Abstract
Context: Rice [Oryza sativa L. (Gramineae)] bran is a rich source of phytochemicals. Its oil also contains several bioactive components that exhibit antioxidative properties such as ferulic acid (F), γ-oryzanol (O), and phytic acid (P) which can be a new source of cosmetic raw materials.
Objective: To evaluate the anti-aging effects of the gel and cream containing niosomes entrapped with the rice bran bioactive compounds.
Materials and methods: The semi-purified rice bran extracts containing F, O, and P which indicated the growth stimulation of human fibroblasts and the inhibition of MMP-2 by sulforhodamine B and gelatin zymography, respectively, were entrapped in niosomes by supercritical carbon dioxide fluid (scCO2) and incorporated in gel and cream formulations. The skin hydration, elasticity, thickness and roughness, and pigmentation in human volunteers after treated with these gel and creams were investigated by corneometer, cutometer, visiometer, and mexameter, respectively.
Results: Gel and cream containing the semi-purified rice bran extracts entrapped in niosomes gave no sign of erythema and edema detected within 72 h on the shaved rabbit skin by the closed patch test investigated by mexameter and visual observation, respectively. These formulations also demonstrated higher hydration enhancement and improvement of skin lightening, thickness, roughness, and elasticity on the skin of 30 human volunteers within the 28–day treatment not more than 9, 27, 7, 3, and 3 times, respectively.
Discussion and conclusions: The formulations containing niosomes entrapped with the rice bran bioactive compounds gave superior clinical anti-aging activity which can be applied as a novel skin product.
Introduction
Skin aging is a complex process involving both intrinsic and extrinsic factors. Intrinsic factors are genetic, hormonal, and biochemical processes that cause irreversible degeneration of skin tissue including flattening of the epidermal dermal interface, loss of skin thickness, reduction in the number of dermal fibroblasts, loss of elastic tissue in the skin (CitationJenkins, 2002; CitationSadick, 2009), whereas extrinsic factors are primarily from the exposure to ultraviolet (UV) radiation result in coarse and rough with deep lines and wrinkles and hyperpigmentation in the skin (CitationFisher et al., 1997). The photo-aging skin has also been shown to increase the activities of matrix metalloproteinase enzyme (MMPs) which has been associated with significant degradation of collagen fibers (CitationBerton et al., 2000). Especially, MMP-2 shows greater specificity toward the degrading constituents of the dermo-epidermal junction leading to the loss of elasticity and dryness of skin (CitationKligman & Kligman, 1986). Therefore, the inhibition of MMP-2 activity and the changes in skin quality and appearances including skin hydration, elasticity, thickness and roughness, and skin pigmentation are important parameters to evaluate skin aging. The new trend of anti-aging cosmetic products is the use of natural antioxidants (CitationKaur, 2007; Gasper et al., 2003). Thailand recently spends a lot of budgets to import many raw materials to formulate cosmetic products because there is no sufficient research and technologies to produce active compounds, especially antioxidants. Rice is one of the major cereals harvested by man in Thailand. Brown rice is polished before eating and 10% of its weight is discharged as rice bran which is a rich source of phytochemicals with striking medicinal properties. Its oil also contains several bioactive components that exhibit antioxidative properties such as ferulic acid (F), γ-oryzanol (O), and phytic acid (P). These compounds can be used as UV filter, radical scavengers, skin elasticity enhancer, and anti-wrinkle in cosmetics (CitationTaniguchi et al., 2003; CitationCoppini et al., 2001; CitationGraf et al., 1987). However, their antioxidative activities are decreased and not sufficient for cosmetics because of low stability to air and light. As known, transfersomes or ethosomes are lipid vesicles (liposomes) with high contents of alcohol or ethanol of up to 45% and are capable of enhancing penetration to the deep tissues and systemic circulation (CitationTouitou et al., 2000; CitationCevc & Blume, 2003). They have been reported to improve skin delivery of various drugs. However, the toxicity of alcohol to skin cells has been reported in many previous works. Even at low concentration, alcohol can increase the percentage of skin cells undergoing apoptosis (CitationNeuman et al., 2002, Citation2010). For niosomes, they are surfactant-based vesicles with more advantageous characteristics of higher stability, smaller particle sizes, and lower cost than the lipid vesicles as well as the transfersomes and ethosomes (CitationHandjani et al., 1979; CitationUchegbu & Vyas, 1998). Many previous studies have shown the advantages of niosomes as topical delivery of many drugs (CitationSchreier & Bouwstra, 1994; CitationKorhonen et al., 2002; CitationManosroi et al., 2009) and cosmetics (CitationHandjani et al., 1989; CitationManosroi et al., 2010). Their advantages include the enhancement of substances accumulated at the site of administration (CitationUchegbu & Vyas, 1998), capability of incorporating a wide variety of hydrophilic and hydrophobic substances (CitationManconi et al., 2006; CitationRangasamy et al., 2008; CitationLasic, 1993; CitationManosroi et al., 2009), improvement of the stability, and avoidance of degradation of the entrapped substances from the environments (CitationYoshioka et al., 1994).
Several types of nonionic surfactants such as polyglycerol alkyl ethers, glucosyl dialkyl ethers, crown ethers, and polyoxyethylene alkyl ethers and esters have been used to prepare niosomal vesicles. The ability of surfactants to form bilayer vesicles can be predicted by the hydrophilic-lipophilic balance (HLB) of surfactants (CitationUchegbu & Vyas, 1998). For instances, sorbitan monostearate (Span) surfactants with HLB of 4–8 can form vesicle (CitationSantucci et al., 1996), whereas Polysorbate 20 with HLB of 16.7 appears to be too hydrophilic to form a bilayer membrane (CitationLawrence et al., 1996). However, nonionic surfactants with HLB > 6 can form bilayer vesicles by the addition of cholesterol. Niosomes can be prepared by various methods, e.g., chloroform film method (CitationBangham, 1965) and reverse phase evaporation (CitationSzoka & Papahadjopoulos, 1978). Most of these methods require large amount of organic solvents that are toxic to human and environments for dissolving of the surfactant. Recently, supercritical carbon dioxide fluids (scCO2) have been used as a one-step preparation for the substitution of organic solvents to form bilayer vesicles with the advantages of being environmental friendly, non-toxic, and non-inflammable (CitationCooper, 2000).
Several niosome-based cosmetic products have already been launched with a great success, e.g., L’Oreal Niosomes™ and Dior’s Capture™. In this study, niosomes were used to entrap the rice bran bioactive compounds and incorporated in gel and cream for topical anti-aging application. In vitro cytotoxicity (sulforhodamine B assay) and inhibition of MMP-2 activity of the rice bran bioactive compounds on human skin fibroblast cells were investigated. Biological safety of gel and cream formulations was evaluated by microorganism colony plate count and in vivo rabbit skin closed patch test. The skin hydration, elasticity, thickness and roughness, and pigmentation which were parameters to evaluate anti-aging effects in human volunteers of the gel and cream containing niosomes entrapped with the rice bran bioactive compounds were performed.
Materials and methods
Materials
The semi-purified rice bran extracts containing the bioactive F, O, and P compounds were from our previous study (CitationManosroi et al., 2010). Human skin fibroblast cells were provided from Dr. Natthanej Luplertlop at the Department of Tropical Hygine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Tween 61 (polyoxyethylene sorbitan monostearate) was purchased from Sigma-Aldrich Co. (St. Louis, MO). Cholesterol was purchased from Serva Feinbiochemica, Heidelberg, Germany. Stearic acid (Srichand United Dispensary Co., Ltd., Thailand), mineral oil (O.V. Chemical & Supply, Thailand), cetyl alcohol (Vidhyasom Co., Ltd., Thailand), white petrolatum (Vechavit, Thailand), isopropyl myristate (Vidhyasom Co., Ltd.), glycerin (O.V. Chemical & Supply, Thailand), triethanolamine (Asia Pacific Specialty Chemicals Ltd., Thailand), methyl paraben and propyl paraben (Vidhyasom Co., Ltd.), propylene glycol (O.V. Chemical & Supply), and carbopol 980 (Namsiang Co., Ltd.) were cosmetic grade. A commercial cream containing soya and oat extracts and vitamin E (Samut Prakan, Thailand) was used as a positive control. Sulforhodamine B (SRB), trichloroacetic acid (TCA), Tris base and ascorbic acid were from Sigma-Aldrich Co.. Alpha-Modified Eagles culture medium, fetal bovine serum (FBS) and tripsin were purchased from Hyclone (Utah, USA). All other chemicals were of analytical reagent grade.
Cytotoxicity testing on the cultured human skin fibroblast of the semi-purified rice bran extracts by the SRB assay
The semi-purified rice bran extracts containing bioactive compounds F, O, and P were tested for cytotoxicity by the SRB assay in a 96-well plate (CitationVoigt, 2005). Briefly, the 1 × 104 human skin fibroblasts (27th passage) were added in the total volume of 200 µL into each well in the 96-well plate and left for cell attachment on the plate for 24 h in a 5% CO2 incubator (Shel Lab, model 2123TC, USA) at 37 ± 1°C. Cells were then treated with 20 µL of the five serial concentrations of the extracts (0.001–10 mg/mL) and kept at the same condition for 24 h. After incubation, the cells were fixed with 50% TCA at 4 ± 1°C for 1 h and washed with distilled water, and air dried over night. The fixed cells were then dyed with 100 µL of 0.4% SRB solution at room temperature (30 ± 1°C) for 30 min. After the dye was removed, the cells were washed with 1% acetic acid and air-dried overnight. The bound dye was solubilized by 100 µL of Tris-solution per well and incubated for 30 min. The absorbances were read by a microplate reader (Model 680, Biorad, USA) at 540 nm. Distilled water was used for the substitution of the extract in the control plate. Ascorbic acid (0.001–10 mg/mL) was used as a positive control. The assays were performed in triplicate. The percentages of cell growth (%G) were calculated by the following equation:
where, Atreat was the absorbance of the treated plates and Acontrol was the absorbance of the control plate, while Ablank was the absorbance of the plates without treatment.
Inhibition of matrix MMPs activity assay of the semi-purified rice bran extracts
The MMP-2 expression in cultured fibroblast cells (27th passage) was determined by gelatin zymograph as previously described (CitationZhang et al., 2008). Briefly, 5 × 105 cells per well were transferred to each well of the 6-well plate and incubated in a 5% CO2 incubator (Shel Lab, model 2123TC, USA) at 37°C for 24 h. After incubation, the cells in each well were treated with 200 µL of the extracts (1 mg/mL) and the total volume was adjusted to 3 mL with Dulbecco’s Modified Eagle Medium (DMEM) without FBS. Then, the plate was incubated under the same condition for 24 h and culture supernatants were collected. The gelatinolytic activity by MMP-2 in the culture supernatants was then identified by vertical gel electrophoresis. Briefly, 20 µL of culture supernatant was loaded onto 9% polyacrylamide gel containing 1 mg/mL gelatin with loading buffer of 0.125 M Tris (pH 6.8), 4% SDS and 0.04% bromophenol blue, and electrophoresed for 180 min (80 V, 4°C). After electrophoresis, gels were washed to remove SDS in renaturing buffer pH 7.4 (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 2.5% Triton X-100) for 30 min. The gels were then incubated in developing buffer pH 7.4 [50 mM Tris (pH 7.5), 5 mM CaCl2, 0.02% NaN3, and 1% Triton X-100] at 37°C for 16 h, subsequently stained with 0.5% Coomassie Brilliant Blue G-250 and destained in 30% methanol and 10% acetic acid (v/v). Gelatinolytic activities were detected as transparent bands against the background of Coomassie blue-stained gelatin imaged by gel document equipment (Bio-Rad Laboratories, UK) and analyzed by Quantity 1-D analysis software (CitationSariahmetoglu et al., 2007; CitationArican & Ceylan, 1999). The area multiplied by intensity (mm2) of the bands on the gel was determined as the relative MMP-2 content. The assay was performed in triplicate. Ascorbic acid was used as a positive control. The percentages of MMP-2 inhibition (%) were calculated by the following equation:
where A was the band intensity of the extract treated sample, B was the band intensity of the control.
Preparation of niosomes entrapped with semi-purified rice bran extracts containing bioactive compounds F, O, and P (Nio FOP)
Nio FOP was prepared by the scCO2 technique on SFE-500 MR-2-C50 system (Thar Instruments Inc., PA, USA) as described in our previous study (CitationManosroi et al., 2008). Briefly, the total amount of 20 mM of Tween 61 mixed with cholesterol together with the mixture of F, O and P (Mixed FOP) at the maximum loading of 0.5, 1.5, and 1.5% w/w, respectively, in 100 mL of distilled water was added into the stirred-view cell. Then, the CO2 gas was introduced into the view cell. The pressure and temperature in the view cell were maintained at 200 bar and 60 ± 1°C, respectively. After 30 min with stirring until equilibrium, the pressure was released and the niosomal dispersions were obtained.
Characteristics of niosomes entrapped with semi-purified rice bran extracts containing bioactive compounds F, O, and P (Nio FOP)
Entrapment efficiency determination
The entrapment efficiencies of the semi-purified rice bran extracts entrapped in niosomes were determined by gel filtration using Sephadex® G-50 (Amersham Biosciences Limited, Sigma-Aldrich) as a packing material and purified water as an eluent. Eluates were collected in tubes using a fractional collector (Foxy JR, Isco Inc., Lincoln, USA) at the flow rate of 7 mL/min. The fractions containing niosomes entrapped with the extracts which were detected at 470 nm, were pooled, collected and dried with a freeze-dryer (Christ, Martin Christ, Germany). The residues were dissolved in absolute ethanol and assayed for F and O contents by HPLC at 320 nm. For P contents, the residues were dissolved in distilled water and assayed by colorimetry using a spectrophotometer at 690 nm. The percentages of entrapment efficiency (%EE) were calculated by the following equation:
Vesicular size determination
The vesicular size and polydispersity index (PDI) of niosomes were measured by a dynamic light scattering (DLS) apparatus (NICOMP 380 ZLS, Particle Sizing Systems, Santa Barbara, CA). The dispersions were diluted to about 100 times with distilled water. The time-dependent correlation function on the scattered light intensity was measured at a scattering angle of 90° and wavelength at 535 nm.
Morphology determination
The dispersion of niosomes was rapidly frozen in liquid propane using cryo-preparation apparatus (Leica EM CPC, Leica Co., Vienna, Austria). The frozen sample was fractured in freeze-replica-making apparatus (FR-7000A, Hitashi Science Co., Tokyo, Japan) at −150°C. The fracture surface was replicated by evaporating platinum at an angle of 45°C and followed by carbon to strengthen the replica. It was placed on a 150 mesh copper grid after washing with acetone and water. The vesicles were observed under a transmission electron microscope (JEM-1200EX, JEOL Co.).
Preparation of gel and cream incorporated with Nio FOP
For gel preparation (Gel nio), carbopol 980 at 0.6% w/w was sparingly dispersed in the niosomal dispersion, then gently stirred by magnetic stirrer until homogeneity. The mixture of mineral oil and conc. paraben (20% of the mixture of methyl and propyl paraben) was added into the dispersion with continuous mixing until homogeneity. The pH of the gel was adjusted to about 6.0–7.0 using triethanolamine at 0.6% w/w with vigorously mixing until the homogeneous gel was obtained. For cream preparation (Cream nio), theoretically, emulsions either O/W or W/O type are formed by heating oil and aqueous phase separately to about 65°C and subsequent mixing together. Therefore, in this study, the oil phase and aqueous phase which contains niosomes were heated to 65 ± 2°C. The oil phase was then poured into the aqueous phase and stirred until the mixture was cooled to 40°C. Conc. paraben was added into the mixture and continuously stirred until the emulsion was completed. Cream RBO was prepared by the same procedure as the Cream nio. It consisted of the crude rice bran extracts at 3% w/w in the oil phase, and 20% w/w of the niosomal dispersion entrapped with 2% w/w crude rice bran extracts in the aqueous phase. The amount of rice bran extracts (3% w/w) in Cream RBO is equal to those in Cream nio. The skin anti-aging effects of the combination of the unentrapped and entrapped rice bran bioactive compounds in Cream RBO was investigated in comparing to that of the entrapped rice bran bioactive compounds in Cream nio.
Chemical stability determination
For stability study of the cosmetic products, temperature variation is the main parameter used to induce rapid chemical and physical alterations in the formulations. Many previous studies have suggested that the storage conditions should be performed at low temperature (4–5°C) and high temperature (40–45°C) for several weeks in comparing to the samples exposed to ambient temperature (25–30°C) (CitationAnchisi et al., 2001; CitationGuaratini et al., 2006). In this study, gel and cream formulations incorporated with Mixed FOP entrapped and not entrapped in niosomes were stored in transparent glass bottles and kept at 4 ± 2, 30 ± 2, and 45 ± 2°C for 12 weeks as well as performed the heating (45°C) and cooling (4°C) for 6 cycles in order to accelerate the condition (CitationMasmoudi et al., 2005) and evaluate the shelf-life of these products. The remaining contents of F and O were determined by HPLC at 320 nm, while those of P were determined by a spectrophotometer at 690 nm (AOAC, 2010) at different storage temperatures for the time intervals of at initial, 2, 4, 8, and 12 weeks and after heating and cooling for 6 cycles. The percentages remaining of F, O, and P in the formulations at various time intervals were compared to the initial stability of the gel and cream containing niosomes entrapped with the rice bran bioactive compounds.
Microbial limit test (colony plate count)
One gram of the sample was added to 9 mL of sterile distilled water in a test tube and mixed thoroughly by a vortex mixer (the 10−1 dilution stock sample). Then, a serial dilution was prepared of up to 10−6. One hundred microliters of each sample (10−2 to 10−6 dilution) was spread on a sterile Petri dish containing LB medium. The experiment was performed in duplicate. After 24–48 h incubation at 37 ± 2°C, the colonies of bacteria, yeasts, and molds were counted and calculated as the following:
In vivo rabbit skin closed patch test
Six male rabbits (New Zealand White, 2.5–3.0 kg) from Royal Project Foundation in Chiang Mai, Thailand, were used to test for the skin irritation of the samples. Animal care and handling throughout the experimental procedure were performed in accordance to the CPCSEA guidelines. The fur on the back of the rabbits was shaved with an electrical clipper 24 h prior sample application. The rabbit’s back was divided into 8 marked areas (6 cm2) for the application of Gel nio, Cream nio, Gel FOP, Cream FOP, Gel base, Cream base, distilled H2O (negative control), and 15% sodium lauryl sulfate solution (SLS) which was used as a positive control. An amount of 0.2 g of the samples was applied on the marked area and covered with a gauze patch for 24 h. Then, the patches were removed and the tested area was rinsed with distilled water. Erythema was measured by mexameter (Courage & Khazaka Electronic GmbH, Germany) at 1, 24, 48, and 72 h after the removal of the patch (CitationDraize et al., 1944). Grading of the severity of erythema and edema formation using erythema scores (CitationKirwin, 1984) which were recorded visually in ranging from 0 to 4 depending on the degree of erythema [No erythema = 0, Slight erythema (barely perceptible-light pink) = 1, Moderate erythema (dark pink) = 2, Moderate to severe erythema (light red) = 3, Severe erythema (extreme redness) = 4]. Degrees of edema were recorded ranging from 0 to 4 as follows: No edema = 0, Very slight edema (barely perceptible) = 1, Slight edema (edges of area well defined by definite raising) = 2, Moderate edema (raising approximately 1 millimeter) = 3, Severe edema (raised more than 1 mm and extending beyond the area of exposure) = 4.
In vivo human skin evaluation
For cosmetic aspects of the rice bran bioactive compounds including F, O, and P, F can be a skin protecting agent or anti-aging supplement for skin due to their special structure providing strong UV absorptive ability (CitationPsotova et al., 2003; CitationTrimbino et al., 2004; CitationLin et al., 2005). O can block the UV rays at the skin’s surface and hinder its transmission (CitationCoppini et al., 2001). Hence, O can be used as a natural UV-filter in sunscreen products. For P, it has been proven efficacy on the increasing of skin elasticity, anti-wrinkle, sebum regulation, and moisturizing (CitationGraf et al., 1987). This present study has aimed at the anti-aging skin effects of topical formulations containing the semi-purified rice bran bioactive compounds entrapped in niosomes. Therefore, the major skin parameters including the skin hydration, elasticity, thickness and roughness, and pigmentation in human volunteers have been evaluated by corneometer, cutometer, visiometer, and mexameter, respectively.
Samples application
These studies were designed as one-sided blind, placebo-controlled studies. A total of 30 healthy volunteers (age 25–40 years old, 5 males and 25 females), who have previously given informed consent, were participated in this study. The samples including Gel nio and Cream nio which were gel and cream incorporated with Nio FOP that were composed of 97% and 85% of niosomes entrapped with the semi-purified rice bran extracts containing F, O, and P at 0.5, 1.5, and 1.5%, respectively. Gel FOP and Cream FOP were gel and cream incorporated with the mixture of the semi-purified rice bran extracts containing F, O, and P at 0.5, 1.5, and 1.5%, respectively. Gel base was composed of carbopol 980, mineral oil, vitamin E acetate, triethanolamine, propyl paraben, and methyl paraben. Cream base was composed of stearic acid, cetyl alcohol, white petrolatum, mineral oil, isopropyl myristate, carbopol 980, glycerin, propylene glycol, triethanolamine, propyl paraben, and methyl paraben. Cream S was a commercial cream in Thai market, containing soya extract, oat kernel extract, and vitamin E. Gel and cream formulations at about 0.2 g were applied on the volar forearm of the volunteers that had no visible skin abnormalities twice daily for 28 days. Area with no sample application was used as a negative control. The volunteers were not allowed to use moisturizers on the tested areas for 1 week prior to, and during, the experiments. These studies were performed during November to December 2009 at the Faculty of Pharmacy, Chiang Mai, Thailand. Approval of the study was obtained from the Ethic Committee of Faculty of Pharmacy, Chiang Mai University in Thailand and complied with the Declaration of Helsinki.
Anti-aging efficacy measurement
The volunteers were accommodated in an air-conditioned room at 25 ± 1°C and 45 ± 5% relative humidity for 15 min before the measurements. The skin hydration, pigmentation, topography, and elasticity were measured prior to the application of samples on day 0 and after sample application at days 14 and 28, and after no sample application for 7 days (wash-out period).
Skin hydration
Skin hydration was measured by a corneometer 825, which was mounted on a multi probe adapter MPA5 (Courage & Khazaka, Electronic GmbH, Cologne, Germany). It was based on the relatively high dielectric constant of water compared to the substances inside the skin (CitationBerardesca & Borroni, 1995). The measurements were performed in triplicate.
Skin pigmentation and erythema
Skin pigmentation (melanin) and redness (erythema) were measured photometrically by a mexameter, which was mounted on a multi probe adapter MPA580 (Courage & Khazaka, Electronic GmbH, Cologne, Germany) based on the remission principle (CitationTaylor et al., 2006). The receiver measured the light reflected by the skin. Melanin and erythema index were calculated.
Skin topography (skin thickness and roughness)
Skin thickness and roughness were measured by a skin visiometer SV600 and calculated according to the Lambert-Beer law from the absorbance of the transmitted light (CitationLee et al., 2006). The absorbances were measured by the CCD chip and transferred to a monitor, which showed the structure of the skin topography.
Skin elasticity
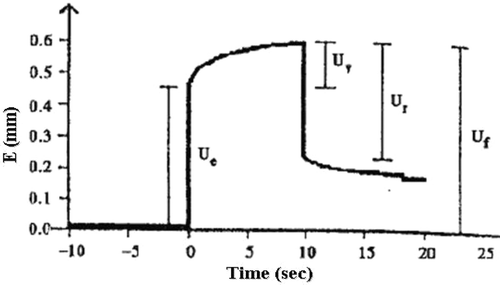
Skin elasticity was performed using a cutometer MPA580 (Courage & Khazaka Electronic GmbH, Cologne, Germany) based on a suction and elongation method (CitationBazin & Fanchon, 2006). The instrument measured the vertical deformation (E) of the skin surface when the skin was pulled into the circular aperture (2 mm in diameter) of the measuring probe with the constant suction pressure of 450 mbar for 2 s. Afterwards, the pressure was cut off and the skin returned to its original position. The resulting curve of each measurement represented the elasticity of the skin (). The net elasticity of the skin (Ur/Ue) was calculated automatically by a built-in database file.
Statistical analysis
All experiments were performed in triplicate. The results of all tests were presented as mean ± SD. ANOVA with Multiple Comparisons was used for the statistical analysis at the significant level of p < 0.05 (Ho: µ1 = µ2 = µn, H1: µ1 ≠ µ2 ≠ µn). The critical region was calculated from F distribution at F > F(1-α, k-1, n-k), whereas k was the number of the treatments and n was the number of the experiment units. For anti-aging efficacy measurement, the 7 samples (k = 7) were treated in 30 volunteers (n = 210). Thus, F > F0.95, 6203 was used in the efficacy analysis.
Results and discussion
This present study has investigated the in vitro anti-skin aging activity and cytotoxicity on skin fibroblasts of the rice bran bioactive compounds using the inhibition of matrix MMPs and SRB assay to confirm their anti-aging effect and safety prior to the clinical anti-aging study in humans as follows.
Cytotoxicity on the cultured human skin fibroblast of the semi-purified rice bran extracts by SRB assay
The semi-purified rice bran extracts containing F, O, and P gave no toxicity on human skin fibroblasts. Moreover, F, O, and P at the concentration of 10 mg/mL showed higher fibroblast growth than no treatment of 1.37 ± 0.281, 1.66 ± 0.233, and 0.97 ± 0.03 times, respectively. Ascorbic acid at the concentration of 10 mg/mL not only showed no stimulation on fibroblast growth but also cytotoxicity at IC50 of 2.99 ± 0.010 mg/mL. The oxidative products of ascorbic acid may gave this more cytotoxicity effect than the extracts (CitationBeatrice et al., 1980). However, the IC50 value of ascorbic acid was still in the acceptance criteria of non-cytotoxic agent (IC50 > 1 mg/mL of samples).
Inhibition of MMP-2 of the semi-purified rice bran extracts
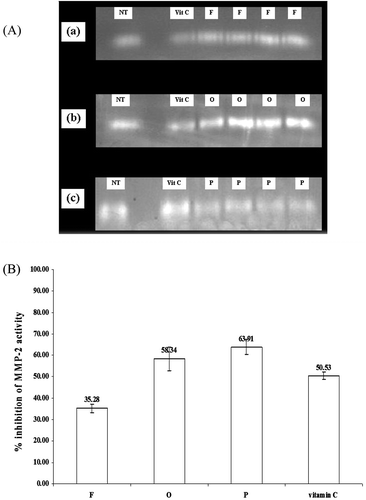
As known, free radicals are one of the causes of skin aging. Many antioxidants can scavenge these radicals. In addition, the MMP-2 is one of the key enzymes in the skin which can degrade the collagen. Therefore, any compounds which can inhibit this enzyme will be able to inhibit the process of aged skin development from the degradation of the skin collagen. There are also many reports associated with the antioxidant activities and cytotoxicity of the rice bran bioactive compounds (CitationIqbal et al., 2005; CitationJittorntrum et al., 2009). The rice bran bioactive compounds have also been widely used as antioxidants in skin care products (CitationXu & Godber, 2001; CitationAhn et al., 2003; CitationTrombino et al., 2004). In our present study, although the semi-purified rice bran extracts containing F gave lower %MMP-2 inhibition on human skin fibroblasts (35.28 ± 2.05%) than ascorbic acid (50.53 ± 1.86%) of 1.43 time (p < 0.05), the extract containing O showed similar activity (58.34 ± 5.47%) to ascorbic acid (p < 0.05). However, the extract containing of P (63.91 ± 3.64%) gave higher activity than ascorbic acid of 1.26 times (). The chelating activity of the rice bran bioactive compounds F, O and P may be responsible for this effect (CitationHynes & O’Coinceanainn, 2004; CitationChotimarkorn et al., 2008; CitationMazzoni et al., 2007). Many previous studies have also indicated that the MMP-2 inhibition activity of different compounds was usually correlated with their metal chelating activities (CitationNewsome et al., 2007; CitationSamia & Karl, 2007).
Figure 2. Inhibition of MMP-2 of the semi-purified rice bran extract by gelatin zymograms, (A) gelatin zymograms of MMP-2 from human skin fibroblasts treated with the semi-purified rice bran extracts containing F, O, and P in comparing to vitamin C and no treatment after incubation for 24 h (a, b, c, respectively). (B) % inhibition of MMP-2 activity of the semi-purified rice bran extracts containing F, O, P, and vitamin C in human skin fibroblasts.
Characteristics of niosomes entrapped with semi-purified rice bran extracts containing bioactive compounds F, O, and P (Nio FOP)
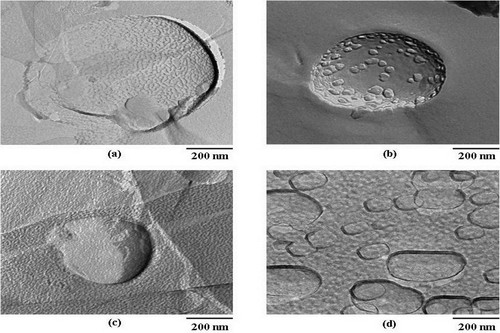
The entrapment efficiencies of the rice bran bioactive compounds (F, O, and P) in niosomes determined by gel filtration technique were 64.47 ± 1.17, 47.54 ± 2.31, and 54.85 ± 0.11%, respectively. The vesicular size and PDI of the niosomal dispersion investigated by DLS apparatus were slightly large with size distribution of 480.9 ± 270.8 nm (PDI = 1.5), which were still in the nanosize range that will be useful for topical application (CitationVerma et al., 2003; CitationBetz et al., 2005). Moreover, from the TEM investigation, the niosomal structures in the emulsion were still intact (). In addition, the niosomes entrapped with rice bran bioactive compounds were stable at high temperature of up to 80°C measured by differential scanning calorimeter (CitationManosroi et al., 2010).
Figure 3. Freeze fracture TEM images of niosomes composed of Tween 61 mixed with cholesterol (20 mM) at 1:1 molar ratio (A) blank niosomes prepared by scCO2 technique (B) niosomes entrapped with the semi-purified mixed extracts containing F, O, and P (C) niosomes entrapped with the extracts incorporated in gel formulation and (D) niosomes entrapped with the extracts incorporated in cream formulation (magnification 12.0K ×, scale bar 200 nm).
Chemical stability
The percentages remaining of F, O, and P in the semi-purified extracts of free and entrapped in niosomes (Nio FOP), Cream nio, and Gel nio after kept at 4 ± 2, 30 ± 2, and 45 ± 2°C for 12 weeks were shown in . At 4 ± 2°C, the percentages remaining of F, O, and P in the unentrapped extracts (12.08 ± 1.10, 15.4 ± 1.19, and 39.54 ± 1.35) were less than those entrapped in niosomes (70.0 ± 1.30, 77.8 ± 1.50, and 75.4 ± 1.15), Cream nio (67.4 ± 1.1, 70.8 ± 1.5, and 75.4 ± 1.5), and Gel nio (77.6 ± 1.1, 66.8 ± 1.5, and 75.4 ± 1.5) of 5.79, 5.05, and 1.90; 5.58, 4.60, and 1.91; 6.42, 4.34, and 2.03 times, respectively. At 30 ± 2°C, the percentages remaining of F, O, and P in the unentrapped extracts (9.39 ± 1.10, 10.5 ± 1.28, and 25.0 ± 1.28) were less than those in niosomes (Nio FOP) (60.0 ± 1.32, 68.0 ± 1.17, and 66.6 ± 1.10), Cream nio (58.3 ± 1.24, 59.0 ± 2, and 65.0 ± 1.7), and Gel nio (65.9 ± 1.15, 58 ± 1.5, and 69.5 ± 1.5) of 6.39, 6.48, and 2.66; 6.21, 5.62, and 2.60; 7.02, 5.52, and 2.78 times, respectively. At 45 ± 2°C, the percentages remaining F, O, and P in the unentrapped extracts (9.39 ± 1.09, 8.9 ± 1.21, and 19.0 ± 1.27) were less than those in niosomes (Nio FOP) (33.33 ± 1.14, 45.0 ± 1.11, and 57.0 ± 1.22), Cream nio (44.0 ± 1.2, 55.0 ± 1.5, and 49.0 ± 1.65), Gel nio (51.0 ± 1.2, 45.4 ± 1.5, and 50.0 ± 1.5) of 3.55, 5.06, and 3.0; 4.69, 6.18, and 2.58; 5.43, 5.10, and 2.63 times, respectively. In all systems, when the storage temperatures increased, the percentages remaining of all bioactive compounds (F, O, and P) decreased. The F and O containing in the semi-purified extracts which were oil soluble may be easily oxidized by heat and sunlight (CitationPalma et al., 2002), while the salt form of P tended to be a thermal stable compound (CitationManconi et al., 2003). The bioactive compound P was more chemical stable than F and O of about 2 times. When the semi-purified extracts containing F, O, and P entrapped in niosomes, their chemical stability was increased more than the unentrapped bioactive compounds of about 2–7 times at all storage temperatures. Tween 61 and cholesterol containing in the niosomal membrane may be oxidized and donate the hydrogen which may retard the autooxidation of F, O, and P (CitationYoshioka et al., 1994). The chemical stability of the semi-purified rice bran extracts containing F, O, and P in niosomes incorporated in gel and cream formulations (Gel nio and Cream nio) was less than the entrapped compounds but not incorporate in gel or cream of about 1 time. The niosomal membrane might be degraded by the oxidative or hydrolysis products from the compositions in gel and cream, thereby increasing the leakage from the niosomes and deterioration of the bioactive compounds (CitationGuilatt et al., 2005). However, the entrapped bioactive compounds incorporated in gel and creams showed more stability than the unentrapped compounds of about 2–6 times. This has indicated that niosomes and gel and cream formulations can increase the shelf-life of the bioactive compounds.
Table 1. The percentages remaining of F, O, and P in the semi-purified extracts of free and the entrapped rice bran bioactive compounds in niosomes (Nio FOP), Cream nio, and Gel nio after kept at 4 ± 2, 30 ± 2, and 45 ± 2°C for 12 weeks.
Microbial limit test (colony plate count)
The colonies of molds (M), bacteria (B), and yeasts (Y) per mL of cream and gel incorporated with niosomes entrapped with the mixture of the semi-purified rice bran extracts (Cream nio and Gel nio) were compared with the cream and gel incorporated with the mixture of the semi-purified rice bran extracts (Cream FOP and Gel FOP) as well as cream and gel base formulations (). At 10−1 dilution, 7 and 10 colonies of molds were found in Cream nio and Gel nio, respectively, and 10 colonies of bacteria were found in Cream nio. At 10−2 dilution, 2 colonies of bacteria and 2 colonies of molds were found in Cream nio and Gel nio, respectively. This might be from the cholesterol in niosomes which was a supplemented diet for molds or bacteria. However, the amounts of molds and bacteria in Cream nio and Gel nio were in the acceptance range for skin care products of less than 100 CFU/mL of the samples according to Cosmetic, Toiletry, and Fragrance Association microbiology guidelines (CitationCurry et al., 1993). The acceptance criteria of total aerobic microbial and total yeasts and molds count of topical products which is less than 100 and 10 CFU/mL of samples, respectively (CitationGrilli, 2009). Moreover, the actives in niosomes were stable for the length of the clinical study of 28 days with the microbial loads of less than 100 CFU. As known, the suitable shelf-life, not only concerning during the clinical test of 28 days but should also cover the storage period on the shelf of the products, which can be evaluated from the physico-chemical stability in different time intervals at various storage temperatures of the contained bioactive compounds, as well as the low microbial loads in the products are required before commercialization as well (CitationBrannan & Geis, 2009; CitationLintner, 2009).
Table 2. The colonies of molds (M), bacteria (B), and yeasts (Y) per mL of Cream nio, Gel nio, Cream FOP, Gel FOP, Cream base, and Gel base.
In vivo rabbit skin closed patch test
The skin irritation in animals was first performed before testing in human (CitationOECD, 1992; Citationvan de Sandt et al., 1997). Thus, the acute skin irritation potential of the semi-purified rice bran extracts, nanovesicles and nanovesicular gel and creams containing the semi-purified rice bran extracts were first evaluated by carrying out the Draize patch test on rabbits (CitationDraize et al., 1944; CitationShah et al., 2007). Then, when these samples did not show any irritation, the skin erythema of our cosmetic products was observed in human volunteers after repeated application for 28 days using mexameter. The results indicated that there were no signs of erythema or edema on the shaved rabbit skin detected from the semi-purified rice bran extracts containing F, O and P within 72 h. Thus, the semi-purified extracts which had the pH values in the range of 5.0–6.0, may do not contain any irritants. All nanovesicular formulations gave no signs of erythema or edema. Therefore, substances in the vesicles appeared to be compatible to the skin. Moreover, the amount of Tween 61 niosomes were about 1–1.5%, which was very low concentration in comparing to 15% of sodium lauryl sulfate (anionic surfactant), which was used as a positive control. The pH values of all nanovesicles were in the range of 5.5–6.5. For the nanovesicular gel and cream formulations, they also gave no signs of erythema. All gel and cream formulations that had the pH in the range of 6.0–7.0 and their compositions were skin compatible. In fact, there are also many alternative approaches to the rabbit Draize test for the skin irritation assessment, such as the rat skin transcutaneous electrical resistance assay and in vitro reconstituted human epidermal (RHE) skin corrosion models which are more acceptable to the cosmetic industry and the various recent regulatory organization (CitationJones et al., 2009).
In vivo human skin evaluation
It is known that “crow’s foot” is much more appropriate for skin aging evaluation than other sites, which is not only subjected to the biological aging process but also to the contraction of the per-orbital muscle in the face and influences of environments (CitationFu et al., 2010). However, anti-aging effect is not only for face but also for the entire body. Thus, the volar forearm was selected for clinical anti-aging assessment in this study in comparing to the anti-aging body lotion containing soya, oat extracts, and vitamin E. Moreover, the volar forearm was considered as a reliable site that was related to intrinsic aging and minimized the influence of physical forces of stretching deformation and the direct effect from UV radiation (CitationYin et al., 2001; CitationKaczvinsky et al., 2010).
Skin hydration
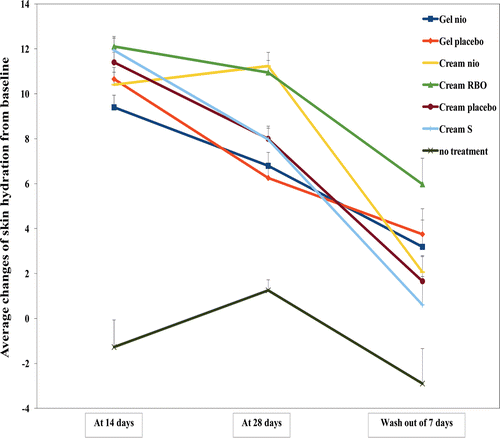
The average changes of skin hydration from the baseline measured by a corneometer after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and the commercial Cream S for 14, 28 days, and wash-out period of 7 days were shown in . Skin hydration after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S at day 14 was higher than no treatment of 8.38, 9.37, 9.17, 10.51, 9.96, and 10.37 times, respectively (p < 0.05). The decrease of skin hydration after treated with Gel nio, Gel placebo, Cream placebo, and Cream S at day 28 was significantly lower than at day 14. This may be from the saturation of the moisturizing effects of gel and cream which usually reaches at day 12–14 of the treatment (CitationCevc & Vierl, 2010). However, the skin hydration after treated with Cream nio and Cream RBO was remained until day 28 because of the sustained release of the rice bran bioactive compounds entrapped in niosomes in the cream formulations (CitationAzeem et al., 2009). Moreover, at the wash-out period of 7 days, the skin hydration treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and the commercial Cream S was higher than no treatment of 2.10, 2.29, 1.71, 3.06, 1.57, and 1.21 times, respectively. This has indicated the long lasting skin hydration effects of gel and cream formulations containing the rice bran bioactive compound entrapped in niosomes. The skin hydration of no treatment at day 14 was not significantly lower than 28 but was higher than the wash-out period of 7 days (day 35). This may be due to the skin cell rejuvenation which usually occurred after 28 days. These results have confirmed the skin hydration enhancing effect of the bioactive compounds containing in the rice bran extracts entrapped in niosomes and the compositions of the gel and cream base formulations. From many previous studies, the nanovesicles were claimed for improving skin moisturization due to an interaction between their lipid components and stratum corneum lipids and permit the nanovesicles to deliver active substances into the skin (CitationHolfland et al., 1995; CitationCoderch et al., 1999). CitationKirjavanen et al. (1996, Citation1999a, Citation1999b) concluded that the vesicles may interact with human skin and produce penetration enhancing effect by fusion with skin lipid to loosen their structure or penetration deep into the skin. The penetration enhancing effect of nanovesicles should be concerned in a difference of components in the vesicles (CitationBetz et al., 2005; CitationElsayed et al., 2007). However, the mechanisms of action of niosomes as the transdermal delivery systems are still not completely clear. In addition, at day 14, Cream RBO gave the highest skin hydration efficacy while the efficacy of Gel nio and Cream nio gave no different to their placebo. At 28 days, the skin hydration after treated with Cream nio and Cream RBO was higher than their placebo, whereas Gel nio gave no different to Gel placebo. At the wash-out period of 7 days, only Cream RBO and Cream nio gave more improvement of skin hydration efficacy compared with their placebo after stop application. The results have indicated that the skin hydration effects depend on types of formulations (gel or cream) and the presence of niosomes and the rice bran bioactive compounds in these formulations. Cream is more suitable for incorporating with niosomes and the rice bran bioactive compounds than gel for skin hydration enhancement investigated by a corneometer.
Skin pigmentation and erythema
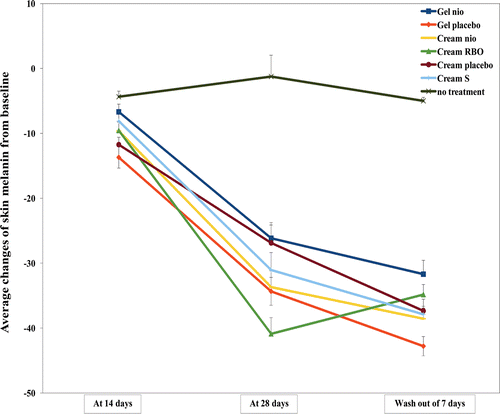
The average changes of skin pigmentation (melanin) from the baseline measured by a mexameter after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S for 14, 28 days, and wash-out period of 7 days were shown in . The skin melanins after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S at day 14 and 28 were lower than no treatment of 1.53, 3.14, 2.21, 2.19, 2.68, and 1.86; 21.22, 27.85, 27.32, 33.15, 21.80, and 25.18 times, respectively (p < 0.05). The sunscreen effect of the rice bran bioactive compounds in the extracts entrapped in niosomes or the compositions of the gel and cream base formulations have been demonstrated by the ex vivo lipid peroxidation inhibition assay in our previous report (CitationManosroi et al., 2010). At the wash-out period of 7 days, the skin melanins treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S were still lower than no treatment of 6.34, 8.56, 7.71, 6.97, 7.47, and 7.58 times, respectively. This has indicated that all gel and cream formulations gave skin lightening effect and sustained this effect even after without the treatment for 7 days. Moreover, the skin redness indicating skin irritation was not observed from all formulations at all time intervals. This result has also demonstrated that the cream is suitable formulation for incorporating with niosomes and the rice bran bioactive compounds more than gel for the skin pigmentation improvement investigated by a mexameter.
Skin topography (skin thickness and roughness)
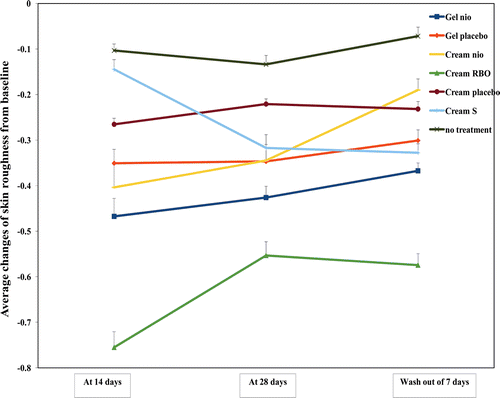
One of the most prominent features of aging is the change of skin topography including the reduced epidermal thickness and the increased skin roughness. With times, keratinocytes comprised 90% of epidermal cell population loss their proliferative capacity thereby decreasing the epidermal turnover rate. Moreover, the elder skin also displayed fine line and wrinkle due to degeneration of dermis elastic fiber (CitationYaar, 2006). The average changes of skin thickness and roughness from the baseline measured by a skin visiometer after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S for 14, 28 days, and wash-out period of 7 days are shown in and , respectively. The skin thickness after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S at day 14, 28, and wash-out period of 7 days were higher than no treatment of 4.70, 6.02, 5.02, 9.04, 5.83, 6.38; 5.85, 6.04, 5.53, 9.96, 5.71, 6.29; 2.16, 2.2, 2.42, 2.45, 2.51, and 2.52, respectively (p < 0.05). Moreover, the skin roughness after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S at day 14, 28, and wash-out period of 7 days was lower than no treatment of 4.51, 3.39, 3.90, 7.29, 2.56, and 1.40; 3.19, 2.59, 2.58, 4.14, 1.65, and 2.37; 5.10, 4.18, 2.64, 7.98, 3.22, and 4.55 times, respectively (p < 0.05). The antioxidative activity of bioactive compounds (F, O, and P) in the gel and cream formulations may be responsible for the improvement of skin texture by limiting the progression of oxidative damage and enhancing the production of collagen and keratinocytes resulting in the greater skin thickness, diminished fine lines and wrinkles, and smoother skin-surface texture (CitationShapiro & Saliou, 2001). Moreover, the composition in gel and creams such as carbopol which is a thickening polymer may protect the skin cell damage from UV exposure by the occlusion effects (CitationCevc et al., 2008). In addition, only Cream RBO gave more improvement of skin topography than cream placebo investigated by a skin visiometer, while Gel nio and Cream nio gave the skin topography improvement similar to their placebo. This may be due to the enrichment of the unentrapped rice bran bioactive compounds in Cream RBO providing more anti-aging effects (CitationMuller et al., 2007). However, this result has confirmed the anti-skin aging activity by increasing the thickness and reducing the roughness of gel and cream incorporated with the rice bran bioactive compounds entrapped in niosomes.
Figure 6. The average changes of skin thickness (topography volume; mm2) from the baseline measured in 30 human volunteers by a skin visiometer after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, Cream S, and no treatment for 14, 28 days, and wash-out period of 7 days.
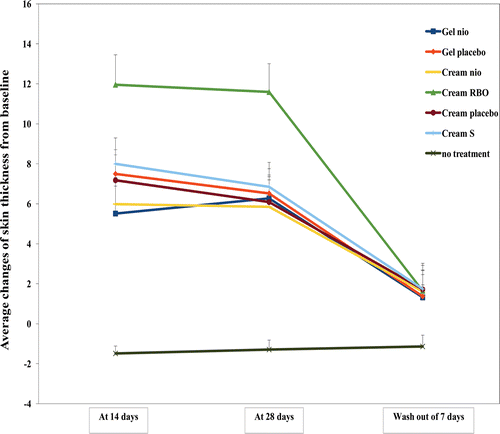
Figure 7. The average changes of skin roughness from the baseline measured in 30 human volunteers by a skin visiometer after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, Cream S, and no treatment for 14, 28 days, and wash-out period of 7 days (*significant different skin hydration of the samples at all time intervals by ANOVA with Multiple Comparisons, p < 0.05, Ho: µ1 = µ2 = µn, H1:µ1 ≠ µ2 ≠ µn).
Skin elasticity
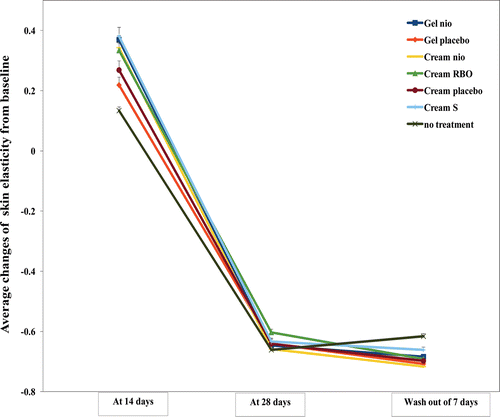
The average changes of skin elasticity from the baseline measured by a cutometer after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S for 14, 28 days, and wash-out period of 7 days were shown in . Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S gave higher skin elasticity than no treatment at 14 days of 2.76, 1.63, 2.56, 2.50, 2.01, and 2.85 times, respectively (p < 0.05). Both gel and cream incorporated with the rice bran bioactive compounds gave more skin elasticity improvement than their placeboes, this might be owing to the presence of niosomes that enhanced the penetration of the rice bran bioactive compounds into the skin (CitationKirjavainen et al., 1999a, Citation1999b). However, at 28 days and the wash-out period of 7 days, all formulations gave no skin elasticity improvement the same as that of no treatment (p < 0.05). Generally, the saturation of the skin elasticity by gel and cream reaches at days 12–14 of the treatment (CitationCevc & Vierl, 2010). At day 28 and wash-out period of 7 days, the skin elasticities after treated with Gel nio, Gel placebo, Cream nio, Cream RBO, Cream placebo, and Cream S were significantly lower than at day 14. This has indicated the cell rejuvenation effects of the volunteers which usually occurred after 28–35 days (CitationSchmitt, 1992). From all of the analysis, it has indicated that the anti-aging effects including skin hydration, pigmentation, topography, and elasticity depend on types of formulations (gel or cream) and the presence of niosomes and the rice bran bioactive compounds in the formulations. Cream give higher skin hydration, pigmentation, and topography enhancement than gel investigated by corneometer, mexameter, and skin visiometer, respectively, while skin elasticity is improved by both of gel and cream applications. Moreover, cream incorporated with the unentrapped and entrapped rice bran bioactive compounds in niosomes (Cream RBO) gave prominently improvement of skin hydration pigmentation, topography, and elasticity, due to the enrichment of the unentrapped rice bran bioactive compounds providing more anti-aging effects. This has confirmed the synergistic skin anti-aging effects of the unentrapped and entrapped rice bran bioactive compounds in niosomes. Hence, Gel nio, Cream nio, and Cream RBO containing the rice bran bioactive compounds entrapped in niosomes can be applied as novel topical products for skin aging due to their superior skin hydration effect and the improvement of skin pigmentation, skin thickness and roughness as well as skin elasticity.
Conclusions
This study has evaluated the gel and cream formulations containing the semi-purified rice bran extracts containing the bioactive compounds F, O, and P entrapped in niosomes (Gel nio, Cream nio, and Cream RBO) for skin aging. These formulations did not only show the stimulation on the growth of human fibroblast and inhibition of MMP-2 activity of the semi-purified rice bran extracts containing F, O, and P but also the improvement of skin properties including hydration, pigmentation, thickness and roughness, and skin elasticity on 30 human volunteers skin for 28 days after the treatment of Gel nio and Cream nio. Moreover, the prominently efficacy of Cream RBO has confirmed the synergistic skin anti-aging effects of the unentrapped and entrapped rice bran bioactive compounds in niosomes. In addition, the chemical stability of the bioactive compounds in the extracts was also enhanced by entrapping in niosomes and incorporated in gel or cream formulations. This study has suggested the synergistic skin anti-aging effects of niosomes, rice bran bioactive compounds, and the composition in the gel and cream formulations. The novel anti-aging formulations with superior in vitro and in vivo skin anti-aging activity and no skin irritation were indicated.
Declaration of interest
This work was supported by the Thailand Research Fund (TRF) under the RGJ-PhD program in Thailand, Natural Product Research and Development Center (NPRDC), Science and Technology Research Institute (STRI), and Nanoscience and Nanotechnology Research Center Project, Faculty of Sciences, Chiang Mai University, Thailand, and Tokyo University of Science (TUS) in Japan.
References
- Anchisi C, Maccioni AM, Sinico C, Valenti D. (2001). Stability studies of new cosmetic formulations with vegetable extracts as functional agents. Farmaco, 56, 427–431.
- Ahn HJ, Kim JH, Yook HS, Byun MW. (2003). Irradiation effects on free radical-scavenging and antioxidant activity of phytic acid. J Food Sci, 68, 2221–2224.
- Arican M, Ceylan C. (1999). Metalloproteinases in canine experimental traumatic keratoconjunctivitis. Zentralbl Veterinarmed a, 46, 527–532.
- Azeem A, Anwer MK, Talegaonkar S. (2009). Niosomes in sustained and targeted drug delivery: Some recent advances. J Drug Target, 17, 671–689.
- Bangham AD, Standish MM, Watkins JC. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol, 13, 238–252.
- Bazin R, Fanchon C. (2006). Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies. Int J Cosmet Sci, 28, 453–460.
- Beatrice Y, Yue T, Niedra R, Bautn JL. (1980). Effects of ascorbic acid on cultured rabbit corneal endothelial cells. Assoc Res Vis Ophthal, 19, 1471–1476.
- Berardesca E, Borroni G. (1995). Instrumental evaluation of cutaneous hydration. Clin Dermatol, 13, 323–327.
- Berton A, Godeau G, Emonard H, Baba K, Bellon P, Hornebeck W, Bellon G. (2000). Analysis of the ex vivo specificity of human gelatinases A and B towards skin collagen and elastic fibers by computerized morphometry. Matrix Biol, 19, 139–148.
- Betz G, Aeppli A, Menshutina N, Leuenberger H. (2005). In vivo comparison of various liposome formulations for cosmetic application. Int J Pharm, 296, 44–54.
- Brannan DK, Geis PA. (2009). Cosmetics microbiology encyclopedia of microbiology. Encyclopedia of Microbiology, 3, 270–280.
- Cevc G, Blume G. (2003). Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, Transfersomes. Biochim Biophys Acta, 1614, 156–164.
- Cevc G, Mazgareanu S, Rother M, Vierl U. (2008). Occlusion effect on transcutaneous NSAID delivery from conventional and carrier-based formulations. Int J Pharm, 359, 190–197.
- Cevc G, Vierl U. (2010). Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J Control Release, 141, 277–299.
- Chotimarkorn C, Benjakul S, Silalai N. (2008). Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem, 111, 636–641.
- Coderch L, de Pera M, Perez-Cullell N, Estelrich J, de la Maza A, Parra JL. (1999). The effect of liposomes on skin barrier structure. Skin Pharmacol Appl Skin Physiol, 12, 235–246.
- Coppini D, Paganizzi P, Santi P, Ghirardini A. (2001). Capacità protettiva nei confronti delle radiazioni solari di derivati di origine vegetale. Cosmetic News, 136, 15–20.
- Cooper AI. (2000). Synthesis and processing of polymers using supercritical carbon dioxide. J Mater Chem, 10, 207–234.
- Curry AS, Graf JG, McEwen GN. (1993). CTFA Microbiology Guidelines. Cosmetic, Toiletry, and Fragrance Association: Washington DC.
- Draize J, Woodard G, Calvery H. (1944). Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther, 82, 377–390.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. (2007). Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int J Pharm, 332, 1–16.
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. (1997). Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med, 337, 1419–1428.
- Fu JJ, Hillebrand GG, Raleigh P, Li J, Marmor MJ, Bertucci V, Grimes PE, Mandy SH, Perez MI, Weinkle SH, Kaczvinsky JR. (2010). A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br J Dermatol, 162, 647–654.
- Gaspar LR, Camargo FB Jr, Gianeti MD, Maia Campos PM. (2008). Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem Toxicol, 46, 3493–3500.
- Graf E, Empson KL, Eaton JW. (1987). Phytic acid. A natural antioxidant. J Biol Chem, 262, 11647–11650.
- Grilli A. (2009). Harmonised Pharmaceutical Microbiology. In SCPDG Local Meeting SGS Life Science Services, March 19.
- Guaratini T, Gianeti MD, Campos PMBGM. (2006). Stability of cosmetic formulations containing esters of Vitamins E and A: Chemical and physical aspects. Int J Pharm, 327, 12–16.
- Rabinovich-Guilatt L, Dubernet C, Gaudin K, Lambert G, Couvreur P, Chaminade P. (2005). Phospholipid hydrolysis in a pharmaceutical emulsion assessed by physicochemical parameters and a new analytical method. Eur J Pharm Biopharm, 61, 69–76.
- Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghe G. (1979). Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cos Sci, 1, 303–314.
- Handjani R, Ribier A., Vanlerberghe G., Zabotto A., Griat J. (1989). Cosmetic and pharmaceutical compositions containing niosomes and a water-soluble polyamide, and a process for preparing these compositions. General Pharmacology: The Vascular System, 20, 5, 1–8.
- Hofland HE, Bouwstra JA, Boddé HE, Spies F, Junginger HE. (1995). Interactions between liposomes and human stratum corneum in vitro: Freeze fracture electron microscopical visualization and small angle X-ray scattering studies. Br J Dermatol, 132, 853–866.
- Hynes MJ, O’Coinceanainn M. (2004). The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J Inorg Biochem, 98, 1457–1464.
- Iqbal S, Bhanger MI, Anwar F. (2005). Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem, 93, 265–272.
- Jenkins G. (2002). Molecular mechanisms of skin ageing. Mech Ageing Dev, 123, 801–810.
- Jittorntrum B, Chunhabundit R, Kongkachuichai R, Srisala S, Visetpanit Y. (2009). Cytoprotective and cytotoxic effects of rice bran extracts on H2O2- induced oxidative damage in human intestinal Caco-2 cells. Thai J Toxicology, 24, 92–100
- Jones P, Goebel C, Dufour E, Rowland J, Araki D, Farkas MC, Hewitt NJ, Hibatallah J, Kirst A, McNamee P, Schellauf F, Scheel J. (2009). A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Skin irritation Martin Macfarlane. Regul Toxicol Pharmacol, 54188–54196.
- Kaczvinsky J, Li J, Crowther J, Surrey E, Mirkovic S, Janson W. (2010). Effect of topical antiaging products on stratum corneum thickness and barrier integrity. J Am Acad Dermatol, 62, Suppl. 1, AB25.
- Kaur IP, Kapila M, Agrawal R. (2007). Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res Rev, 6, 271–288.
- Kirjavainen M, Urtti A, Jääskeläinen I, Suhonen TM, Paronen P, Valjakka-Koskela R, Kiesvaara J, Mönkkönen J. (1996). Interaction of liposomes with human skin in vitro–the influence of lipid composition and structure. Biochim Biophys Acta, 1304, 179–189.
- Kirjavainen M, Urtti A, Valjakka-Koskela R, Kiesvaara J, Mönkkönen J. (1999a). Liposome-skin interactions and their effects on the skin permeation of drugs. Eur J Pharm Sci, 7, 279–286.
- Kirjavainen M, Mönkkönen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A. (1999b). Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Release, 58, 207–214.
- Kirwin CJ. (1984). Eye and skin local toxicity testing. In: Sperling F, ed. Toxicology Principles and Practice. Sperling a Wiley-Interscience publication: New York, 169–175.
- Kligman LH, Kligman AM. (1986). The nature of photoaging: Its prevention and repair. Photodermatol, 3, 215–227.
- Korhonen M, Lehtonen J, Hellen L, Hirvonen J, Yliruusi J. (2002). Rheological properties of three component creams containing sorbitan monoesters as surfactants. Int J Pharm, 247, 103–114.
- Lasic DD. (1993). Liposomes: From Physics to Applications, Elsevier: Amsterdam.
- Lawrence MJ, Chauhan S, Lawrence SM, Barlow DJ. (1996). The formation, characterization and stability of nonionic surfactant vesicles. STP Pharm Sci, 6, 49–60.
- Lee MS, Lee KH, Sin HS, Um SJ, Kim JW, Koh BK. (2006). A newly synthesized photostable retinol derivative (retinyl N-formyl aspartamate) for photodamaged skin: Profilometric evaluation of 24-week study. J Am Acad Dermatol, 55, 220–224.
- Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, Monteiro-Riviere NA, Grichnik JM, Zielinski J, Pinnell SR. (2005). Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol, 125, 826–832.
- Lintner K. (2009). Cosmetic ingredients: Definitions, legal requirements, and an attempt to harmonize (global?) characterization, Global Regulatory Issues for the Cosmetics Industry, 31–53.
- Manconi M, Valenti D, Sinico C, Lai F, Loy G, Fadda AM. (2003). Niosomes as carriers for tretinoin. II. Influence of vesicular incorporation on tretinoin photostability. Int J Pharm, 260, 261–272.
- Manconi M, Sinico C, Valenti D, Lai F, Fadda AM. (2006). Niosomes as carriers for tretinoin. III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm, 311, 11–19.
- Manosroi A, Chutoprapat R, Abe M, Manosroi J. (2008). Characteristics of niosomes prepared by supercritical carbon dioxide (scCO2) fluid. Int J Pharm, 352, 248–255.
- Manosroi A, Chutoprapat R, Sato Y, Miyamoto K, Hsueh K, Abe M, Manosroi W, Manosroi J. (2010). Antioxidant activities and skin hydration effects of rice bran bioactive compounds entrapped in niosomes. J Nanosci Nanotechnol, 10, 1–9.
- Manosroi A, Chutoprapat R, Abe M, Manosroi J. (2010). Characteristics of niosomes entrapped with rice bran bioactive compounds prepared by supercritical carbon dioxide. In: Proceeding of International Conference of Nanoscience and Nanotechnology (ICONN2010).
- Manosroi A, Khositsuntiwong N, Götz F, Werner RG, Manosroi J. (2009). Transdermal enhancement through rat skin of luciferase plasmid DNA loaded in elastic nanovesicles. J Liposome Res, 19, 91–98.
- Masmoudi H, Dréau YL, Piccerelle P, Kister J. (2005). The evaluation of cosmetic and pharmaceutical emulsions aging process using classical techniques and a new method: FTIR. Int J Pharm, 289, 117–131.
- Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. (2007). Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res, 86, 436–440.
- Müller RH, Petersen RD, Hommoss A, Pardeike J. (2007). Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev, 59, 522–530.
- Neuman MG, Haber JA, Malkiewicz IM, Cameron RG, Katz GG, Shear NH. (2002). Ethanol signals for apoptosis in cultured skin cells. Alcohol, 26, 179–190.
- Neuman MG, Oruña L, Coto G, Lago G, Nanau R, Vincent M. (2010). Hyaluronic acid signals for repair in ethanol-induced apoptosis in skin cells in vitro. Clin Biochem, 43, 822–826.
- Newsome AL, Johnson JP, Seipelt RL, Thompson MW. (2007). Apolactoferrin inhibits the catalytic domain of matrix metalloproteinase-2 by zinc chelation. Biochem Cell Biol, 85, 563–572.
- OECD. (1992). Acute dermal irritation/corrosion. In: OECD Guidelines for Testing of Chemicals. Guideline 404, 6 pp. Paris, France: OECD.
- Palma S, Lo Nostro P, Manzo R, Allemandi D. (2002). Evaluation of the surfactant properties of ascorbyl palmitate sodium salt. Eur J Pharm Sci, 16, 37–43.
- Psotová J, Lasovský J, Vicar J. (2003). Metal-chelating properties, electrochemical behavior, scavenging and cytoprotective activities of six natural phenolics. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 147, 147–153.
- Rangasamy M, Ayyasamy B, Raju S, Gummadevelly S, Shaik S. (2008). Formulation and in vitro evaluation of niosome encapsulated acyclovir. J Pharm Res, 1, 163–166.
- Sadick NS, Karcher C, Palmisano L. (2009). Cosmetic dermatology of the aging face. Clin Dermatol, 27, S3–S12
- Samia M, Karl E. (2007). Evaluation of the stability of blends of sunflower and rice bran oil. Eur J Lipid Sci Tech, 109, 531–535.
- Santucci E, Carafa M, Coviello T, Murtas E, Riccieri FM, Alhaique F, Modesti A, Modica A. (1996). Vesicles from polysorbate-20 and cholesterol-a simple preparation and a characterisation. STP Pharm Sci, 6, 29–32.
- Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R. (2007). Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. Faseb J, 21, 2486–2495.
- Schmitt WH. (1992). Skin care product. In: Williams DF, Schmitt WH, eds.; Chemistry and Technology of the Cosmetics and Toiletries Industry. Blackie Academic and Professional: London, 99–101.
- Schreier H, Bouwstra J. (1994). Liposomes and niosomes as topical drug carriers: Dermal and transdermal drug delivery. J Control Release, 30, 1–15.
- Shah KA, Date AA, Joshi MD, Patravale VB. (2007). Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int J Pharm, 345, 163–171.
- Shapiro SS, Saliou C. (2001). Role of vitamins in skin care. Nutrition, 17, 839–844.
- Szoka F Jr, Papahadjopoulos D. (1978). Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci usa, 75, 4194–4198.
- Taniguchi H, Nomura E, Hosoda A. (2003). Useful application of ferulic acid produced from rice bran. Yukigouseikagakukyoukaishi, 61, 310–321.
- Taylor S, Westerhof W, Im S, Lim J. (2006). Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol, 54, S282–S290.
- Touitou E, Godin B, Weiss C. (2000). Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Devel Res, 50, 406–415.
- Trombino S, Serini S, Di Nicuolo F, Celleno L, Andò S, Picci N, Calviello G, Palozza P. (2004). Antioxidant effect of ferulic acid in isolated membranes and intact cells: Synergistic interactions with alpha-tocopherol, beta-carotene, and ascorbic acid. J Agric Food Chem, 52, 2411–2420.
- Uchegbu IF, Vyas SP. (1998). Non-ionic surfactant-based vesicles (niosomes) in drug delivery. Int J Pharm, 172, 33–70.
- van de Sandt J, Roguet R, Cohen C, Esdaile D, Ponec M, Corsini E, Barker C, Fusenig N, Liebsch M, Benford D, de Fraissinette AB, Fartasch M. (1997). The use of human keratinocytes and human skin models for predicting skin irritation. The Report and Recommendations of ECVAM Workshop 38, ATLA, 27, 515–537.
- Verma DD, Verma S, Blume G, Fahr A. (2003). Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm, 258, 141–151.
- Voigt W. (2005). Sulforhodamine B assay and chemosensitivity. Methods Mol Med, 110, 39–48.
- Xu ZM, Godber JS. (2001). Antioxidant activities of major components of c-oryzanol from rice bran using a linoleic acid model. J Am Oil Chem Soc, 78, 645–649.
- Yaar M. (2006). Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Springer Berlin Heidelberg: Germany, 9–19.
- Yin L, Morita A, Tsuji T. (2001). Skin premature aging induced by tobacco smoking: The objective evidence of skin replica analysis. J Dermatol Sci, 27 Suppl 1, S26–S31.
- Yoshioka T, Sternberg B, Florence AT. (1994). Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int J Pharm, 105, 1–6.
- Zhang W, Xu X, Yin C, Zhou L, Zheng S. (2008). MMP2 promoter polymorphism (C-1306T) and risk of recurrence in patients with hepatocellular carcinoma after transplantation. Clin Genet, 73, 273–278.