Abstract
Context: Plants are known to play a crucial role in African traditional medicine for the treatment of infection diseases.
Objectives: To investigate the claimed antimicrobial properties of plants traditionally used in African countries, providing scientific validation for their use.
Materials and methods: Eighty-three polar and non-polar extracts from 22 medicinal plants were screened for their antibacterial activity against Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) and Mycobacterium smegmatis using the broth microdilution method.
Results and Discussion: In vitro antibacterial activity against one or more tested bacteria was shown by 83% of the extracts. The highest activity was obtained with the methanol extracts of the aerial parts of Acacia karroo Hayne (Fabaceae) and Anacardium occidentale L. (Anacardiaceae) and the roots of Bridelia cathartica G. Bertol (Euphorbiaceae), against S. aureus (minimum inhibitory concentration (MIC) = 7.5 µg/mL). The same MIC values were exhibited against E. faecalis by the methanol extract of A. occidentale, the dichloromethane and methanol extracts of B. cathartica and the ethyl acetate extract of Momordica balsamina l. (Curcubitaceae) leaves. Gram-negative bacteria were less sensitive; the growth of P. aeruginosa was significantly inhibited (MIC = 31 µg/mL) by the n-hexane and methanol extracts of Gomphocarpus fruticosus (l.) Ait. (Asclepiadaceae) fruits and by the dichloromethane extract of Trichilia emetica Vahl (Meliaceae) seeds. Most of the active extracts were rich in fenols/flavonoids.
Conclusion: This study supports the use of most of the studied plants in traditional medicine, for the treatment of infectious diseases. Some of them are worthy of further investigation.
Introduction
Plants have been used as medicines for thousands of years, playing a crucial role in drug discovery and development. The importance of natural products is particularly evident in the area of infectious diseases, where over 60% of antimicrobial agents are of natural origin (CitationNewman et al., 2003; CitationNewman & Cragg, 2007). The large number of natural product-derived antibiotics may be due, in part, to the evolution of secondary metabolites as a defense strategy against the environmental stresses. This feature, coupled with their huge structural diversity and great biodiversity, makes natural products an outstanding and challenging subject to drug discovery and development (CitationRollinger et al., 2006). It is estimated that infectious diseases are directly responsible for 26% of annual deaths worldwide (CitationMorens et al., 2008). The impact of bacterial diseases is particularly significant in Africa, where drugs are limited and the emergence of drug resistance has made many of the currently available drugs ineffective. Bacterial resistance to antibiotics is a serious and growing problem of public health representing a global threat. In fact, infections diseases are becoming more difficult to treat due to multi-drug resistant bacteria especially Gram-positive pathogens. There is, therefore, an urgent need for new antibiotics (CitationGootz, 2010; CitationMarquez, 2005).
Nowadays, 80% of people living in Sub-Saharan Africa are almost completely dependent on folk medical practices for their primary healthcare needs, and higher plants are known to play a crucial role in traditional medicine (CitationWHO, 2002). This is the case of Mozambique, where there is a strong dependence on plants as medicines, particularly in the rural areas (CitationBandeira et al., 2001). As well as in other African countries, medicinal plants are sold in Mozambican markets or prescribed by traditional healers. Therefore, it would be important to study their safety and effectiveness.
This study investigated the claimed antimicrobial properties of a number of species traditionally used in African countries, providing scientific validation for their use. Thus, polar and non-polar extracts from different plant species were screened for antibacterial activity.
Material and methods
Plant materials
The information on the plant material is summarized in and . These plant species are commonly found in Mozambique flora being, however, some of them are also cultivated in Portuguese botanical gardens. Therefore, the plant material was collected in both countries, Mozambique and Portugal. The species collected in Mozambique (2006; see ) were identified by the botanist Dr. Silva Mulhovo and the voucher specimens have been deposited at the herbarium (LMA) of Instituto de Investigação Agronomica of Mozambique. The remaining species (collected in 2005 and 2006; see ) were identified by Dr. Teresa Vasconcelos of the Instituto Superior de Agronomia, University of Lisbon and the voucher specimens have been deposited at the herbarium of the Instituto Superior de Agronomia, Lisbon, Portugal. Voucher specimen numbers are given in .
Table 1. Ethnobotanical data of the plants studied.
Table 2. Plant data and MIC values (µg/mL) of plant extracts.
Preparation of plant extracts
Different plant parts (aerial parts, bark, roots, leaves, twigs, fruits) or whole plants were shade dried, at room temperature, after collection (). Crude plant extracts were prepared according to the following standard procedure (CitationCos et al., 2006a). Briefly, 15–50 g of dried powdered plant material was extracted sequentially with 150–500 mL of hexane, dichloromethane, ethyl acetate and methanol for 48 h, at room temperature. After filtration, the extracts were concentrated to dryness, under reduced pressure at 40–45°C, and stored at 4°C until use. The yield of the dried extracts (as w/w percentage of the starting dried material) is available in . For some plants [Anacardium occidentale L. (Anacardiaceae), Gomphocarpus fruticosus (L.) Ait. (Asclepiadaceae), Leonotis leonurus (L.) R.Br. (Lamiaceae), Litogyne gariepina (DC.) Anderb (Asteraceae), Salvadora australis Schweick, Salvadora persica L. (Salvadoraceae), Tecomaria capensis (Thunb.) Spach (Bignoneaceae) and Trichilia emetica Vahl (Meliaceae); leaves] only the n-hexane and methanol extracts were prepared.
Phytochemical screening
In order to carry out a preliminary evaluation of the major phytochemicals of each active extract, qualitative screenings were carried out by using standard protocols. Briefly, thin layer chromatography (TLC), in adequate developing solvent systems, was performed. The developed chromatograms were sprayed with specific reagents, namely natural products polyethylene glycol reagent (NP/PEG) for flavonoids, Draggendorff reagent for alkaloids, fast blue salt (FBS) reagent for phenolics, and anisaldehyde-sulfuric acid (AS) for terpenes (CitationWagner & Blader, 1996). Results were displayed qualitatively in a range between (−), absence and (+++) strongly positive (CitationWagner & Blader, 1996; ).
Table 3. Phytochemical screening of the antibacterial active extracts.
Microorganisms
The antibacterial assays were carried out by using Gram-positive strains Staphylococcus aureus ATCC 6538 and Enterococcus faecalis ATCC 51299, and Gram-negative Escherichia coli ATCC 25922, Pseudomonas aeruginosa CIP 9027 and Klebsiella pneumoniae ATCC 9997 and Mycobacterium smegmatis ATCC 607.
Antibacterial testing
Plant extracts were dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 2 mg/mL. Concentrations of plant extracts were used in a range of 1–3.5 µg/mL.
The antibacterial activity evaluation was performed by determination of the minimum inhibitory concentration (MIC). MICs were determined by the microplate broth microdilution method according to Clinical and Laboratory Standards Institute recommendations (CitationCLSI, 2008). The MIC, defined as the lowest concentration of compound at which no growth (turbidity) was present, was determined after 24 h of incubation at 37°C. The bacterial growth was evaluated by both an Absorbance Microplate Reader (ELX808TM – BioteK, USA) at 630 nm and by direct observation of wells turbidity. Samples with a MIC value ≤125 µg/mL were considered to have antibacterial activity. The MIC for each extract was determined at least three separate times.
Results and discussion
A total of 83 extracts, from 22 different plant species belonging to several families, were screened for their potential antibacterial properties. The plants were selected mainly according to their use in Mozambique traditional medicine to treat infectious diseases (). The extracts were prepared by sequentially extracting the plant material with n-hexane, dichloromethane, ethyl acetate and methanol. For some plants only n-hexane and methanol extracts were prepared.
The selected bacteria panel includes drug-sensitive reference strains of Gram-positive and Gram-negative bacteria, representing common pathogenic species of different classes commonly used for primary screening, as proposed by Cos et al. (2006a). M. smegmatis, a fast-growing avirulent, saprophytic mycobacterium, was also selected as a simple model for testing antimycobacterial activity.
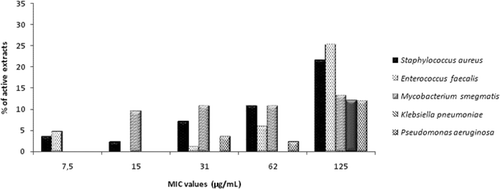
The results are summarized in and . It should be noted that MIC endpoint criteria are not consensual. In fact, some authors consider values of 250 µg/mL as strong antibacterial activity (CitationAligiannis et al., 2001; CitationTalib & Mahasneh, 2010), whereas others use a stricter endpoint criteria (CitationCos et al., 2006a, Citation2006b), in which crude extracts with MIC values less than 100 µg/mL can be considered as active and are worthy for further studies. Taking into account the different criteria, extracts with a MIC value ≤125 µg/mL were considered to be active in this study. As can be observed in , all the tested plants showed antibacterial activity; 83% of the extracts were active against one or more bacteria. Cassia abbreviata Oliv. (Fabaceae) and Bridelia cathartica G. Bertol. (Euphorbiaceae), methanol extracts showed the broadest spectra of activity, inhibiting the growing of four out of six tested bacteria strains ().
The highest numbers of active extracts were found against the Gram-positive bacteria S. aureus and M. smegmatis (47 and 45%, respectively) as displayed in . Regarding E. faecalis, 37% of the extracts were found to be active. For Gram-negative bacteria, similar results were obtained for P. aeruginosa (36%). Conversely, only 12% of the extracts inhibited the growth of K. pneumoniae and E. coli was resistant to all plant extracts. The lowest MIC values were obtained against both S. aureus and E. faecalis. The best results against the former were obtained with the methanol extracts of Acacia karroo Hayne (Fabaceae), A. occidentale and B. cathartica, which exhibited a very promising activity (MIC = 7.5 µg/mL). A strong inhibition of the growth of S. aureus was also observed for C. abbreviata and Parkinsonia aculeata L. (Fabaceae) methanol extracts, which showed MIC values of 15 µg/mL. Among the active extracts against the E. faecalis strain, A. occidentale (methanol extract), B. cathartica (both dichloromethane and methanol extracts) and Momordica balsamina L. (Curcubitaceae) (ethyl acetate extract) showed the lowest MIC values (7.5 µg/mL).
The development of M. smegmatis was strongly inhibited by the methanol extracts of Aloe parvibracteata Schonland (Asphodelaceae), A. occidentale, B. cathartica, G. fruticosus, and T. emetica and by the hexane extracts of Leonotis leonurus, L. gariepina and Senna didymobotrya (Fresen.) H.S.Irwin & Barneby (Fabaceae) (MIC = 15 µg/mL).
Most of the extracts exhibited weaker activities against Gram-negative bacteria than those found for Gram-positive ones and M. smegmatis. P. aeruginosa was the most sensitive Gram-negative strain. Its growth was significantly inhibited (MIC = 31 µg/mL) by extracts of G. fruticosus fruits (hexane and methanol extracts) and T. emetica seeds (dichloromethane extract). Significant results were also found for extracts of Pittosporum tobira [Dryand.] W.T. Aiton (Pittosporaceae) and Schefflera actinophylla (Endl.) Harms (Araliaceae) (MIC = 62 µg/mL). Some of the extracts had weak/moderate activity against K. pneumoniae (MIC = 125 µg/mL) and, as mentioned above, E. coli was resistant to all extracts (MIC > 250 µg/mL).
The results obtained with Gram-negative species can be partially explained by the morphological differences observed in bacteria cell wall. In fact, Gram-negative bacteria have an extra outer membrane, highly hydrophobic, that acts as a permeability barrier to a large number of compounds, mainly of hydrophilic nature (CitationStavri et al., 2007).
When analyzing the results of the phytochemical screening (), a strong presence of phenolics/flavonoids was found in most of the active extracts. Therefore, they might be responsible, at least partially, for the observed antibacterial properties (CitationCazarolli et al., 2008). This class of compounds has been frequently found in plants of Anacardiaceae family (CitationKubo et al., 2003), species of Bridelia and Acacia genus (CitationAdebayo & Ishola, 2009; CitationTung et al., 2007), and were also isolated from C. abbreviata (CitationDehmlow et al., 1998). Moreover, terpenoids, present in almost all extracts, may also play an important role in the antibacterial activity (CitationCowan, 1999). This was corroborated by low MIC values obtained for M. balsamina extracts. In fact, the methanol extracts of this species were found to be rich in cucurbitane-type triterpenoids (Ramalhete et al., 2009a, 2009b).
To the best of our knowledge, this is the first report of determination of MIC values for most of these plants. In some cases, the reported data were carried out for different plant parts/extracts only by diffusion methods or described no significant MIC values (CitationAl-Fatimi et al., 2007; CitationStafford et al., 2005; CitationKambizi & Afolayan, 2001; CitationKumar et al., 1997). However, significant antibacterial activity was previously described for T. emetica (CitationShai et al., 2008; Germanò et al., 2005), Cassia occidentalis L. (Fabaceae) (CitationYadav et al., 2010) and Tabernaemontana elegans Stapf.(Apocynaceae) (CitationPallant & Steenkamp, 2008).
In conclusion, the results of this study support the use of most the studied plants in traditional medicine, for the treatment of infectious diseases. This correlation can be observed, for instance, by the inhibition of E. faecalis growth displayed by B. cathartica, traditionally used in diarrhea pathologies and by the activity presented by C. ambrosioides against S. aureus, used in abscesses treatment. Therefore, the plant extracts that have shown promising activity are worthy of further phytochemical and toxicological investigation.
Acknowledgments
The authors thank Dr. Teresa Vasconcelos, Instituto Superior de Agronomia, University of Lisbon, Portugal, for providing and identifying some of the plants. They also are grateful to Dr. Catarina Arruda and Dr. Isabel Pestana from the Portuguese Embassy in Mozambique, as well as the Portuguese Office of International Affairs for plant transport.
Declaration of interest
This study was supported by FCT, Portugal (BD/22321/2005).
References
- Adebayo EA, Ishola OR. (2009). Phytochemical and antimicrobial screening of the crude extracts from the root, stem bark and leaves of Bridelia ferruginea. Afr J Biotechnol, 8, 650–653.
- Adonizio AL, Downum K, Bennett BC, Mathee K. (2006). Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol, 105, 427–435.
- Al-Fatimi M, Wurster M, Schröder G, Lindequist U. (2007). Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol, 111, 657–666.
- Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. (2001). Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem, 49, 4168–4170.
- Bandeira SO, Gaspar F, Pagula FP. (2001). African ethnobotany and healthcare: Emphasis on mozambique. Pharm Biol, 39 Suppl 1, 70–73.
- Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, Pizzolatti MG, Silva FR. (2008). Flavonoids: Prospective drug candidates. Mini Rev Med Chem, 8, 1429–1440.
- CLSI-Clinical Laboratory Standards Institute. (2008) M100–S18. Performance standards for antimicrobial susceptibility testing: 18th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- Cos P, Vlietinck AJ, Berghe DV, Maes L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol, 106, 290–302.
- Cos P, Maes L, Sindambiwe JB, Vlietinck AJ, Berghe VD. (2006b). Bioassays for antibacterial and antifungal activities. Biological screening of plant constituents. Trieste, Italy: UNIDO-ICS (United Nations Industrial Development Organization and the International Centre for Science and High Technology).
- Cowan MM. (1999). Plant products as antimicrobial agents. Clin Microb Rev, 12, 564–582.
- Dehmlow EV, van Ree T, Guntenhoner M. (1998). 2,4-trans-7,4′-dihydroxy-4-methoxyflavan from Cassia abbreviata. Phytochemistry, 49, 1805–1806.
- Cowan MM. (1999). Plant products as antimicrobial agents. Clin Microbiol Rev, 12, 564–582.
- Gootz TD. (2010). The global problem of antibiotic resistance. Crit Rev Immunol, 30, 79–93.
- Grace OM, Simmonds MSJ, Smith GF, Van Wik AE. (2008). Therapeutic uses of Aloe L. (Asphodelaseae) in southern Africa. J Ethnopharmacol, 119, 604–614.
- Kambizi L, Afolayan AJ. (2001). An ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in Guruve District, Zimbabwe. J Ethnopharmacol, 77, 5–9.
- Kumar S, Bagchi GD, Darokar MP. (1997). Antibacterial activity observed in the seeds of some coprophilous plants. Int J Pharm, 35, 179–184.
- Kubo I, Nihei K, Tsujimoto K. (2003). Antibacterial action of anacardic acids against methicillin resistant Staphylococcus aureus (MRSA). J Agric Food Chem, 51, 7624–7628.
- Marquez B. (2005). Bacterial efflux systems and efflux pumps inhibitors. Biochimie, 87, 1137–1147.
- Mølgaard P, Nielsen SB, Rasmussen DE, Drummond RB, Makaza N, Andreassen J. (2001). Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol, 74, 257–264.
- Morens DM, Folkers GK, Fauci AS. (2008). Emerging infections: A perpetual challenge. Lancet Infect Dis, 8, 710–719.
- Neuwinger HD. (2000). African Traditional Medicine. A Dictionary of Plants Uses and Applications. First ed. Stuttgart, Germany Medpharm: Scientific Publications.
- Newman DJ, Cragg GM. (2007). Natural products as sources of new drugs over the last 25 years. J Nat Prod, 70, 461–477.
- Newman DJ, Cragg GM, Snader KM. (2003). Natural products as sources of new drugs over the period 1981-2002. J Nat Prod, 66, 1022–1037.
- Pallant C, Steenkamp V. (2008). In-vitro bioactivity of Venda medicinal plants used in the treatment of respiratory conditions. Hum Exp Toxicol, 27, 859–866.
- Protabase record. Available at: http://www.prota4u.org/. Accessed in December 2010.
- Ramalhete C, Molnár J, Mulhovo S, Rosário VE, Ferreira MJ. (2009). New potent P-glycoprotein modulators with the cucurbitane scaffold and their synergistic interaction with doxorubicin on resistant cancer cells. Bioorg Med Chem, 17, 6942–6951.
- Ramalhete C, Mansoor TA, Mulhovo S, Molnár J, Ferreira MJ. (2009). Cucurbitane-type triterpenoids from the African plant Momordica balsamina. J Nat Prod, 72, 2009–2013.
- Rollinger JM, Langer T, Stuppner H. (2006). Strategies for efficient lead structure discovery from natural products. Curr Med Chem, 13, 1491–1507.
- Stafford GI, Jäger AK, van Staden J. (2005). Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J Ethnopharmacol, 97, 107–115.
- Shai LJ, Mcgaw LJ, Masoko P, Eloff JN. (2008). Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans. S Afr J Bot, 74, 677–684.
- Stavri M, Piddock LJ, Gibbons S. (2007). Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother, 59, 1247–1260.
- Talib WH, Mahasneh AM. (2010). Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules, 15, 1811–1824.
- Tung YT, Wu JH, Kuo YH, Chang ST. (2007). Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour Technol, 98, 1120–1123.
- Wagner H, Blader S. (1996). Plant Drug Analysis. Second ed. Berlin, Germany: Springer.
- WHO. (2002). Traditional and alternative medicine, Fact Sheet 271, World Health Organization, Geneva [Online]. Available at: http://www.who.int/mediacentre/news/releases/release38/en/. Accessed on December 2010.
- Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S. (2010). Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia, 81, 223–230.