Abstract
Context: Conjugated linoleic acids (CLAs) are a mixture of positional and geometric isomers of linoleic acid (LA) and believed to have many positive biological activities.
Objective: The present study was undertaken to assess the antioxidant activity of cis-9, trans-11 and trans-10, cis-12 as single or mixed CLA isomers at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11).
Materials and methods: A microplate reader was used to determine the free radical scavenging properties of CLAs against DPPH radical in ethanol.
Results: The kinetic reactions of CLA-DPPH• showed that all tested CLAs have exerted radical scavenging activities in a dose-dependent manner and observed to immediately react and quench DPPH radicals at all tested levels and no lag phase was noticed in CLA-DPPH• reactions. The median inhibitory concentration (IC50) value for cis-9, trans-11 CLA was observed to be more effective than other tested CLA. Total antioxidant capacity (TAC) of all tested CLAs were less effective radical scavengers as compared to vitamin E and butylated hydroxytoluene, although all tested CLAs were quenched a high amount (P < 0.05) of DPPH free radicals.
Discussion and conclusion: All tested CLAs have the ability to directly react and quench DPPH free radicals in ethanol. Furthermore, trans-10, cis-12 CLA has greater maximal efficacy than other tested CLAs as free radical scavenger, while cis-9, trans-11 CLA is the most potent isomer to directly react and quench free radicals at low concentrations in the system, suggesting that the free radical scavenging activity of CLA isomers may contribute to their diverse biological activities.
Introduction
Conjugated linoleic acids (CLAs) are dietary fatty acids produced by ruminant animals and have been shown in vivo and in vitro to exhibit anti-carcinogenic effect (CitationGuo et al., 2007; CitationSeo et al., 2008). Conjugated linoleic acids are unique, because they are present in food from animal sources such as dairy foods and meats, while the majority of naturally occurring substances that have been demonstrated to have anti-carcinogenic activity are of plant origin. Furthermore, CLAs have also many other important properties that regulate physiological and metabolic responses such as antidiabetic (CitationBelury et al., 2003), anti-atherogenic (CitationValeille et al., 2006), anti-obesity (CitationLee et al., 2006), and have immune system enhancement effects (CitationNtambi et al., 2002).
Antioxidant activities including free radical scavenging capacity, may give more explanations for CLA beneficial effects, even though the mechanisms which are responsible for these beneficial effects of CLA are still unclear (CitationYu, 2001; CitationLeung & Liu, 2000). Various forms of CLA isomers were found in food content and animal products. Evidence has indicated that cis-9, trans-11 and trans-10, cis-12 CLA isomers and the mixture between them may contribute differently to their beneficial effects and may act in a different way in the biological systems (CitationPariza et al., 2000).
Park and others (1999) reported that trans-10, cis-12 CLA was more effective than cis-9, trans-11 CLA in changing body composition by reducing body fat and enhancing body water, protein and ash in mice. In another study, trans-10, cis-12 CLA showed a higher activity than the mixture isomers of CLA in reducing incorporation of fatty acids into cell triglycerides and inducing apoptosis, demonstrating that trans-10, cis-12 CLA is a major anti-adipogenic isomer of CLA. In addition, trans-10, cis-12 CLA was observed to be more effective in suppressing hepatic triglycerides secretion than cis-9, trans-11 CLA in Hep G2 cell cultures (CitationLin et al., 2001). Moreover, trans-10, cis-12 CLA showed a strong antioxidant at concentration 2–20 µM as compared to cis-9, trans-11 CLA or α-tocopherol, while cis-9, trans-11 CLA possessed a weak antioxidant activity under the same concentrations (CitationLeung et al., 2000).
In contrast, cis-9, trans-11 CLA showed a higher activity than trans-10, cis-12 CLA and the mixture of these two CLA isomers in inducing hepatic peroxisome proliferation in Sprague-Dawley rats (CitationMoya et al., 1999). In another study, cis-9, trans-11 was more effective than trans-10, cis-12 CLA in enhancing the growth of young rodents (CitationPariza et al., 2000). Furthermore, cis-9, trans-11 CLA was chosen over trans-10, cis-12 CLA incorporation into tissue lipids (CitationMartin et al., 2000).
The mixture of cis-9, trans-11 and trans-10, cis-12 CLA isomers was found to possess higher biological activity than the individual CLA isomers. Truitt and collaborators (1999), reported that a mixture of cis-9, trans-11 and trans-10, cis-12 CLA isomers was more effective than either cis-9, trans-11 CLA or trans-10, cis-12 CLA as anti-aggregatory in induced human platelet aggregation. It was also observed that the mixture of CLA isomers had a greater inhibitory activity for platelet cyclooxygenase than either cis-9, trans-11 CLA or trans-10, cis-12 CLA as measured by [14C] thromboxane B2 formation.
Therefore, the general objective of this study was to determine the free radical scavenging properties of trans-10, cis-12 and cis-9, trans-11 as single or mixed CLA isomers at two selected ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11), against the stable 2,2-diphenyl-1-picryhydrazyl radical (DPPH) in ethanol. Since the milk fat from the ruminant is considered as the main source of CLA, these two ratios of CLA isomers were obtained previously from cow milk fat of Mafriwal and Jersey breeds that can be provided naturally for human consumption. Moreover, the specific objectives of the present study were:
to assess the kinetics of selected CLA isomers against DPPH radicals.
to determine the median inhibitory concentration (IC50) of selected CLA isomers on DPPH free radical.
to estimate and compare the total antioxidant capacity (TAC) of CLA with selected antioxidants.
Materials and methods
DPPH radical scavenging assay
The DPPH assay was carried out using a developed method that was used by CitationShe et al. (2010) with slight modification. Solution of 500 µM DPPH free radical was prepared by dissolving DPPH (Sigma Chemical Co., USA) in 99% ethanol (Sigma Chemical Co., USA) with hard shaking and then allowed to stand for 30 min in the dark at room temperature. The reactions of DPPH free radical with tested materials were carried out in a 96-well microplate and the absorbance at 517 nm was set using a microplate reader (ASYS UVM 340, Austria). A sample reaction well containing 100 µL of CLA isomers was added with 100 µL of 500 µM DPPH free radical solution. DPPH free radical solution was added to each tested sample in microplate to initiate the reaction between the antioxidant and DPPH radical. Absorption was red at 517 nm immediately after the addition of DPPH free radical solution on the microplate wells. An absorption reading at 517 nm was also established for a mixture of 100 µL of solvent (ethanol) and 100 µL of 500 µM DPPH free radical solutions as a control. All samples were analyzed at least in triplicate, and fresh DPPH free radical stock solution was prepared daily.
Kinetics of CLA-radical reactions
The kinetic reactions of CLAs against the stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) in ethanol were determined by measuring the disappearances of DPPH radicals in the reaction mixtures in using the developed method of CitationYu (2001). Six concentrations (2.5, 5, 10, 20, 40 and 80 mg/mL ethanol) of trans-10, cis-12 (purity ≥ 98%) (Cayman Chemical Ltd, USA) and cis-9, trans-11 (purity ≥ 96%) CLA isomers (Cayman Chemical Ltd, USA) and the selected two mixtures of CLA were used in the kinetic study. CLA solution (100 µL) was mixed into 100 µL of 500 µM DPPH• ethanol solution. Absorbance at 517 nm was measured for each reaction mixture against an ethanol blank at 0, 5, 10, 20, 40, 80, 160, 320, and 1400 min. The reaction of CLA-DPPH• was obtained by plotting the remaining amount of DPPH• against time at each level to demonstrate the kinetic properties of CLA isomers and the mixtures. The values of % DPPH• remaining at different reaction times for various levels of CLA were obtained using the following formula:
Where:
A sample = absorbance of the certain concentration of a selected antioxidant (CLA-DPPH•) at 517 nm measured at certain reaction time.
A control = absorbance of the control (DPPH•) at 517 nm measured at certain reaction time.
Median inhibitory concentration (IC50) quantification
The IC50 of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) were determined by plotting the percent DPPH• remaining at steady state of the reaction against the corresponding CLA concentration. Then, from concentration-response curve for each tested CLA, the IC50 values were obtained by using the following formula:
Where:
A% = the % inhibition immediately above 50%.
B% = the % inhibition immediately below 50%.
CA = the concentration with the % inhibition immediately above 50%.
CB = the concentration with the % inhibition immediately below 50%.
X = IC50 value.
Total antioxidant capacity quantification
Total antioxidant capacity (TAC) of trans-10, cis-12 and cis-9, trans-11CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) CLA was estimated and compared to vitamin E (Sigma Chemical Co., USA) and butylated hydroxytoluene (BHT) (Sigma Chemical Co., USA) according to the method described by CitationWinston et al. (1998) using the stable 2,2-diphenyl-1-picryhydrazyl radical (DPPH•). 500 µM DPPH• solution (100 µL) were added into 100 µL of ethanol solutions of vitamin E, BHT, trans-10, cis-12 CLA, cis-9, trans-11 CLA and the selected two combinations of trans-10, cis-12 and cis-9, trans-11CLA isomers at ratios 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) to start the reaction. The final concentration was 50 mM for each test compound except for the control containing pure ethanol according to the method described by CitationYu (2001). All tests were conducted at room temperature in triplicate and the absorbance at 517 nm was measured by using a microplate reader. The area under the kinetic curve for each tested compound and the control against the time was calculated by integration. Then, total antioxidant capacity (TAC) was determined according to the following formula:
Where:
∫ SA = the integrated areas of the curve defining the sample reactions.
∫ CA = the integrated areas of the curve defining the control reactions.
Statistic analysis
Data were reported as mean ± Standard error (SE) and differences among the mean (n = 3) of tested compounds were determined by using Kruskal-Wallis H test and one-way analysis of variance (ANOVA) after verification of the normal distribution of the data followed by Tukey post test. All statistical tests were performed by using SPSS 15 software package (SPSS, 2006). Significant differences were tested at P < 0.05 level.
Results
Kinetics of CLA-radical reaction
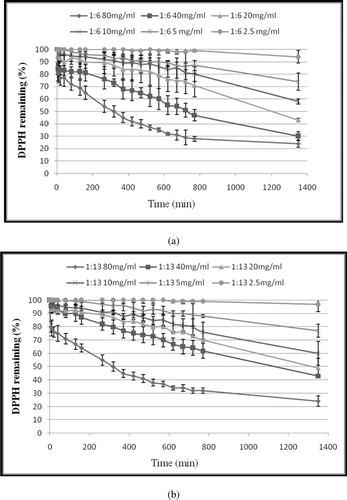
The kinetic reactions of trans-10, cis-12 and cis-9, trans-11 single CLA isomers against the stable 2,2-diphenyl-1-picryhydrazyl radical (DPPH•) in ethanol are shown in , while the kinetic reactions of mixed CLA isomers against DPPH free radical at ratios 1:6 and 1:13 of trans-10, cis-12/cis-9, trans-11 CLAs are shown in . The free radical scavenging activity of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) CLA was detected at all tested concentrations, and the lowest concentration was 2.5 mg of CLA/mL in ethanol. Dose and time dependence were observed under the same testing conditions. The higher concentration of CLA at 80 mg/mL was more effective in quenching free radicals than other tested concentrations in the system. All tested CLAs immediately reacted and quenched DPPH free radicals at all tested concentrations without exhibiting any lag phase in CLA-DPPH• reactions.
Figure 1. Kinetic curve of single CLA isomers against DPPH free radical (a) cis-9, trans-11 CLA (b) trans-10, cis-12 CLA. The final concentrations of CLA were 2.5, 5, 10, 20, 40 and 80 (mg/mL) in the reaction mixtures. DPPH• concentration was 500 µM in all reaction mixtures. Values are mean ± SE (n = 3).
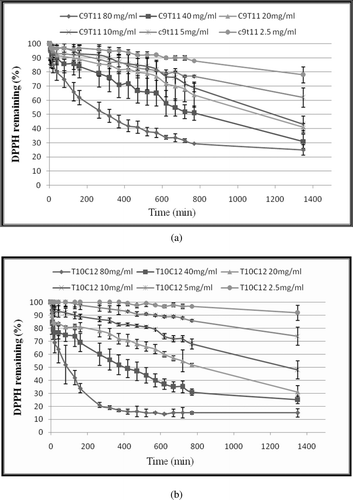
Figure 2. Kinetic curve of mixed CLA isomers against DPPH free radical (a) at ratio 1:6 of trans-10, cis-12/cis-9, trans-11. (b) at ratio 1:13 of trans-10, cis-12/cis-9, trans-11. The final concentrations of CLA were 2.5, 5, 10, 20, 40 and 80 (mg/mL) in the reaction mixtures. DPPH• concentration was 500 µM in all reaction mixtures. Values are mean ± SE (n = 3).
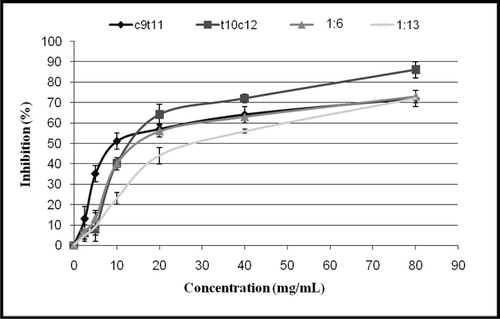
Median inhibitory concentration (IC50) of CLAs
The inhibition percent of DPPH• at steady state against the corresponding concentrations of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at the two tested ratios is shown in . The IC50 value for cis-9, trans-11 CLA (11.1 ± 3.8 mg/mL) was observed to be more effective than other tested CLAs followed by trans-10, cis-12 CLA (12.6 ± 3.4 mg/mL), the mixtures of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:6 (16.3 ± 1.1 mg/mL) and the mixtures of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:13 (27.9 8 mg/mL). No significant (P > 0.05) difference existed between all tested CLAs except the IC50 value for the mixtures of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:13, which was observed to be significantly less effective than other tested CLAs. Furthermore, cis-9, trans-11 CLA quenched significantly more DPPH radicals at low concentrations (5 and 10 mg/mL) against DPPH radicals than that of trans-10, cis-12 CLA and the two mixtures of trans-10, cis-12 and cis-9, trans-11 at the ratios of 1:6 and 1:13. Meanwhile, trans-10, cis-12 CLA quenched significantly more (P < 0.05) DPPH radicals at high concentrations (40 and 80 mg/mL) against DPPH radicals than other tested CLAs ().
Table 1. Inhibition % of DPPH• radical at steady state among different concentrations of selected CLAs, and median inhibitory concentration (IC50) value for each selected CLA.
Comparison of total antioxidant capacity
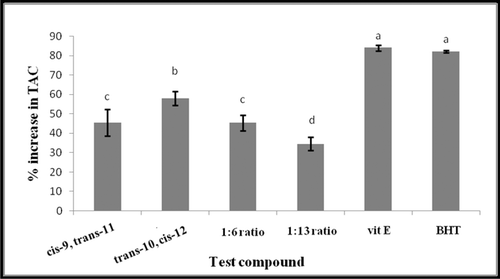
Total antioxidant capacity (TAC) of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at the two tested ratios was measured and compared with widely used antioxidants such as vitamin E and BHT (). Total antioxidant capacity of trans-10, cis-12 CLA (57.87 ± 3.5%) was observed to be significantly more effective (P < 0.05) than other tested CLAs followed by cis-9, trans-11CLA (45.48 ± 6.9%) and the mixture of trans-10, cis-12 and cis-9, trans-11 at ratio 1:6 (45.41 ± 4%) then 1:13 (34.58 ± 3.5%). Meanwhile, all tested CLAs were less effective radical scavengers as compared to vitamin E (83.96 ± 1.5%) and BHT (82.07 ± 0.6%), although all tested CLAs were quenched a significant high amount (P < 0.05) of DPPH free radicals, on the same per mole basis. All antioxidants were tested at a level of 50 mM against 500 µM DPPH free radicals.
Figure 4. Comparison of TAC of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) with Selected antioxidants vitamin E (Vit E) and butylated hydroxytoluene (BHT). All antioxidants were compared at 50 mM final concentration against 500 µM DPPH radical. Vertical bars represent the standard error of experimental data (n = 3). Columns marked with the same letter are not significantly different (P < 0.05).
Discussion
There is a growing interest in the usage of antioxidants that in the attempt to prevent oxidative damage caused by of free radicals in the human body. This oxidative damage may be a causative agent in the occurrence and development of several chronic diseases, like cancer, diabetes and atherosclerosis diseases (CitationKeller et al., 2002; CitationSenthilkumar & Subramanian, 2007; CitationShirwaikar et al., 2007). Most of natural antioxidants are extracted from plants that contain various antioxidant compounds such as flavonoids, phenolics, carotenes and vitamins. Therefore, most of the antioxidant methods to determine the antioxidant activity of plant extract are screening for these compounds. Furthermore, plant extract can be prepared in organic and inorganic solvents, which give more chances to apply more than one antioxidant assay. Conjugated linoleic acids are unique compounds, because they have been found predominantly in animal products (e.g., meat, milk). They are oily and pure compound, which make the applications of the antioxidant assays very limited due to its lipid-soluble nature. Therefore, this study was carried out using just DPPH assay, but with different parameters to evaluate the free radical scavenging activity of trans-10, cis-12 and cis-9, trans-11 as single or mixed CLA isomers at two selected ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11). These two ratios of CLA isomers were selected based on them natural availability in the milk fat of Mafriwal and Jersey breeds (CitationYassir et al., 2010).
The kinetics of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) of the CLA-DPPH• reactions showed dose and time dependence in their reactions at all tested concentrations (2.5, 5, 10, 20, 40 and 80 mg/mL in ethanol). Furthermore, no lag phase was observed for all tested CLAs against DPPH free radical in ethanol. These data indicate that all tested CLAs can provide immediate protection against free radicals and were in agreement with previous study reported by CitationYu (2001) that the mixture of CLA isomers exerted kinetic reactions without a lag phase unlike linoleic acid. This lag phase indicated the delay of radical quenching actions.
In the present study, the IC50 values of cis-9, trans-11 and trans-10, cis-12 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) were determined. The IC50 value is defined as the level of the tested CLA that caused 50% inhibition of DPPH radical formation under the experimental conditions. The lowest IC50 indicates potent antioxidant to act as DPPH scavengers. The IC50 value for cis-9, trans-11 CLA was observed to be more effective than other tested CLAs followed by trans-10, cis-12 CLA, the mixtures of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:6 and 1:13. These data indicate that the individual CLA isomers may have a greater free radical scavenging capacity than the mixture of them. In other words, individual CLA isomers may have inhibitory effects between them in their reactions against the free radicals.
Similar inhibitory effect between trans-10, cis-12 and cis-9, trans-11 CLA isomers was observed in a previous study (CitationMoya-Camarena et al., 1999). In that study, the mixture of CLA isomers containing 41% of cis-9, trans-11/trans-9, cis-11 CLA and 44.1% of trans-10, cis-12 CLA, was significantly a weaker activator for peroxisome proliferator-activated receptor α (PPARα) than single isomers of cis-9, trans-11; trans-10, cis-12; or trans-9, cis-11 CLA using a rat hepatoma cell line.
In contrast to the inhibitory effect, the mixture of trans-10, cis-12 and cis-9, trans-11 CLA isomers was found to be more effective for the inhibition of cyclooxygenase on human platelet thromboxane B2 formation from exogenously added arachidonic acid than the single CLA isomers, followed by trans-10, cis-12 CLA (CitationTruitt et al., 1999), suggesting that there may be a synergistic effects between these CLA isomers. Martin and co-workers (2000) investigated the CLA effect on lipid-metabolizing enzymes in male rats by determining selected enzyme activities in different tissues after cellular subfractionation. The authors found that the mixture of CLA isomers was a weaker substrate for the mitochondrial carnitine palmitoyl transferase I (CPT-I) compared to either trans-10, cis-12 or cis-9, trans-11 CLA isomers. The same authors also reported that the mixture of CLA isomers was a poorer substrate for the acyl-CoA oxidase (ACO) than trans-10, cis-12 CLA, but more favored than cis-9, trans-11 CLA. These results demonstrated that interactions between trans-10, cis-12 and cis-9, trans-11 CLA isomers might be more complex than synergistic or inhibitory effects.
Measuring the percent of inhibition of DPPH• at steady state and determining the IC50 values of CLA as single or mixed isomers might have led to a conclusion that trans-10, cis-12 CLA has greater maximal efficacy than other tested CLAs as free radical scavenger, while cis-9, trans-11 CLA was most potent isomer to directly react and quench free radicals than other tested CLAs at low concentrations in the system. However, all tested CLAs possessed as free radical scavenger, but they act at different concentrations in their reactions with free radicals. This observation is consistent only with the data for trans-10, cis-12 CLA isomer, but not for other CLAs as suggested by CitationLeung and Liu (2000).
In a previous study, CitationLeung and Liu (2000) stated that the antioxidant activity of trans-10, cis-12 CLA was comparable to that of vitamin E and higher than cis-9, trans-11 CLA at low concentrations (2–200 µM) measured by TBARS assay. In the current study, the total antioxidant capacity (TAC) of all tested CLAs were less effective radical scavengers as compared to vitamin E and BHT at the concentration of 50 mM as measured by DPPH assay. However, all tested CLAs were quenched a significant high amount of DPPH free radicals. This may be due to the different concentrations of CLAs used and the concept of the assay. In the present study, the DPPH assay has measured the antioxidant activity of the sample’s reaction itself with DPPH free radicals, while in the previous study, TBARS assay was carried out to measure the antioxidant activity by determining the resistance of lipid or lipid emulsions to oxidation in the presence of the antioxidant being tested. Conversely, TAC of trans-10, cis-12 CLA was observed to be significantly more effective than other tested CLAs followed by cis-9, trans-11 CLA, the mixture of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:6 and the mixture of trans-10, cis-12 and cis-9, trans-11 at ratio of 1:13. These results supported the conclusion, which obtained from measuring the percent of inhibition of DPPH• at steady state indicating that trans-10, cis-12 CLA has greater maximal efficacy than other tested CLAs as free radical scavenger, and cis-9, trans-11 CLA was most potent isomer to directly react and quench free radicals at low concentrations in the system.
The present study demonstrated that trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) were shown to exhibit antioxidant capacity to directly react and quench DPPH free radicals at different concentrations without observing any lag phase in CLA-DPPH• reactions. Trans-10, cis-12 CLA has greater maximal efficacy than other tested CLAs as free radical scavenger, while cis-9, trans-11 CLA was most potent isomer to directly react and quench free radicals than other tested CLAs at low concentrations in the system. All tested CLAs were less effective radical scavengers as compared to vitamin E and BHT at the concentration of 50 mM, although all tested CLAs quenched a significant high amount of DPPH free radicals. In general, these findings suggest that the free radical scavenging activity of CLA isomers may contribute to their diverse biological activities.
Conclusions
The present study demonstrated that trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) were shown to exhibit antioxidant capacity to directly react and quench DPPH free radicals at different concentrations without observing any lag phase in CLA-DPPH• reactions. Trans-10, cis-12 CLA has greater maximal efficacy than other tested CLAs as free radical scavenger, while cis-9, trans-11 CLA was most potent isomer to directly react and quench free radicals than other tested CLAs at low concentrations in the system. All tested CLAs were less effective radical scavengers as compared to vitamin E and BHT at the concentration of 50 mM, although all tested CLAs quenched a significant high amount of DPPH free radicals. In general, these findings suggest that the free radical scavenging activity of CLA isomers may contribute to their diverse biological activities.
Acknowledgments
The authors gratefully acknowledge the laboratory assistants, technicians and officers of Universiti Putra Malaysia (UPM) for their technical support, helpfulness and co-operation in providing all the equipments and facilities needed for this study.
Declaration of interest
This work was supported by the Ministry of Science, Technology and Innovation (MOSTI), Malaysia through grant NO. 05-01-04-SF0373.
References
- Belury MA, Mahon A, Banni S. (2003). The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr, 133, 257S–260S.
- Guo DD, Moon HS, Arote R, Seo JH, Quan JS, Choi YJ, Cho CS. (2007). Enhanced anticancer effect of conjugated linoleic acid by conjugation with Pluronic F127 on MCF-7 breast cancer cells. Cancer Lett, 254, 244–254.
- Keller C, Maillard M, Keller J, Hostettmann K. (2002). Screening of European fungi for antibacterial, antifungal, larvicidal, molluscicidal, antioxidant and free-radical scavenging activities and subsequent isolation of bioactive compounds. Pharm Biol, 40, 518–525.
- Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y, Park JH. (2006). Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta, 1761, 736–744.
- Leung YH, Liu RH. (2000). Trans-10, cis-12-conjugated linoleic acid isomer exhibits stronger oxyradical scavenging capacity than cis-9, trans-11-conjugated linoleic acid isomer. J Agric.Food Chem, 48, 5469–5475.
- Lin Y, Schuurbiers E, Van der Veen S, De Deckere EA. (2001). Conjugated linoleic acid isomers have differential effects on triglyceride secretion in HepG2 cells. Biochim Biophys Acta, 1533, 38–46.
- Martin JC, Grégoire S, Siess MH, Genty M, Chardigny JM, Berdeaux O, Juanéda P, Sébédio JL. (2000). Effects of conjugated linoleic acid isomers on lipid-metabolizing enzymes in male rats. Lipids, 35, 91–98.
- Moya-Camarena SY, Vanden HJP, Belury MA. (1999). Conjugated linoleic acid activates peroxisome proliferator-activated receptor α and β subtypes but does not induce hepatic peroxisome proliferation in Sprague-Dawley rats. BBA-Mol Cell Biol L, 1436, 331–342.
- Ntambi JM, Choi Y, Park Y, Peters JM, Pariza MW. (2002). Effects of conjugated linoleic acid (CLA) on immune responses, body composition and stearoyl-CoA desaturase. Can J Appl Physiol, 27, 617–628.
- Pariza MW, Park Y, Cook ME. (2000). Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med, 223, 8–13.
- Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. (1999). Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids, 34, 235–241.
- Senthilkumar G, Subramanian S. (2007). Evaluation of antioxidant potential of Terminalia chebula. Fruits studied in streptozotocin-induced diabetic rats. Pharm Biol, 45, 511–518.
- Seo JH, Moon HS, Kim IY, Guo DD, Lee HG, Choi YJ, Cho CS. (2008). PEGylated conjugated linoleic acid stimulation of apoptosis via a p53-mediated signaling pathway in MCF-7 breast cancer cells. Eur J Pharm Biopharm, 70, 621–626.
- She GM, Hu JB, Zhang YJ, Yang CR. (2010). Phenolic constituents from Rhopalocnemis phalloides with DPPH radical scavenging activity. Pharm Biol, 48, 116–119.
- Shirwaikar A, Rajendran K, Barik R. (2007). Effect of Garuga pinnata. Roxb aqueous bark extracts on tissue antioxidant activity in streptozotocin-nicotinamide-induced type II diabetic rats. Pharm Biol, 45, 205–209.
- Truitt A, McNeill G, Vanderhoek JY. (1999). Antiplatelet effects of conjugated linoleic acid isomers. Biochim Biophys Acta, 1438, 239–246.
- Valeille K, Ferezou J, Parquet M, Amsler G, Gripois D, Quignard-Boulange A, Martin J. (2006). The natural concentration of the conjugated linoleic acid, cis-9, trans-11, in milk fat has antiatherogenic effects in hyperlipidemic hamsters. J Nutr, 136, 1305–1310.
- Winston GW, Regoli F, Dugas AJJr, Fong JH, Blanchard KA. (1998). A rapid gas chromatographic assay for determining oxyradical scavenging capacity of antioxidants and biological fluids. Free Radic Biol Med, 24, 480–493.
- Yassir MA, Arifah AK, Yaakub H, Zuraini A, Zakaria ZA. (2010). Comparison of conjugated linoleic acid and other fatty acid content of milk fat of Mafriwal and Jersey cows. J Animal Vet Adv, 9, 1318–1323.
- Yu L. (2001). Free radical scavenging properties of conjugated linoleic acids. J Agric Food Chem, 49, 3452–3456.