Abstract
Context: Enicosanthellum pulchrum (King) Heusden (Annonaceae) is a coniferous tree that is confined to mountain forests. The chemical constituents of this species have been studied previously; however, its biological activity has never been investigated before and is reported here for the first time.
Objective: The extracts, fractions and compounds from the roots of E. pulchrum were investigated for their inhibitory effects on platelet-activating factor (PAF) receptor binding to rabbit platelets using 3H-PAF as a ligand.
Materials and methods: The PAF receptor binding inhibitory effect using rabbit platelets was determined in vitro by measuring the difference between total amount of bound 3H-PAF in the presence and the absence of excess unlabelled PAF. The compounds were isolated by bioassay-guided fractionation and their structures were elucidated by spectroscopic techniques.
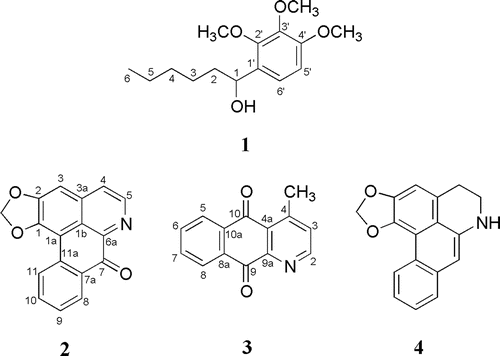
Results and discussion: Among the extracts tested, the ethyl acetate extract was the most active with 85.6% inhibition, while hexane and methanol extracts showed 40.2 and 42.5% inhibition, respectively. Fractionation of the ethyl acetate extract using vacuum liquid chromatography (VLC) yielded six fractions AEA(I-–VI). Chromatography fraction AEA(VI) yielded a new compound, 1-(2′,3′,4′-trimethoxyphenyl)hexan-1-ol, while fraction AEA(III) afforded three compounds, namely liriodenine, cleistopholine and dehydroanonaine. 1-(2′,3′,4′-Trimethoxyphenyl)hexan-1-ol, cleistopholine and dehydroanonaine showed relatively strong inhibition with IC50 values of 26.6, 50.2 and 45.4 µM, respectively.
Conclusion: The results suggest that these compounds could be responsible for the PAF antagonistic activity of the ethyl acetate extract of this plant.
Introduction
Enicosanthellum pulchrum (King) Heusden (Annonaceae) was first discovered at the Coteau area on the border of Thailand and Malaysia. It is a coniferous tree that can grow up to about 3–5 m tall. E. pulchrum produces green flowers with gentle smell blossom on the top. The propagation is quite difficult either by seeding or grafting. This tree is confined to mountain forests at an altitude of 1200–1500 m (CitationBurkill, 1966; CitationDavid, 1989). Several isoquinoline alkaloids have been isolated from this species such as (−)-asimilobine, (−)-anonaine, (−)-norliridine, liriodenine and (−)-scoulerine (CitationLavault et al., 1990). However, to our knowledge, no biological activity of this species has been reported so far.
Platelet-activating factor (PAF), produced by a variety of cells such as macrophages, polymorphonuclear neutrophils (PMN), basophils and platelets, may participate in a number of physiological responses, which include aggregation (CitationKuijpers et al., 1991), chemotaxis (CitationVerghese et al., 1987), granule secretion, oxygen radical generation from leukocytes and the adherence of leukocytes to the endothelium (CitationO’Flaherty et al., 1981; CitationShaw et al., 1981; CitationCamussi et al., 1988; CitationZimmerman et al., 1992). PAF is also involved in several pathophysiology of various inflammatory diseases, including asthma, systemic lupus erythematosus, rheumatoid arthritis and Crohn’s disease (CitationImarzumi et al., 1995; CitationZimmerman et al., 2002). The development of potent and selective PAF receptor antagonists has been particularly valuable for studies on the pathophysiology of PAF (CitationFeuerstein et al., 1997). A number of natural PAF antagonists have been identified including ginkgolides from Ginkgo biloba L. (Ginkgoaceae) (CitationBraquet et al., 1985), kadsurenone from Piper futokadsurae Sieb.et Zucc (Piperaceae) (CitationShen et al., 1985) and gliotoxin related products from fungi and actinomycetes (Okomoto et al., 1986a,b,c,d,e). Recently, we isolated a potent PAF antagonist, 5-(Z-heptadec-4′-enyl)resorcinol from Ardisia elliptica Thunb. (Myrsinaceae) (CitationJalil et al., 2004).
In our preliminary screening, the methanol extract of the roots of E. pulchrum showed a strong inhibitory effect on PAF receptor binding in vitro. This paper reports the bioassay-guided isolation and structure elucidation of a new compound, 1-(2′,3′,4′-trimethoxyphenyl)hexan-1-ol, from E. pulchrum and its effect on the binding of 3H-PAF to washed rabbit platelets.
Materials and methods
General
Centrifuge: Hettich. UV spectrophotometer: Shimadzu. Cell harvester: Skatron Co. Liquid Scintillation counter: Packard. Vacuum liquid chromatography (VLC): silica gel H (10–40 µm). Column chromatography (CC): silica gel 60 (230–400 mesh), Sephadex LH20. Thin-layer chromatography (TLC) and prep. TLC: precoated silica gel 60 F254. Hexane, ethyl acetate, chloroform (CHCl3) and methanol (MeOH) used were of analytical grades. Radiolabeled PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, 125 Ci/mmol) was purchased from Amersham (UK). Unlabeled PAF and cedrol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Bovine serum albumin (BSA) was purchased from Boehringer Mannheim Co. (Germany). Other chemicals were purchased from Merck Co. (Germany) and BDH Laboratory Supplies (UK).
Plant material
The roots of E. pulchrum were collected in June, 2007 from Cameron Highlands mountain forest, Pahang, Malaysia. The plant was identified by Prof. Dr. Kamarudin Mat Salleh and a voucher specimen (SM 769) was deposited at the Herbarium of the Faculty of Science and Technology, Universiti Kebangsaan Malaysia (UKM).
Bioassay-guided isolation
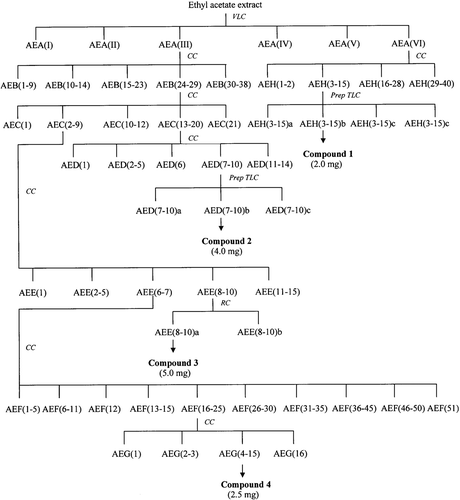
The roots (515 g) were air-dried and ground to mesh size 40–60 before extracted with hexane, ethyl acetate and methanol successively using Soxhlet apparatus. Evaporation of the solvents using rotary evaporator gave crude hexane, ethyl acetate and methanol extracts, respectively. Each extract was tested for PAF receptor binding antagonistic activity at a concentration of 18.2 µg mL−1. The isolation scheme of the active compounds is shown in . The ethyl acetate extract (9.3 g) which showed the highest inhibition was subjected to VLC over silica gel H eluted with hexane–CHCl3 to CHCl3–MeOH gradient. Fractions of 250 mL were collected and combined into six fractions AEA (I–VI), according to their TLC profiles. All fractions were also tested for PAF receptor binding assay. Among them, fraction AEA(VI) and AEA(III) were selected for further isolation work. Fraction AEA(VI) was chromatographed on silica gel 60 with gradient elution of hexane–CHCl3–MeOH to yield 40 fractions, AEH(1–40). Fraction AEH(3–15) were combined according to their TLC profiles and was further purified by preparative TLC to afford compound 1 (2.0 mg). Fraction AEA(III) was subjected to silica gel 60 column chromatography to give 21 fractions, AEC(1–21). Combined fractions AEC(13–20) was further chromatographed on silica gel 60 eluted with hexane–CHCl3 and CHCl3–MeOH in increasing polarity to yield 14 fractions, AED(1–14). Fraction AED(7–10) was subjected to preparative TLC to afford compound 2 (4.0 mg). Fraction AEC(2–9) was rechromatographed on silica gel 60 eluted with hexane–CHCl3–MeOH to give 15 fractions, AEE(1–15). Fraction AEE(8–10) was separated by radial chromatography to obtain compound 3 (5.0 mg). Repeated column chromatography of fraction AEE(6–7) afforded compound 4 (2.5 mg). Structural elucidation of the compounds was performed by spectroscopic methods and the PAF antagonistic activity of each compound was determined.
Preparation of rabbit platelets
Six volume of blood was collected from each New Zealand White rabbit, medial ear artery directly into one volume of ACD solution (0.15 M Sodium citrate, 0.075 M citrate acid, pH 5.2). The procedure was under approval of the Animal Ethics Committee of UKM (approval no. FSKB/2007/Juriyati/10-July/192). The blood was centrifuged at 270 × g for 10 min and the top platelet-rich plasma (PRP) was removed carefully. The PRP was centrifuged at 500 × g for 15 min. Then, the platelet pellets were washed two times in buffer A (20% ACD solution, 60% K2HPO4 buffer, 20% sodium citrate, pH 6.8) and centrifuged at 500 × g (15 min), followed by washing in buffer B (50 mL K2HPO4, 0.1 g BSA, pH 7.0) at 50 × g (10 min). The top whitish layer was removed and centrifuged at 500 × g (15 min) to obtain platelets. The final concentration was adjusted to 3 × 108 platelets mL−1.
PAF receptor binding inhibitory assay (PAF assay)
The assay was carried out according to the method described previously (CitationJantan et al., 2001b). Cedrol, a known PAF antagonist was used as a positive control (CitationYang et al., 1995), while 0.2% dimethyl sulfoxide (DMSO) in normal saline was used as a negative control. Each extract (1 mg) was dissolved in DMSO and ethanol (1:1). The stock solution was then diluted by a serial dilution with normal saline to give final concentration of 18.2, 9.1, 4.5, 1.8 or 0.9 µg mL−1. The reaction mixtures consisted of 200 µL of washed rabbit platelet suspension, 25 µL of 3H-PAF (2.0 nM) with or without unlabeled PAF (2.0 M), and 25 µL of sample or control were incubated at room temperature for 1 h. The free and bound ligands were separated by a filtration technique using Whatman GF/C glass fiber filters. The radioactivity was measured by a scintillation counter. The difference between total radioactivities of bound 3H-PAF in the presence and the absence of excess unlabelled PAF is defined as specific binding of 3H-PAF.
Statistical analysis
The percentage inhibition values are reported as the means ± SEM of three separate experiments. Data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s test to determine statistical significance. P values less than 0.05 (p < 0.05) were considered as statistically significant. The IC50 values were determined by using Probit analysis with 95% confidence intervals.
Results and discussion
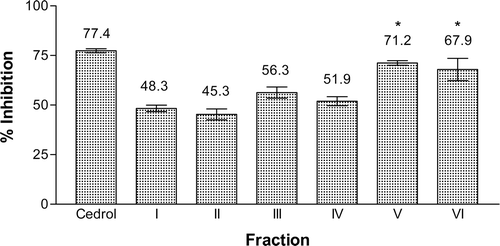
The hexane, ethyl acetate, and methanol extracts from the roots of E. pulchrum were investigated for their PAF receptor binding inhibitory activity on rabbit platelets at the concentration of 18.2 μg mL−1. Among them, ethyl acetate extract showed the strongest activity with 85.6% inhibition, while the methanol and hexane extracts gave 42.5 and 40.2% inhibition, respectively (). The ethyl acetate extract was then chromatographed on silica gel H using a VLC technique to yield six fractions, AEA(I) to AEA(VI). Fraction AEA(V) possessed the highest inhibition on PAF receptor binding of 71.2%, followed by fraction AEA(VI) with 67.9% inhibition. Other fractions displayed moderate inhibitory activity between 40 and 60% (). Isolation work on fraction AEA(V) had not led to any pure compound. Therefore, fraction AEA(VI) and fraction AEA(III) were selected for detailed chemical investigation. Extensive chromatographic separation of these fractions had led to the isolation of compounds 1 from fraction AEA(VI) and compounds 2, 3, and 4 from fraction AEA(III) ().
Figure 2. Inhibitory effect of the hexane, ethyl acetate and methanol extracts of the roots of E. pulchrum on the PAF receptor binding to rabbit platelets at 18.2 μg mL−1. F(3, 4) = 24.202, p < 0.05. *No significant difference as compared with cedrol.
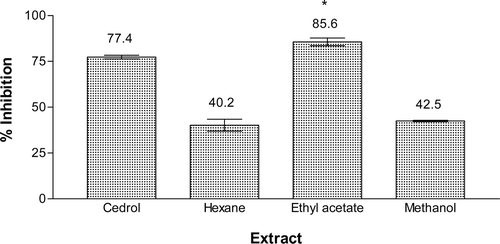
Figure 3. Inhibitory effect of the VLC fractions of the ethyl acetate extract from E. pulchrum on the PAF receptor binding to rabbit platelets at 18.2 μg mL−1. F(6, 7) = 19.505, p < 0.05. *No significant difference as compared with cedrol.
The structure of compound 1 was elucidated by using 1H NMR and 13C NMR techniques, in combination with EI mass spectrometry. The known alkaloids 2, 3 and 4 were identified as liriodenine (CitationGuinaudeau et al., 1983; CitationWijeratne et al., 1996), cleistopholine (CitationWaterman & Muhammad, 1985; CitationCave et al., 1987) and dehydroanonaine (CitationLenz & Koszyk, 1984), respectively, by comparison of their physicochemical and spectroscopic data with those reported in the literature. Compound 3 and 4 are reported for the first time from this genus. The 1H NMR spectrum of compound 1 showed a 9H singlet at δ 3.68 attributable to methoxy protons. A signal at δ 4.12 was assigned to hydroxyl proton bonded to the alkyl chain. A doublet (2H, J = 8.8 Hz) was observed at lower magnetic field (δ 6.85) indicating the presence of ortho-coupled protons between H-5′ with H-6′. A part of that, the presence of methyl protons as well as methylene protons can be observed in the region δ 0.81–0.90 (3H) and 1.21–1.70 (8H), respectively. These unsaturated alkyl substituent appeared as multiplet at higher magnetic field (). The 13C NMR spectrum showed the presence of carbons corresponding to compound 1. The presence of methyl and methoxy carbons was observed at δ 14.3 and δ 61.8, respectively. Four signals were detected at δ 22.9, 24.9, 29.9 and 32.2, attributable to methylene carbons. The signals for oxygenated aromatic carbons were observed at 139.0 (C-3′), 147.2 (C-4′), and 150.3 (C-2′), while signals for non-oxygenated aromatic carbons were recorded at δ 113.9 (C-5′), 114.0 (C-1′), and 126.9 (C-6′) (). The structure elucidation of compound 1 was also supported by EI mass spectral data. The mass spectrum showed a molecular ion [M]+ at m/z 268 corresponding to C15H24O4. The spectrum further showed the presence of fragment ion peak at m/z 251 which was due to the lost of a hydroxyl radical. The peak at m/z 197 was formed by the lost of a pentyl radical via α-cleavage at C-1/C-2. The lost of a propyl radical via C-3/C-4 cleavage gave fragment ion peak at m/z 225. Based on these spectroscopic data, compound 1 was characterized as 1-(2′,3′,4′-trimethoxyphenyl)hexan-1-ol. Compound 1 is a new compound.
Table 1. 1H-NMR and 13C-NMR spectral data of compound 1, 2, 3 and 4.
PAF receptor binding inhibitory effects of compounds 1–4 were evaluated at 18.2 μg mL−1. The results showed that compound 1 displayed strong inhibitory activity with 75% inhibition, while compound 3 and 4 gave comparable percentage inhibition of 65.0 and 62.6%, respectively. Compound 2 exhibited the lowest inhibitory activity of 40%. Compounds, 1, 3, and 4 were further investigated at various concentrations of 18.2, 9.1, 4.5, 1.8, and 0.9 μg mL−1. The compounds exhibited dose-dependent responses, that is, as the concentration of the compound increased, the percentage inhibition increased. The IC50 values of compounds 1, 3, and 4 were 26.6, 50.2, and 45.4 µM, respectively (). The IC50 values of these compounds were higher than that of cedrol (13.1 µM), but comparable to the reported value of ginkgolide J (54 µM) from G. biloba (CitationBraquet et al., 1985). The weak activity of the compound 2, and relatively strong activity of compound 4 were in agreement with the results obtained from a previous study on aporphine alkaloids (CitationJantan et al., 2001a). Structure–activity analysis of aporphine alkaloids had revealed that the coplanar conformation of oxoaporphines (i.e., liriodenine) was not preferable in binding to the receptor while the presence of a methylenedioxy group at C-1 and C-2 of compound 4 (i.e., dehydroanonaine) resulted in a slight increase in the binding affinity.
Table 2. Inhibitory effects of compounds 1, 3, and 4 on the PAF receptor binding to rabbit platelets at various concentrations.
Conclusion
The results indicate that 1-(2′,3′,4′-trimethoxyphenyl)hexan-1-ol (1), cleistopholine (3), and dehydroanonaine (4) isolated from the roots of E. pulchrum were relatively strong PAF receptor binding inhibitors. Further studies will be carried out on the PAF antagonistic activity of derivatives of 1-(2′,3′,4′-trimethoxyphenyl)hexan-1-ol (1) to identify lead molecules for the development of therapeutic agents in a variety of inflammation, respiratory, immunological, and cardiovascular disorders.
Declaration of interest
We wish to thank the Ministry of Higher Education, Malaysia for financial support under research grant UKM-NN-03-FRGS0001-2006.
References
- Braquet P, Spinnewyn B, Braquet M, Bourgain RH, Taylor JE, Etienne A, Drieu K (1985): BN 52021 and related compounds: A new series of highly specific PAF-acether receptor antagonist isolated from Ginkgo biloba. Blood Vess,16, 559–572.
- Burkill IH.(1966). A Dictionary of Economic Products of the Malay Peninsular. Kuala Lumpur, Malaysia: Ministry of Agriculture & Cooperatives.
- Camussi G, Tetta C, Bussolino F, Baglioni C. (1988). Synthesis and release of platelet-activating factor is inhibited by plasma alpha 1-proteinase inhibitor or alpha 1-antichymotrypsin and is stimulated by proteinases. J Exp Med, 168, 1293–1306.
- Cave A, Cassels BK, Leboeuf M. (1987). Kinabaline and the aporphinoid biogenesis of azaanthracene and azafluorene alkaloid. Phytochemistry, 26, 537–541.
- David MJ. (1989). Revision of Disepalum (Annonaceae). Brittonia, 41, 359–378.
- Feuerstein G, Rabinovici R, Leor J, Winkler JD, Vonhof S. (1997). Platelet-activating factor and cardiac diseases: therapeutic potential for PAF inhibitors. J Lipid Mediat Cell Signal, 15, 255–284.
- Guinaudeau H, Leboeuf M, Cave A. (1983). Aporhinoid alkaloids III. J Nat Prod, 46, 761–835.
- Imaizumi TA, Stafforini DM, Yamada Y, McIntyre TM, Prescott SM, Zimmerman GA. (1995). Platelet-activating factor: A mediator for clinicians. J Intern Med, 238, 5–20.
- Jalil J, Jantan I, Shaari K, Abdul Rafi IA. (2004). Bioassay-guided isolation of a potent platelet-activating factor antagonist alkenyiresorcinol from Ardisia elliptica. Pharm Biol, 42, 457–461.
- Jantan I, Rafi IA, Jalil J. (2001a). Inhibition of platelet-activating factor receptor binding by aporphine and phenanthrenoid alkaloids from Aromadendron elegans. Planta Med, 67, 466–467.
- Jantan I, Jalil J, Abdul Warif NM. (2001b). Platelet-activating factor (PAF) antagonistic activities of compounds isolated from Guttiferae species. Pharm Biol, 39, 243–246.
- Kuijpers TW, Hakkert BC, Hoogerwerf M, Leeuwenberg JF, Roos D. (1991). Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol, 147, 1369–1376.
- Lavault M, Guinaudeau H, Bruneton J, Sevenet T, Hadi HA. (1990). (-)-Thaipetaline, a tetrahydroprotoberberine from a Malayan Annonaceae. Phytochemistry, 29, 3845–3847.
- Lenz GR, Koszyk FJ. (1984). A biomimetic synthesis of the novel 6, 7-oxazine ring-fused dehydroaporphine alkaloid duguenaine. J Chem Soc Perkin Trans, 1, 1273–1277.
- O’Flaherty JT, Wykle RL, Miller CH, Lewis JC, Waite M, Bass DA, McCall CE, DeChatelet LR. (1981). 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: A novel class of neutrophil stimulants. Am J Pathol, 103, 70–78.
- Okamoto M, Yoshida K, Nishikawa M, Ando T, Iwami M, Kohsaka M, Aoki H. (1986a). FR-900452, a specific antagonist of platelet activating factor (PAF) produced by Streptomyces phaeofaciens. I. Taxonomy, fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot, 39, 198–204.
- Okamoto M, Yoshida K, Nishikawa M, Kohsaka M, Aoki H. (1986b). Platelet activating factor (PAF) involvement in endotoxin-induced thrombocytopenia in rabbits: Studies with FR-900452, a specific inhibitor of PAF. Thromb Res, 42, 661–671.
- Okamoto M, Yoshida K, Uchida I, Nishikawa M, Kohsaka M, Aoki H. (1986c). Studies of platelet activating factor (PAF) antagonists from microbial products. I. Bisdethiobis(methylthio)gliotoxin and its derivatives. Chem Pharm Bull, 34, 340–344.
- Okamoto M, Yoshida K, Uchida I, Kohsaka M, Aoki H. (1986). Studies of platelet activating factor (PAF) antagonists from microbial products. II. Pharmacological studies of FR-49175 in animal models. Chem Pharm Bull, 34, 345–348.
- Okamoto M, Yoshida K, Nishikawa M, Hayashi K, Uchida I, Kohsaka M, Aoki H. (1986e). Studies of platelet activating factor (PAF) antagonists from microbial products. III. Pharmacological studies of FR-900452 in animal models. Chem Pharm Bull, 34, 3005–3010.
- Shaw JO, Pinckard RN, Ferrigni KS, McManus LM, Hanahan DJ. (1981). Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol, 127, 1250–1255.
- Shen TY, Hwang SB, Chang MN, Doebber TW, Lam MH, Wu MS, Wang X, Han GQ, Li RZ. (1985). Characterization of a platelet-activating factor receptor antagonist isolated from haifenteng (Piper futokadsura): specific inhibition of in vitro and in vivo platelet-activating factor-induced effects. Proc Natl Acad Sci USA, 82, 672–676.
- Verghese MW, Charles L, Jakoi L, Dillon SB, Snyderman R. (1987). Role of a guanine nucleotide regulatory protein in the activation of phospholipase C by different chemoattractants. J Immunol, 138, 4374–4380.
- Waterman PG, Muhammad I. (1985). Sesquiterpenes and alkaloids from Cleistopholis patens. Phytochemistry, 24, 523–527.
- Wijeratne EMK, Hatanaka Y, Kikuchi T, Tezuka Y, Gunatilaka AAL. (1996). A dioxo-aporphine and other alkaloids of two annonaceous plants of Sri Lanka. Phytochemistry, 42, 1703–1706.
- Yang HO, Suh DY, Han BH. (1995). Isolation and characterization of platelet-activating factor receptor binding antagonists from Biota orientalis. Planta Med, 61, 37–40.
- Zimmerman GA, Prescott SM, McIntyre TM. (1992). Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today, 13, 93–100.
- Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. (2002). The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med, 30, S294–S301.