (132.38, 75.027 mg AscAE/g) and a high percentage of DPPH radical scavenging activity (68.14%). The TAA and FRAP assays showed positive and significantly high correlation (R2 = 0.646). The total phenolic content in the seagrass extracts showed a better correlation with reducing power (R2 = 0.597) than the DPPH radical-scavenging activity (R2 = 0.495).
Discussion and conclusions: The antioxidant capacities of the seagrasses showed potential rich sources of natural antioxidants. Further studies are necessary for isolation and characterization of the active antioxidant compounds, which can be used to treat various oxidative stress-related diseases.
Introduction
Antioxidants in biological systems have multiple functions which include protection from oxidative damage and in the major signaling pathways of cells. The major action of antioxidants in cells is to prevent damage caused by the action of reactive oxygen species (ROS). ROS, such as, superoxide radicals (O2), hydroxyl (OH), peroxide (ROO) and nitric acid radicals are generated in living organisms during excessive metabolism (CitationAruoma & Cuppette, 1997) and they cause extensive oxidative damage to cells leading to age-related degenerative diseases, cancer, and a wide range of other human diseases (CitationReaven & Witzum, 1996; CitationAruoma, 1999).
Several synthetic antioxidants, such as butylated hydroxyanisol (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) are commercially available and are currently in use. However, their use is now restricted due to their side effects. It has been shown that they promote the development of cancerous cells in rats. These findings have reinforced the efforts for the development of alternative antioxidants of natural origin (CitationHuang & Wang, 2004). In this regard, many natural antioxidants have already been isolated from various natural resources, such as oilseeds, cereal crops, vegetables, spices, and herbs (CitationRamarathnam et al., 1995). Seagrasses specifically produced bioactive compounds that reportedly have antibacterial (CitationHarrison & Chan, 1980; CitationBernard & Pesando, 1989; CitationDevi et al., 1997; CitationBhosale et al., 2002; CitationRagupathi Raja Kannan et al., 2010a), antialgal (CitationHarrison, 1982), antifungal (CitationBallesteros et al., 1982; CitationJensen et al., 1998), antiviral (CitationPremanathan et al., 1992; CitationRowley et al., 2002), antiprotozoal (CitationOrhan et al., 2006), antiinflammatory (CitationHua et al., 2006), and antidiabetic (CitationGokce et al., 2008) activities. More recently, reports have revealed that seagrasses are rich sources of antioxidant compounds (CitationHasina et al., 2003; CitationKolenchenko et al., 2005; CitationGokce et al., 2008; CitationSureda et al., 2008; CitationRagupathi Raja Kannan et al., 2010b, Citation2010c).
In folk medicine, seagrasses have been used for a variety of remedial purposes, such as, the treatment of fever and skin diseases, muscle pains, wounds, and stomach problems, remedy against stings of different kinds of rays and, as tranquillizer for babies (Citationde la Torre-Castro & Rönnbäck, 2004), and so on. The seeds of the tropical seagrass Enhalus acoroides (Linnaeus f.) Royle have been traditionally eaten in the Philippines. The raw seeds are described as crunchy and sweet, while boiled seeds contain more starch and taste like cooked sweet potato (CitationMontaño et al., 1999). In addition to being edible, the seeds are thought to have aphrodisiac and contraceptive properties (CitationAliño et al., 1991). Halophila ovalis was used by the fishing communities of Cuddalore and Nagapattinam districts of Tamil Nadu, South India as medicine to treat various skin diseases, burns, and boils (CitationKannan et al., 1999).
In this paper, we report the antioxidative potential of eight species of seagrasses from the coasts of Gulf of Mannar (India) by measuring the total antioxidant activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, ferric reducing antioxidant power (FRAP), and the total phenolic content in ethanolic extracts.
Materials and methods
Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich. Gallic acid, ascorbic acid, FeCl3, AlCl3, Folin-Ciocalteus’s phenol reagent, and sodium carbonate were purchased from Merck (Mumbai, India). All the chemicals used including the solvents were of analytical grade.
Plant material
The fresh leaves of Enhalus acoroides (Linnaeus f.) Royle, Halophila ovalis (R. Br.) Hook. f. (Hydrocharitaceae), Halophila ovata Gaidich (Hydrocharitaceae), Halophila stipulacea (Forssk.) Aschers (Hydrocharitaceae), Thalassia hemprichii (Ehrenberg) Ascherson (Hydrocharitaceae), and Syringodium isoetifolium (Ascherson) Dandy (Cymodoceaceae), Cymodocea serrulata (R. Brown) Ascherson & Magnus (Cymodoceaceae), Halodule pinifolia (Miki) den Hartog (Cymodoceaceae) were collected from the intertidal region of the Mandapam coast (Lat. 09˚ 17.417′N; Long. 079˚ 08.558′E) during March 2009 and immediately brought to the laboratory in plastic bags containing seawater to prevent dehydration of plants. The plants were washed thoroughly with tap water to remove all sand particles and epiphytes. The samples were shadedried at room temperature for five days until a constant weight was obtained and ground in an electric mixer. The powdered samples were kept in air tight containers and stored in refrigerator for further use.
Preparation of seagrass extracts
Dried finally crushed powdered samples (10 g) were extracted for 24 h in 200 ml of ethanol at room temperature under dark condition. The extraction was twice repeated and filtered using Whatmann No. 1 filter paper. Each filtrate was concentrated to dryness under reduced pressure using a rotary flash evaporator.
Determination of total phenolic content
Total phenolic content was estimated as gallic acid equivalents (GAE) according to the Folin-Ciocalteu Reagent (CitationVelioglu et al., 1998). A 1.0 mL aliquot of each sample (0.1mg/ml in ethanol) was added to 1.5 ml deionized water and 0.5 mL of 0.1 M Folin-Ciocalteu reagent and thoroughly mixed. After 1 min, 1.0 ml of 20% sodium carbonate solution was added, and the mixture was mixed thoroughly. The control contained all the reaction reagents except the sample. After 30 min of incubation at 37°C, the absorbance was measured at 750 nm, using the PerkinElmer Lamda 25 UV-VIS Spectrophotometer. Total phenolic content was expressed as mg/g gallic acid equivalent. A calibration curve of gallic acid was prepared and the total phenolic content was standardized against gallic acid and was expressed as mg GAE per gram of sample on a dry weight (DW) basis.
Total antioxidant activity
Total antioxidant activities of the crude extracts were determined according to the method of CitationPrieto et al. (1999). Briefly, 0.3 ml of sample solution (0.1mg/ml) was mixed with 3.0 ml reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). Reaction mixture was incubated at 95°C for 90 min in a water bath. Absorbance of all the sample mixture was measured at 695 nm. Total antioxidant activity is expressed as the number of equivalence of ascorbic acid. A calibration curve of ascorbic acid was prepared and the total antioxidant activity was standardized against ascorbic acid and was expressed as mg ascorbic acid equivalents per gram of sample on a dry weight (DW) basis.
DPPH radical-scavenging activity
The scavenging effects of samples for DPPH radical were monitored according to the method of CitationYen and Chen (1995). Briefly, a 2.0 ml of aliquot of test sample solution was added to 2.0 ml of 0.16 mM DPPH methanolic solution. The mixture was vortexed for 1 min and then left to stand at room temperature for 30 min in the dark, and its absorbance was read at 517 nm. The ability to scavenge the DPPH radical was calculated using the following equation:
Where the Acontrol is the absorbance of the control (DPPH solution without sample), the Asample is the absorbance of the test sample (DPPH solution plus test sample), and the Asample blank is the absorbance of the sample only (sample without DPPH solution). Synthetic antioxidants, gallic acid, and ascorbic acid were used as positive controls.
FRAP assay
The reducing power of the crude extract was determined by the method prescribed by CitationOyaizu (1986). Briefly, 1.0 ml of sample was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide (1%). The reaction mixture was incubated at 50°C for 20 min. After incubation, 2.5 ml of trichloroacetic acid (10%) was added and centrifuged (650g) for 10 min. From the upper layer, 2.5 ml solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%). Absorbance of all the sample solutions was measured at 700 nm. Ascorbic acid is used as a positive control. Reducing power is expressed as the number of equivalence of gallic acid. A calibration curve of gallic acid was prepared, and the FRAP was standardized against gallic acid and was expressed as mg GAE per gram of sample on a dry weight (DW) basis.
Statistical analysis
Three replicates of each sample were used for statistical analysis and the values were reported as mean ± SD. Statistical analysis were performed by one-way analysis of variance followed by Duncan test. Pearson’s correlation test was used to assess correlations between means. A significant difference was considered at the level of p < 0.05. All computations were done by employing the statistical software (SPSS, version 16.0).
Results and discussion
Total phenolic content in seagrasses
Phenolic compounds are well known, as high-level antioxidant constituents because of their high ability to scavenge toxic free radicals and reactive species such as superoxide radical (O2), hydroxyl radical (OH), peroxide radical (ROO) and nitric oxide (NO) radicals (CitationHall & Cuppett, 1997). The total phenolic content of the extracts are presented in . Halophila stipulacea showed a significantly higher amount of phenols (1.3987 ± 0.055) when compared to other seagrasses followed by H. pinifolia (0.6468 ± 0.040). The presence of phytoconstituents, such as phenols, flavonoids and tannin in seaweeds and seagrasess indicated a possible role that its extracts may have antioxidant activity. This activity was believed to help in preventing a number of diseases through free-radical scavenging activity (CitationAnggadiredja et al., 1997; CitationRuberto et al., 2001). Similarly, some earlier reports reveal that seagrasses especially their polyphenols have the antioxidant activity (CitationHasina et al., 2003; CitationKolenchenko et al., 2005; CitationGokce et al., 2008; CitationSureda et al., 2008; CitationRagupathi Raja Kannan et al., 2010b, Citation2010c). It is also reported that the presence of condensed tannins in seagrasses may act as deterrents against herbivore feeding as well as against fungal and bacterial invasion (CitationMcMillan, 1984).
Table 1. Levels of total phenolic content in seagrasses.
Antioxidant capacities of the seagrass species
The total antioxidant activities in ethanol extracts of the eight seagrasses are presented in . In the phosphomolybdenum method, molybdenum VI (Mo6+) is reduced to form a green phosphate/Mo5+ complex. Higher activity of 132.382, 75.027, and 32.415 mg ascorbic acid equivalent/g was observed respectively in H. pinifolia, H. stipulacea, and C. serrulata. The total antioxidant activity was significantly different among the eight seagrasses. Published reports on the in vitro total antioxidant activity of seagrasses are limited. Similarly, H. pinifolia, showed stronger antioxidant activity than T. hemprichii, E. acoroides and S. isoetifolium when tested by thin layer chromatography (CitationRagupathi Raja Kannan et al., 2010c). CitationAthiperumalsami et al. (2010) have reported highest antioxidant activity in the methanolic extract of Halophila ovalis than H. pinifolia tested by the NO scavenging method. CitationRagupathi Raja Kannan et al. (2010b) have reported antioxidant activity of 11.77 mg ascorbic acid equivalent/g of the leaf of Enhalus acoroides collected from Chinapallam, Gulf of Mannar, India which is higher than the value of the present study (8.439 mg ascorbic acid equivalent/g). However, CitationKumaran and Karunakaran (2007) have reported total antioxidant activity in the range of 245 to 376 mg ascorbic acid equivalents/g in some Phyllanthus species. CitationGanesan et al. (2008) reported higher activity (32.01 mg ascorbic acid equivalent/g) in ethyl acetate fraction of the red seaweed Acanthophora spicifera. CitationYe et al. (2009) on the other hand reported higher antioxidant activity (30.50µmol FeSo4/mg) in the ethanol extract of the brown seaweed Sargassum pallidum. It appears that solvents used for extraction dramatically influence the chemical composition of the extracts (CitationYuan et al., 2005).
Table 2. Antioxidant activity of seagrass extracts determined by TAA and FRAP assays.
The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability. DPPH is a stable free radical and it accepts an electron or hydrogen radical to become a stable diamagnetic molecule. When a DPPH solution is mixed with a substrate acting as a hydrogen atom donor, a stable non-radical form of DPPH is obtained with the simultaneous change of the violet color to pale yellow (CitationMolyneux, 2004). Hence, DPPH has been used extensively as a free radical to evaluate reducing substances (CitationCotelle, 1996) and is a useful reagent for investigating the free radical scavenging activities of compounds (CitationDuan et al., 2006). DPPH radical scavenging activities (%) of eight species of seagrasses are presented in . In the present study, higher DPPH radical scavenging activities was recorded in Halodule pinifolia (68.066%) followed by H. stipulacea (67.413%) and C. serrulata (61.853%). The minimum DPPH radical scavenging activity was recorded in H. ovata (16.926%). The scavenging effect of standards on the DPPH radical decreased in the order: ascorbic acid > gallic acid, which was 57.28 and 55.753%, respectively.
Table 3. Antioxidant activity of seagrasses.
In the FRAP assay, antioxidants in the sample reduced ferric (III) to ferrous (II) in a redox-linked colourimetric reaction (CitationLi et al., 2006) that involves single electron transfer. The reducing power indicates that the antioxidant compounds are electron donors and reduce the oxidized intermediate of the lipid peroxidation process, so that they can act as primary and secondary antioxidants (CitationYen & Chen, 1995). shows the results of FRAP assay of the seagrasses. In this present study, Halophila stipulacea (46.289 ± 1.002), and Halodule pinifolia (42.611 ± 0.800) had the highest ability for reducing Fe3+ compared to the other seagrasses. The same trend was obtained for total phenolic content. The positive control ascorbic acid showed significantly higher antioxidant activity than all the seagrass samples. A similar trend has been reported by CitationKumaran and Karunakaran (2007) in methanol extracts of Phyllanthus species.
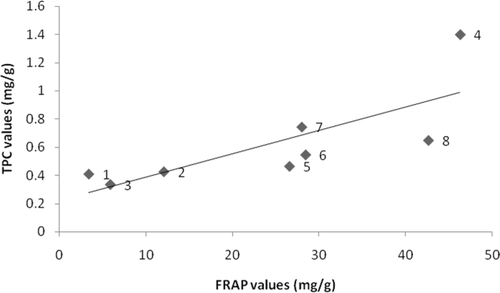
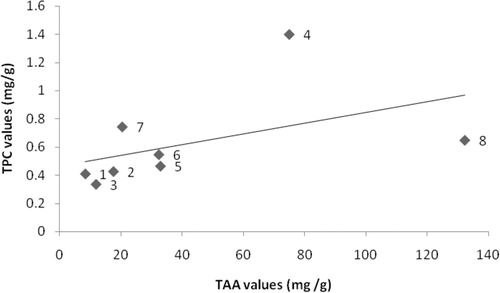
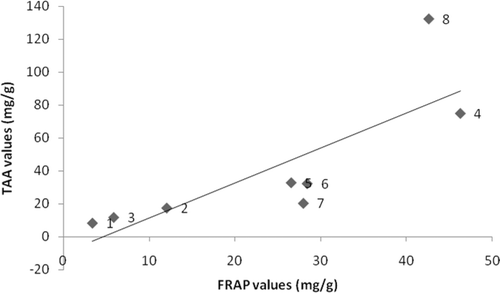
The correlation between the total phenolic content and antioxidant assays were studied and the results are presented in . In this study, the positive correlation between TAA and FRAP assays results (R2 = 0.646) indicated the compounds present in the ethanolic extracts of seagrasses which were capable of reducing the ferric radical were also able to reduce ferric ions. In agreement with previous studies (CitationDuan et al., 2006), there was a significant correlation between antioxidant activity and phenolic content of these eight species of seagrasses. The total phenolic content in the seagrass extracts showed a higher correlation with reducing power (R2 = 0.597) than the DPPH radical-scavenging activity (R2 = 0.495). According to Athiperumalsamy et al. (2010), other than phenolic compounds some other phytoconstituents like vitamin A, C, E and β-carotene have been implicated as antioxidants in the scavenging of radicals in seaweeds and seagrasses.
Figure 1. Correlation of FRAP and TAA values of seagrass ethanolic extracts from (1) Enhalus acoroides, (2) Halophila ovalis, (3) H. ovata, (4) H. stipulacea, (5) Syringodium isoetifolium, (6) Cymodocea serrulata, (7) Thalassia hemprichii, and (8) Halodule pinifolia.
Conclusions
From the present findings it was concluded that the seagrasses have strong antioxidant properties due to the total phenolic content. Several studies reported that phenols are the major contributor for the antioxidant capacities of seagrasses. Also, studies are being carried out on other species of seagrasses of different habitats in order to provide more comprehensive data on the antioxidant activity. Further research studies are needed to study the polyphenols of these seagrasses using HPLC-DAD and NMR technique and those results can be extrapolated to clinical studies.
Acknowledgment
The authors are grateful to Prof. T. Balasubramanian, Dean, CAS in Marine Biology, Faculty of Marine Sciences and authorities of Annamalai University for providing the necessary facilities.
Declaration of interest
The authors report no conflict of interest. The authors are alone responsible for the content and writing of the paper.
References
- Aliño PM, Cajipe GJB, Ganzon-Fortes E, Licuanan WRY, Montaño NE, Tupas LM. (1991). The use of marine organisms in folk medicine and horticulture: A preliminary study. SICEN Leaflet, 1, 1–8.
- Anggadiredja J, Andyani R, Murawanah H. (1997). Antioxidant activity of Sargassum polycystum (Phaeophyta) and Laurencia obtuse (Rhodophyta) from Seribu Islands. J Appl Phycol, 9, 477–479.
- Aruoma IO. (1999). Antioxidant action of plant foods. Use of oxidative DNA damage, as a tool for studying antioxidant efficacy. Free Rad Res, 30, 419–427.
- Aruoma IO, Cuppette SL. (1997). Antioxidant methodology: in vivo and in vitro concepts. IL: AOAS Press.
- Athiperumalsami T, Devi Rajeswari V, Hastha poorna S, Kumar V, Louis Jesudass L. (2010). Antioxidant activity of seagrasses and seaweeds. Bot Mar, 53, 251–257.
- Ballesteros D, Martin D, Uriz MJ. (1982). Biological activity of extracts from Mediterranean macrophytes. Bot Mar, 35, 481–485.
- Bernard P, Pesando D. (1989). Antimicrobial and antifungal activity of extracts from the rhizomes of the Mediterranean seagrass Posidonia flavano (L.) Delile. Bot Mar, 32, 85–88.
- Bhosale SH, Nagle VL, Jagtap TG. (2002). Antifouling potential of some marine organisms from India against species of Bacillus and Pseudomonas. Mar Biotechnol, 4, 111–118.
- Cotelle N, Bernier JL, Catteau JP, Pommery J, Wallet JC, Gaydou EM. (1996). Antioxidant properties of hydroxy-flavones. Free Radic Biol Med, 20, 35–43.
- de la Torre-Castro M, Rönnbäck P. (2004). Links between humans and seagrasses-an example from tropical East Africa. Ocean & Coastal Management, 47, 361–387.
- Devi P, Solimabi W, D’Souza L, Sonak S, Kamat Y, Singbal SYS. (1997). Screening of some marine plants for antiviral activity against marine fouling bacteria. Bot Mar, 40, 87–91.
- Duan XJ, Zhang WW, Li XM, Wang BG. (2006). Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem, 95, 37–43.
- Ganesan P, Kumar CS, Bhaskar N. (2008). Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol, 99, 2717–2723.
- Gokce G, Haznedaroglu MZ. (2008). Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol, 115, 122–130.
- Hall CA, Cuppett SL. (1997). Structure-activities of natural antioxidants, in antioxidant methodology. In: Auroma OI and Cuppett SL, ed. In Vivo and In Vitro Concepts, Champaign, IL: AOCS Press, 141–170.
- Harrison PG. (1982). Control of microbial growth and of amphipod grazing by water-soluble compounds from leaves of Zostera marina. Mar Biol, 67, 225–230.
- Harrison,PG Chan, AT. (1980). Inhibition of the growth of microalgae and bacteria by extracts of eelgrass (Zostera marina) leaves. Mar Biol, 61, 21–26.
- Hasina EI, Kolenchenko EA, Sgrebneva MN, Kovalev VV, Khotimchenko YuS. (2003). Antioxidant activities of a low etherified pectin from the seagrass Zostera marina. Russian J Mar Biol, 29, 259–261.
- Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, Chao LK. (2006). Study on the antiinflammatory activity of methanol extract from seagrass Zostera japonica. J Agric Food Chem, 54, 306–311.
- Huang HL, Wang BG. (2004). Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J Agric Food Chem, 52, 4993–4997.
- Jensen PR, Jenkins KM, Porter D, Fenical W. (1998). Evidence that a new antibiotic flavone glycoside chemically defends the sea grass Thalassia testudinum against Zoosporic Fungi. Appl Environ Microbiol, 64, 1490–1496.
- Kannan L, Thangaradjou T, Anantharaman P. (1999). Status of seagrasses of India. Seaweed Res Utiln, 21, 25–33.
- Kolenchenko EA, Sonina LN, Khotimchenko Yu S. (2005). Comparative estimation of the antioxidant activity of low-etherified pectin, obtained from the Eelgrass Zostera marina and antioxidants in vitro. Russian J Mar Biol, 31(5): 331–334.
- Kumaran A, Karunakaran RJ. (2007). In vitro antioxidant properties of methanol extracts of five Phillanthus species from India. LWT-Food Sci Technol, 40, 344–352.
- Li YF, Guo CJ, Yang JJ, Wei JY, Xu J, Cheng S. (2006). Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem, 96, 254–260.
- Mc Millan C. (1984). The condensed tannins (proanthocyanidins) in seagrasses. Aquat Bot, 20, 351–357.
- Molyneux P. (2004). The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklankarin J Sci Technol, 26(2), 211–219.
- Montaño NME, Bonifacio RS, Rumbaoa RGO. (1999). Proximate analysis of the flour and starch from Enhalus acoroides (L.f) Royle seeds. Aquat Bot, 65, 321–325.
- Orhan I, Sener B, Atici T, Brun R, Perozzo R, Tasdemir D. (2006). Turkish freshwater and marine macrophytes extracts show in vitro antiprotozoal activity and inhibit Fabl, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine, 13, 735–739.
- Oyaizu M. (1986). Studies on product of browning reaction prepared from glucose amine. Japanese J Nut, 44, 307–315.
- Premanathan M, Chandra K, Bajbai SK, Kathiresan K. (1992) A survey of some Indian medicinal plants for antiviral activity. Bot Mar, 35, 321–324.
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem, 269, 337–341.
- Ragupathi Raja Kannan R, Arumugam R, Anantharaman P. (2010a). Antibacterial potential of three seagrasses against human pathogens. Asian Pacific J Trop Med, 11, 890–893.
- Ragupathi Raja Kannan R, Arumugam R, Anantharaman P. (2010b). In vitro antioxidant activities of Enhalus acoroides. Asian Pacific J Trop Med, 11, 898–901.
- Ragupathi Raja Kannan R, Arumugam R, Meenakshi S, Anantharaman P. (2010c). Thin layer chromatography analysis of antioxidant constituents of seagrasses of Gulf of Mannar Biosphere Reserve, South India. Int J ChemTech Res, 2(3), 1526–1530.
- Ramarathnam N, Osawa T, Ochi H, Kawakishi S. (1995). The contribution of plant food antioxidants to human health. Trends Food Sci Technol, 6, 75–82.
- Reaven PD, Witztum JL. (1996). Oxidized low density lipoproteins in atherogenesis: Role of dietary modification. Annu Rev Nutr, 16, 51–71.
- Rowley DC, Hansen MS, Rhodes D, Sotriffer CA, Ni H, McCammon JA, Bushman FD, Fenical W. (2002). Thalassiolins A-C: new marine-derived inhibitors of HIV cDNA integrase. Bioorg Med Chem, 10, 3619–3625.
- Ruberto G, Baratta MT, Biondi DM, Amico V. (2001). Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J Appl Phycol, 13, 403–407.
- Sureda A, Box A, Terrados J, Deudero S, Pons A. (2008). Antioxidant response of the seagrass Posidonia oceanica when epiphytized by the invasive macroalgae Lophocladia lallemandii. Mar Environ Res, 66, 359–363.
- Velioglu YS, Mazza G, Gao L, Oomah BD. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem, 46, 4113–4117.
- Ye H, Zhou C, Sun Y, Zhang X, Liu J, Hu Q, Zeng X. (2009). Antioxidant activities in vitro of ethanol extract from brown seaweed Sargassum pallidum. Eur Food Res Technol, 230(1), 101–109.
- Yen GC, Chen HY. (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem, 43, 27–37.
- Yuan YV, Bone DE, Carrington MF. (2005). Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem, 91, 485–494.