Abstract
Context: Vitex negundo Linn. (Verbenaceae) seeds are pepper substitute and occasionally used as a condiment for edible purposes. The seeds also find use for analgesia, sedation, rheumatism and joint inflammation in folk medicine.
Objective: To isolate and characterize bioactive constituents from V. negundo seeds.
Materials and methods: The ethanol extract of V. negundo seeds was subjected to repeated column chromatography. Chemical structures were elucidated by detailed spectroscopic analyses on the basis of NMR, IR, and MS data. Several pathogenic fungi isolates were employed to evaluate the antifungal activity of the isolated compound.
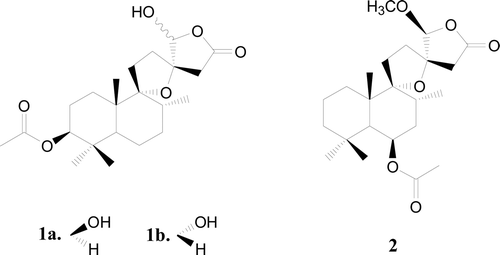
Results: Chemical investigations of the seed extract afforded a new labdane diterpenoid, named negundol (1a + 1b), as an inseparable mixture of two diastereoisomers in a 5:4 ratio. Their structures were identified as (rel 3S, 5S, 8R, 9R, 10S, 13S, 16S)-3-acetoxy-9, 13-epoxy-16-hydroxy-labda-15, 16-olide (1a), and (rel 3S, 5S, 8R, 9R, 10S, 13S, 16R)-3-acetoxy-9, 13-epoxy-16-hydroxy-labda-15, 16-olide (1b). Compound 1 was active as an antifungal agent with MIC80 values in the range of 16–64 μg/mL.
Discussion and conclusion: The presence of compound 1 in V. negundo is of chemotaxonomic significance, since plants under the genus Vitex are chemically characterized with labdane diterpenoids. Compound 1 exhibited potential antifungal activity and may be considered a lead compound for promising antifungal agent.
Keywords::
Introduction
Vitex negundo Linn. (Verbenaceae), a small aromatic plant with a typical five foliolate leave pattern, flourishes abundantly in wastelands and is widely distributed in tropical to temperate regions, being a native of South Asia, China, Japan, Indonesia, East Africa, and South America. V. negundo seeds are pepper substitute and occasionally used as a condiment for edible purpose (CitationKunkel, 1984; CitationFacciola, 1990). The seeds have also been reported to possess anti-inflammatory (CitationChawla et al., 1992; CitationZheng et al., 2009), analgesic (CitationZheng et al., 2010), antioxidant (CitationOno et al., 2004), antiandrogenic activity (CitationBhargava, 1989), antimicrobial and insecticidal activity (CitationYuan et al., 2004; Citation2006). Previous phytochemical studies on the seeds focused on terpenoids (CitationChawla et al., 1992; CitationOno et al., 2004), lignans (CitationZheng et al., 2009; CitationOno et al., 2004) and flavonoids (CitationBhargava, 1989; CitationChawla et al., 1991). In continuation of our studies on this medicinal seed, we report herein the details of isolation, structure elucidation and antifungal activity of a new labdane diterpenoid, negundol (1), the structure of which () was corroborated by detailed spectroscopic analysis (1H, 13C, 2D-NMR, MS, and IR).
Materials and methods
General
Optical rotations were acquired with a Perkin-Elmer 341 polarimeter. UV spectra were run on a Varian Cary Eclipse 300 spectrophotometer. IR spectra were recorded on a Bruker Vector 22 spectrometer with KBr pellets. NMR spectra were recorded on a Bruker Avance 600 NMR spectrometer with TMS as an internal standard. ESIMS were measured on an Agilent LC/MSD Trap XCT mass spectrometer, whereas HRESIMS were measured using a Q-TOF micro mass spectrometer (Waters, USA). Materials for CC were silica gel (100–200 mesh; Huiyou Silical Gel Development Co. Ltd. Yantai, China), silica gel H (10–40 μm; Yantai), Sephadex LH-20 (40–70 μm; Amersham Pharmacia Biotech AB, Uppsala, Sweden), and YMC-GEL ODS-A (50 μm; YMC, Milford, MA). Preparative TLC (0.4–0.5 mm) was conducted on glass plates precoated silica gel GF254 (Yantai).
Plant material
V. negundo seeds were collected during October 2006 from the Wanglang National Nature Reserve, Sichuan province. Prof. Han-Chen Zheng (School of Pharmacy, Second Military Medical University) identified the specimens; a sample (#2006-168) was deposited in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University, Shanghai, China.
Extraction and isolation
The air-dried and powdered seeds (25 kg) were extracted with hot aqueous ethanol (80% v/v) under reflux three times for 2 h each time. After removal of the solvent under reduced pressure, the residue was partitioned sequentially with petroleum ether, CH2Cl2, EtOAc, and n-BuOH, respectively. According to previous reports, the CH2Cl2 extract of V. negundo seeds exhibited significant antimicrobial and insecticidal activity (CitationYuan et al., 2004; Citation2006), which therefore prompted us to isolate antifungal metabolites from it. The CH2Cl2 extract (260 g) was subjected to silica gel chromatography using mixtures of petroleum ether-EtOAc mixtures (50:1, 20:1, 10:1, 5:1, 3:1, 1:1, 0:1 v/v) to afford fractions A-G. Fraction C (6.5 g) was subjected on ODS column chromatography using MeOH–H2O (80%) as eluent to give five fractions (C.1–C.5). Fraction C.4 (580 mg) was rechromatographed on Sephadex LH-20 with MeOH-H2O (80%) followed by preparative TLC (petroleum ether-EtOAc, 5:2) to give 1 (26 mg, 0.01%).
Negundol (1a + 1b): colorless syrup; ![]() +21.0° (c 0.2, MeOH); UV λmax (MeOH) nm: 209; IR νmax 3503, 2969, 1788, 1704, 1384, 1280, 1113, 973 cm−1; 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data (see ); (+)-HRESIMS m/z [M + Na]+ 417.2256 (calcd for C22H34O5Na, 417.2253).
+21.0° (c 0.2, MeOH); UV λmax (MeOH) nm: 209; IR νmax 3503, 2969, 1788, 1704, 1384, 1280, 1113, 973 cm−1; 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data (see ); (+)-HRESIMS m/z [M + Na]+ 417.2256 (calcd for C22H34O5Na, 417.2253).
Table 1. 1H NMR and 13C NMR data of negundol (1a + 1b) in CDCl3
Antifungal activity assay
The antifungal activities of the samples were individually tested against pathogenic fungi including Candida albicans (ATCC 76615), Cryptococcus neoformans (ATCC 32609), Trichophyton rubrum and Aspergillus fumigatus by using serial dilution method (CitationAta et al., 2009). Briefly, SDA (Sabouraud-Dextrose-Agar) slant medium was employed for pathogenic fungal growth. Dilutions of compound 1 were prepared in dimethyl sulfoxide. The tested solutions were serially diluted (two-fold) in 96-well plates, resulting in the concentrations 128, 64, 32, 16, 8, 4, 2, 1, and 0.5 μg/mL. Organisms at a concentration of ∼1–5 × 103 colony forming units (CFU)/mL were then added to each well. Plates were made in triplicate and incubated at 35°C for about 24 h for C. albicans, about 72 h for C. neoformans and about 168 h for T. rubrum and A. fumigatus, and then their turbidity were obtained by measuring optical density at 630 nm. The standard antifungal agent amphotericin B was used as a positive control and experiments were repeated at least three times. Test substance concentrations at which fungi proliferation was reduced by 80% are given as MIC80 values and the values presented are an average of triplicate.
Results and discussion
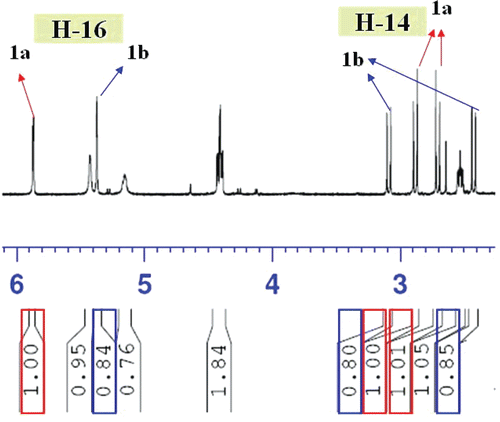
Negundol (1) was obtained as an inseparable mixture of two diastereoisomers in a 5:4 ratio, as determined by integration of well-resolved 1H NMR resonances for H-16 [δH 5.87, s in 1a (1.00); δH 5.37, s in 1b (0.84)] and H-14 [δH 2.88, 2.71, d, J = 17.4 Hz in 1a (1.00); δH 3.09, 2.42, d, J = 16.8 Hz in 1b (0.80)] of each isomer (). Exhaustive efforts to separate this mixture employing column chromatography and HPLC using various stationary and mobile phases were unsuccessful. Therefore, the structure elucidation of 1 was performed on the mixture. The molecular formula of 1 was determined as C22H34O6 (6 degrees of unsaturation) by analysis of its HRESIMS [m/z 417.2256 (M + Na)+] and NMR data (). Absorptions at 3503 and 1788 cm−1, the IR spectrum of 1, along with the 1H, 13C, and HMQC NMR spectroscopic data as discussed below, indicated that 1a and 1b are labdane-type diterpeoids, possessing a hydroxyl group and a γ-lactone group.
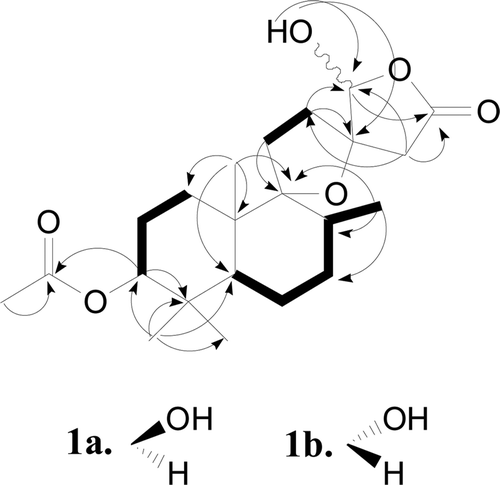
The NMR data for the major and minor isomers were considered separately for structure elucidation purpose. The 1H NMR spectrum of the major isomer (1a) indicated signals due to three tertiary methyl groups (δH 0.86, 0.86, 0.91), one secondary methyl group (δH 0.82, d, J = 6.0 Hz), one acetyl group (δH 2.06), two oxygenated methine protons (δH 4.40, dd, J = 12.0, 4.2 Hz; δH 5.87, s), and two methylene protons (δH 2.88, d, J = 17.4 Hz; δH 2.71, d, J = 17.4 Hz) adjacent to the carbonyl group. The 13C NMR spectrum of 1a gave 22 carbon signals, including one carboxyl carbon (δC 171.7), one acetal carbon (δC 103.2), one oxygenated methine carbon (δC 81.3) and two oxygenated quaternary carbons (δC 94.5, 88.9). These data suggested that 1a was a labdane-type diterpene having one each of a spiro-tetrahydrofuran ring, γ-spiro-lactone ring, hydroxyl group and acetoxy group. Further comparison of the 1H and 13C NMR data of 1a with those of (rel 5S, 6R, 8R, 9R, 10S, 13S, 16S)-6-acetoxy-9, 13-epoxy-16-methoxy-labdan-15, 16-olide (2), which was previously isolated from the fruits of Vitex rotundifolia (CitationOno et al., 2001), led to the assumption that 1a is a 16-O-demethyl regioisomer of 2 with an acetoxyl group at C-3 instead of C-6, which was confirmed by detailed HMBC and 1H–1H COSY spectroscopic analysis (). In the NOESY spectra of 1a, correlations were observed between H-8/H-20, H-19/H-20, H-3/H-18, H-5/H-3, H-14/H-17 and H-1/H-16, indicating a β-orientation for H-8, H-19, and H-20 and an α- orientation for H-3, H-5, H-17, and H-18. The β-configuration for the hydroxyl group at C-16 was confirmed from the low-field resonance of Ha-12 (δH 2.53), which was deshielded by the hydroxyl group at C-16 (CitationKusumi et al., 1986; CitationOno et al., 1999). Compound 1a was therefore characterized as (rel 3S, 5S, 8R, 9R, 10S, 13S, 16S)-3-acetoxy-9, 13-epoxy-16-hydroxy-labda-15, 16-olide.
The 1H and 13C NMR spectra of 1b together with DEPT and 1H-13C COSY analysis indicated the presence of general structural features in common with 1a of the same molecular formula C22H34O6. The major differences were the signals corresponding to an acetal proton (δH 5.37, s in 1b; δH 5.87, s in 1a) and two methylene protons adjacent to the carbonyl group (δH 3.09, 2.42, d, J = 16.8 Hz in 1b; δH 2.88, 2.71, d, J = 17.4 Hz in 1a). In addition, the configuration of the hydroxyl group at C-16 was determined to be α on the basis of NOESY spectra, which was further evidenced by the non-deshielded signal of H-12 (δH 2.12, Ha; 1.99, Hb) compared to that of 1a (δH 2.53, Ha; 1.83, Hb) (CitationOno et al., 1999). Key NOSEY correlations were detected between H-16/H-12, H-8/H-20, H-18/H-3, H-3/H-5, H-5/H-18, and H-14/H-17, indicating a β-orientation for H-8, H-16, H-19, and H-20 and an α-orientation for H-3, H-5, H-17, and H-18. Compound 1b was therefore recognized as an isomer at C-16 of 1a and defined as (rel 3S, 5S, 8R, 9R, 10S, 13S, 16R)-3-acetoxy-9, 13-epoxy-16-hydroxy-labda-15, 16-olide.
The presence of compound 1 in V. negundo is of chemotaxonomic significance since plants under the genus Vitex are chemically characterized with labdane diterpenoids (CitationLi et al., 2005). Compound 1 was tested for its antifungal activity against several pathogenic fungi isolates and showed good results (), especially for C. albicans (MIC80 64 μg/mL), C. neoformans (MIC80 16 μg/mL), and T. rubrum (MIC80 32 μg/mL). Further studies are needed to investigate this compound, which may be considered as a lead compound for promising antifungal agent.
Table 2. Antifungal activity of negundol [MIC80 (μg/mL)].
Declaration of interest
The authors are grateful to the financial support from the National Natural Science Fund of China (No. 81102773). We also thank professor Yang Genjin, Center of Analysis and Measurement, Second Military Medical University, for the NMR analysis and technical assistance.
Supplementary data
Spectroscopic data of compound 1, including 1H-, 13C-NMR and 2D NMR, is provided as supplementary information.
Supplementary Material
Download PDF (270.6 KB)References
- Ata A, Gale EM, Samarasekera R. (2009). Bioactive chemical constituents of Caesalpinia bonduc (Fabaceae). Phytochem Lett, 2, 106–109.
- Bhargava SK. (1989). Antiandrogenic effects of a flavonoid-rich fraction of Vitex negundo seeds: A histological and biochemical study in dogs. J Ethnopharmacol, 27, 327–339.
- Chawla AS, Sharma AK, Handa S. (1991). Chemical investigation and anti-inflammatory activity of Vitex negundo seeds: Part I. Indian J Chem, 30B, 773–776.
- Chawla AS, Sharma AK, Handa SS, Dhar KL. (1992). Chemical investigation and anti-inflammatory activity of Vitex negundo seeds. J Nat Prod, 55, 163–167.
- Facciola S. (1990). Cornucopia-A Source Book of Edible Plants. Vista Calif.:kampong Publications.
- Kunkel G. (1984). Plants for Human Consumption. Koenigstein. (Germany): Koeltz Scientific Books.
- Kusumi T, Muanza-Nkongolo D, Goya M, Ishitsuka M, Iwashita T, Kakisawa H. (1986). Structures of crenulacetals A, B, C, and D. The new diterpenoids from the brown algae of Dictyotaceae. J Org Chem, 51, 384–387.
- Li CZ, Su YF, Ji XJ. (2005). Advances in studies on chemical constituents from plants of Vitex L. and their bioactivities. Chin Tradit Herbal Drugs, 36, 930–938.
- Ono M, Nishida Y, Masuoka C, Li JC, Okawa M, Ikeda T, Nohara T. (2004). Lignan derivatives and a norditerpene from the seeds of Vitex negundo. J Nat Prod, 67, 2073–2075.
- Ono M, Yamamoto M, Masuoka C, Ito Y, Yamashita M, Nohara T. (1999). Diterpenes from the fruits of Vitex rotundifolia. J Nat Prod, 62, 1532–1537.
- Ono M, Yamamoto M, Yanaka T, Ito Y, Nohara T. (2001). Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem Pharm Bull, 49, 82–86.
- Yuan L, Xue M, Xin J, Li CH. (2004). Toxicity of Vitex negundo extracts to several insect pests. Pesticides, 43, 70–72.
- Yuan L, Xue M, Liu Y, Wang HS. (2006). Toxicity and oviposition-deterrence of Vitex negundo extracts to Plutella xylostella. Chin J Appl Ecol, 17, 695–698.
- Zheng CJ, Huang BK, Han T, Zhang QY, Zhang H, Rahman K, Qin LP. (2009). Nitric oxide scavenging lignans from Vitex negundo seeds. J Nat Prod, 72, 1627–1630.
- Zheng CJ, Huang BK, Han T, Zhang QY, Zhang H, Rahman K, Qin LP. (2010). Antinociceptive activities of the liposoluble fraction from Vitex negundo seeds. Pharm Biol, 48, 651–658.