Abstract
Context: Polyherbal formulations containing different plants are used for the treatment of various diseases. Romix® powder is a polyherbal formulation consisting of 14 traditionally used herbs and is used as a food supplement. There is no information about pharmaceutical activities of Romix®.
Objective: This study determined the total phenolic and total flavonoid content, and investigated the antioxidant and anti-inflammatory activities and acute toxicity of Romix®.
Material and methods: The total phenolics in the extracts were determined colorimetrically by using the Folin-Ciocalteu reagent. The total flavonoid content of the extracts was evaluated by a spectrophotometric method. The quercetin content of the extract was analyzed using the high-performance liquid chromatography (HPLC) method. Antioxidant activity of the extracts was determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assays. The anti-inflammatory activity was evaluated by the carrageenan-induced paw edema test in the rat.
Results: The flavonoid and phenolics contents of Romix® were 50.58 and 265.83 mg/g in ethanol extract and 18.60 and 222.50 mg/g in water extract, respectively. Total quercetin content of Romix® was determined as 2.857 mg/g. Antioxidant activity results showed that ethanol extract in 1 mg/mL concentration (4.49775 µg/mL) had moderate antioxidant activity than water extract in the same concentration (4.28191 µg/mL). Intraperitoneal administration of 25 mg/kg Romix® extract exhibited anti-inflammatory activity and inhibited paw swelling at 1, 2, 3, 4, 5 and 6 h in rats with no acute toxicity.
Conclusion: These findings suggest that Romix® due to its antioxidant and anti-inflammatory activities may be useful in the prevention or treatment of aging-related and inflammatory diseases.
Introduction
Medicinal plants constitute the main source of new pharmaceuticals and healthcare products. A whole range of plant-derived dietary supplements, phytochemicals and pro-vitamins assist in maintaining good health. The widespread use of traditional herbs and medicinal plants has been traced to the occurrence of natural products with medicinal properties. Various medicinal plants have been used for years in daily life to treat diseases all over the world.
Oxidative damage of biological molecules in the human body are involved in degenerative or pathological processes such as aging, coronary heart disease and cancer. This oxidative damages could be retarded by endogenous defense systems such as catalase, superoxide dismutase, and the glutathione peroxidase system, but these systems are not completely efficient (CitationBergendi et al., 1999). In the past decade, many epidemiological studies have confirmed that intake of exogenous antioxidants was effective in preventing or suppressing such diseases (CitationBlock et al, 1992; CitationSingh & Downing, 1995). Phenolic components have been known to act as antioxidants not only because of their ability to donate electrons, but also because of their stable radical intermediates, which can effectively prevent oxidation at the cellular and physiological level (CitationCuvrelier et al., 1992). Moreover, phenolic compounds show different biological activities such as antibacterial, anticarcinogenic, anti-inflammatory, antiviral, anti-allergic, estrogenic and immune-stimulating agents (CitationLarson, 1988).
Natural antioxidants or phytochemical antioxidants are secondary metabolites of plants. One of the most important groups of these metabolites may be counted as phenolic compounds. Flavonoids, carotenoids, cinnamic acids, benzoic acids, folic acid, ascorbic acid and tocopherols, and tocotrienols, are some of the antioxidants produced by the plants for various functions. Of the secondary metabolites, flavonoids, a group of naturally available plant phenolics, have been shown to possess several biological properties, such as antioxidant, hepatoprotective, antithrombotic, antihypertensive, anti-inflammatory, anti-allergic, antitumor, bactericidal and antiviral activities (CitationMorel et al., 1993; CitationMiddleton & Kandswami, 1994; Citationvan Acker et al., 1996). In recent years, there has been a growing interest in antioxidant properties of phenolic compounds.
Herbal drugs are rapidly becoming popular as an alternative therapy. The polyherbal formulations containing different plants/extracts have to be tested again in the formulation form. Numerous polyherbal formulations are used for the treatment of some diseases. Hence, there is a need to establish a simple and sensitive screening method for contents and activity as the quality control of polyherbal formulations continue to develop.
Romix® powder is a polyherbal formulation consisting of 14 traditionally used herbs, Juniperus communis Linn (Cupressaceae), Peganum harmala Linn. (Zygophyllaceae), Curcuma longa Val. (Zingiberaceae), Thymus sipyleus Boiss. (Lamiaceae), Origanum majorana Linn. (Lamiaceae), Nigella sativa Linn. (Ranunculaceae), Zingiber officinale Rosc. (Zingiberaceae), Hypericum perforatum Linn. (Hypericaceae), Foeniculum vulgare Mill. (Apiaceae), Pimpinella anisum Linn. (Apiaceae), Valeriana officinalis Linn. (Valerianaceae), Cassia angustifolia Vahl. (Caesalpiniaceae), Eugenia caryophyllata Linn. Merr. & Perry. (Myrtaceae), Salvia triloba Linn. (Lamiaceae). This powder is approved as a food supplement by The Ministry of Agriculture, Turkey. Solely, quality control studies of this product were done. There is no information about pharmaceutical activities of Romix®. The objectives of the present study were to determine total phenolic, total flavonoid and quercetin content, and to investigate antioxidant and anti-inflammatory activities and acute toxicity of Romix®.
Materials and methods
Plant material
Romix® (Lot number: 10.09.01) was generously donated by Naturin Natural Products, Drug and Drug Raw Material Ltd. The querter of 9 Eylül, 342 street, no 6, Aker Plaza, Gaziemir, Izmir, Turkey.
Preparation of extract
For determination of total phenolic and flavonoid contents and antioxidant capacity of Romix® powder, ethanol and water extracts were separately prepared. The powder (5 g) was extracted with ethanol, under stirring for 2 days. However, water extract of it was prepared by 5% infusion. The extraction solvent was evaporated under reduced pressure to dryness (CitationUnver et al., 2005). The yields of ethanol and water extracts of Romix® were 21.46 and 27.52%, respectively. Sample solutions were prepared by dissolving the extracts in the extraction solvent (1 mg/mL and 0.5 mg/mL). All chemicals used were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
For assessment of anti-inflammatory activity, Romix® powder (15 g) placed in a Soxhlet apparatus. Romix® powder was extracted with pure ethanol in a Soxhlet extractor. Extraction was performed with 300 mL of an appropriate solvent for 16 h (ethanol in Soxhlet extractor until colorless). After extraction, the extract was concentrated in a rotary vacuum evaporator at 40°C and then lyophilized (CitationBimakra et al., 2011). The yield was calculated to be 33% w/w. The extract was administered to rats immediately after suspension in saline. All doses are expressed as w/v of the plant extract. The extracts (6.75, 12.5 and 25 mg/kg) were intraperitoneally administered to rats immediately after suspension in saline.
Determination of total phenolic and flavonoid contents
Total phenolic content was determined by Folin-Ciocalteu method (CitationMeda et al., 2005). Briefly, 0.1 mL of extracts (0.5 mg/mL and 1 mg/mL) were mixed with 2.8 mL deionized water. This solution was mixed with 2 mL 2% sodium carbonate and 0.1 mL of 0.1 N Folin-Ciocalteu reagent. After incubation at room temperature for 30 min, the absorbance of the mixture was measured at 750 nm against a deionized water blank on a UNICAM 8625 UV/Vis spectrophotometer. Gallic acid was chosen as a standard. The data expressed as milligram gallic acid equivalents.
Total flavonoid content was determined by the aluminum chloride colorimetric method described by CitationChang et al. (2002). 0.5 mL of the extracts (0.5 mg/mL and 1 mg/mL) were mixed with 1.5 mL of ethanol, 0.1 mL of 10% aluminum chloride and 2.8 mL of distilled water. The mixture was kept at room temperature for 30 min and the absorbance was recorded at 415 nm with the help of UNICAM 8625 UV/Vis spectrophotometer. Quercetin equivalent (QE) was chosen as a standard. The amount of flavonoid was expressed as QE.
HPLC analysis
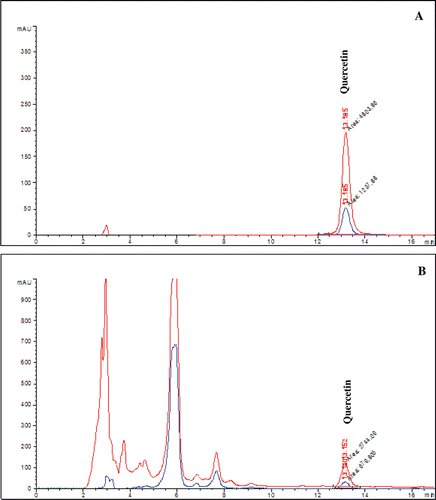
Sample solutions were prepared by dissolving the extract in methanol at a concentration of 0.1 g/mL in a sonicator. The solution was passed through a 0.22 μm pore diameter filter before use. The quercetin content of the extract was analyzed using the HPLC method (Agilent 1100 HPLC-DAD) (CitationSpácil et al., 2008). An Ace C18 column (25 cm × 4.6 mm × 5 µm) was used. The mobile phase comprised water and formic acid (95:5, v/v). The detection wavelength, flow rate and column temperature were set to 350 nm, 0.9 mL/min and 25°C, respectively. For all solutions (samples and standards), a quantity of 10 µL was injected. The quercetin was identified by comparing the retention time and spectral characteristics of its peaks with that’s of standard.
Aalibration curve was established by diluting a quercetin stock solution with methanol. Within the concentration range injected (10, 50, 100, 250 and 500 mg/mL), the detector response was linear (R2 ≥ 0.9996). The sensibility of the method evaluated determining the limits of detection (LOD) and limits of quantification (LOQ). LOD and LOQ are 1.951 mg/L and 6.506 mg/L, respectively.
DPPH radical scavenging activity
The capacity of the plant extract to scavenge the DPPH radical was measured according to Blois method with a slight modification (CitationTurkoglu et al., 2006). 1000 µL of the extracts (0.5 mg/mL and 1 mg/mL) were added to a 4 mL of a 0.004% methanol solution of DPPH. After 30 min of incubation in dark at room temperature, the absorbance was read against a blank at 517 nm. Inhibition (I) of a free radical by DPPH in percent I (%) was calculated as follows:
where, Ablank is the absorbance of the control reaction and Asample is the absorbane of the test compound. Tocopherol was used for comparison.
Experimental procedures
Experiments were performed on male Wistar rats (weighing 150–200 g each). The protocol was approved by Ege University, Local Ethical Committee of Animal Experiment (26.02.2010, no. 2010–40). Animals were housed in a room maintained at 22 ± 1°C with an alternating 12 h light-dark cycle. Food and water were available ad libitum. The animals were transported to a quiet laboratory at least 1 h before the experiment. All experiments conformed to ethical guidelines for investigation of experimental pain in conscious animals (CitationZimmermann, 1983). The number of animals and the intensity of noxious stimuli were the minimum possible with which to demonstrate reliable effects of the agents tested. Each animal was used once only and was humanely sacrificed immediately after completion of testing.
Assessment of anti-inflammatory activity
The anti-inflammatory activity was evaluated by the carrageenan-induced (Sigma Chemical Co., St. Louis, USA) paw edema test in the rat (CitationWinter et al., 1962; CitationSchapoval et al., 1998). Male Wistar rats were deprived of food overnight and treated intraperitoneally with saline (as control group) and Romix® (6.75, 12.5 and 25 mg/kg), 30 min before 0.1 mL 1% carrageenan in isotonic saline was injected subplantarly into the left hind paw. The contra lateral paw was injected with 0.1 mL saline and used as a control. Paw volume was measured by water plethysmometer (Lettica, LE 7500, Barcelona, Spain) before and 1, 2, 3, 4, 5 and 6 h after the injection of carrageenan into the plantar region of the left hind paw. The anti-inflammatory test was repeated with 10 mg/kg indomethacin (Sigma Chemical Co., St.Louis, USA) administration.
Acute toxicity
In this study, the acute intraperitoneal (i.p.) toxicity of the ethanol extract of Romix® was assessed using the limit test in the rat (CitationDerelanko & Hollinger, 1995; CitationBarile 2008). The limit dose (2000 mg/kg body weight) for acute i.p. toxicity according to EPA/OECD was used. In the test, male and female Wistar albino rats weighing 150–200 g each were used (n = 5 for each group).
Statistics
Results are reported as means ± SEM (n), with n indicating the number of animals. Data were analyzed using the Student’s t-test, ANOVA, or nonparametric tests, as appropriate. A probability value of p < 0.05 was considered to denote a statistically significant difference.
Results
Total flavonoid, quercetin and polyphenol contents
The results showed that ethanol extract of Romix® had high total flavonoid and polyphenol contents than water extract (). The quercetin content of the extract was determined by HPLC and chromatograms of standart quercetin (A) and Romix® extract (B) were shown in . Total quercetin content of Romix® extract was calculated as 2.857 mg/g.
Table 1. Content of flavonoid and total phenolic compounds in Romix® extracts.
Antioxidant activity
The antioxidant activities of the Romix® extracts are reported in . The results showed that ethanol extract exerted higher antioxidant activity than water extract.
Table 2. Total antioxidant activity of Romix® extracts.
Anti-inflammatory activity
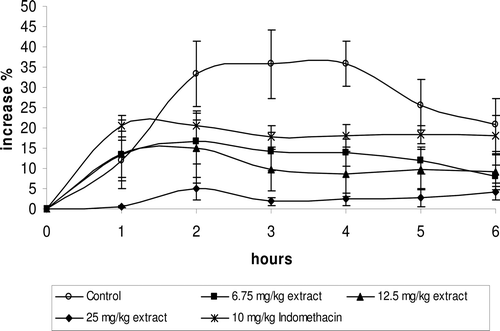
The intraplantar injection of carrageenan caused a time dependent paw edema in the rat, although saline injection caused no swelling (data not shown). Intraperitoneal administration of 12.5 mg/kg Romix® extract inhibited paw swelling at 2, 3, 4, and 5 h (p < 0.05) while 25 mg/kg Romix® extract inhibited paw swelling at 1, 2, 3, 4, 5 and 6 h (p < 0.05). Similarly, a significant inhibition after 2, 3, 4 and 5 h (p < 0.05) was obtained by indomethacin administration (). Percent increment in paw swelling was calculated by using the values before carrageenan injection.
Figure 2. Percent increase in carrageenan-induced paw edema in control rats treated with control (saline), in rats treated with 6.75, 12.5, and 25 mg/kg i.p. Romix® extract, and 10 mg/kg i.p. indomethacin. Percent increment in paw swelling was calculated by using the values before carrageenan injection (n = 7 for each group). Intraperitoneal administration of 12.5 mg/kg Romix® extract inhibited paw swelling at 2, 3, 4, and 5 h (p < 0.05) while 25 mg/kg Romix® extract inhibited paw swelling at 1, 2, 3, 4, 5 and 6 h (p < 0.05). Similarly, a significant inhibition after 2, 3, 4 and 5 h (p < 0.05) was obtained by indomethacin administration.
Acute toxicity
No lethality was observed among rats treated with i.p. doses of the ethanol extract of Romix®.
Discussion
Several medicinal plants have been extensively used in the traditional system of medicine. In this study, the effect of ethanol extract of Romix® was studied in an animal model for the evaluation of anti-inflammatory activity. The antioxidant capacity of infusions prepared from Romix® was also evaluated by using radical scavenging capacity and the results clearly demonstrated that Romix® infusion had an antioxidant activity. Total flavonoid with the quercetin content and total phenol contents were also determined. The results showed that ethanol extract had higher antioxidant activity than water extract which may be correlated with the high total flavonoid and polyphenol contents. It is already well known that polyphenols are the major plant compounds with antioxidant activity. Flavonoids are among the important polyphenolic components detected in plant extracts (CitationRice-Evans et al., 1996; CitationMoure et al., 2001; CitationBouaziz et al., 2005).
Carrageenan-induced paw edema test is a suitable test for the evaluation of anti-inflammatory activity. This method has frequently been used to assess the antiedematous effects of natural products (CitationSegura et al., 1998). Several mechanisms of action were proposed to explain the in vivo activity, including inhibition of phospholipase A2, cyclooxygenases (COX), lipoxygenases, and modulation of proinflammatory gene expression such as COX-2 and inducible nitric oxide synthase (CitationKim et al., 2004). Investigations demonstrated that carrageenan edema was effectively decreased by COX inhibitors (CitationDimartino et al., 1987). We have observed that Romix® extract significantly inhibited carrageenan-induced paw edema at 12.5 and 25 mg/kg, i.p. doses. It may be assumed that the anti-edema effects of the extract might be due to possible inhibition of the COX pathway. Some flavanoids, such as quercetin, blocked both the COX and lipoxygenase pathways at relatively high concentrations, while at lower concentrations the lipoxygenase pathway was the primary target of inhibitory anti-inflammatory activity (CitationLandolfi et al.,1984; CitationGuardia et al., 2001). Anti-inflammatory effect of quercetin has been shown in several in vitro and in vivo studies (CitationSato et al., 1997; CitationPelzer et al., 1998; CitationShen et al., 2002; CitationMorikawa et al., 2003). Our results have shown that suppressing inflammatory reaction in vivo can result from the flavanoids such as quercetin.
The role of oxygen-derived free radicals, such as OH− radical and superoxide radical, in the inflammatory process is well known (CitationFantone & Ward, 1982). It is also generally assumed that most of the antioxidants possess anti-inflammatory effects (CitationJang et al., 1997). A number of plant polyphenols have been shown to have antioxidant and anti-inflammatory properties (CitationRao et al., 1982). There is much evidence accumulated that flavonoids possess important effects on various biological systems, which may explain their widespread therapeutic uses (CitationDi Carlo et al., 1999). Flavonoids demonstrate various pharmacological activities. Among these actions, the anti-inflammatory activity of flavonoids may be mediated by the inhibition of the AA-metabolizing enzymes, COX/LOX, as well as by their antioxidative properties (CitationChi et al., 2001). We suppose that the anti-inflammatory effect of the ethanol extract of Romix® could be related to radical scavenging activity and that it depends on a synergic action of all the components of the ethanol extract. Recent studies report that quercetin suppresses inflammatory response in association with antioxidative capacity (CitationSánchez de Medina et al., 2002). Our data suggested that the anti-inflammatory effect of quercetin may be due to its antioxidative property.
The acute LD50 value of the extract of Romix® after i.p. administration in mice was >2000 mg/kg in 24 h. Because the anti-inflammatory effect of Romix® extract was induced at doses far below the LD50 value, this effect is of potential therapeutic use and this extract deserves further pharmacological investigation.
Conclusion
In conclusion, up to our knowledge this is the first report of the antioxidant and anti-inflammatory activities of Romix® extracts. It was clearly demonstrated in our study that ethanol extract of Romix® exhibits anti-inflammatory activity in rats. These findings suggest that Romix® due to its antioxidant and anti-inflammatory activities may be useful in the prevention or treatment of aging-related and various inflammatory diseases.
Acknowledgments
This work was supported by the Naturin Natural Products, Drug and Drug Raw Material Ltd. Izmir, Turkey.
Declaration of interest
The authors declared no conflict of interest.
References
- Barile FA. (2008). Principles of Toxicology Testing. CRC Press, Inc., Taylor & Francis Group. New York, USA.
- Bergendi L, Benes L, Duracková Z, Ferencik M. (1999). Chemistry, physiology and pathology of free radicals. Life Sci, 65, 1865–1874.
- Bimakra M, Rahmana RA, Taip FS, Ganjloob A, Md Salleha L, Selamat J, Hamid A, Zaidul ISM. (2011). Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Process, 89, 67–72.
- Block G, Patterson B, Subar A. (1992). Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer, 18, 1–29.
- Bouaziz M, Grayer RJ, Simmonds MS, Damak M, Sayadi S. (2005). Identification and antioxidant potential of flavonoids and low molecular weight phenols in olive cultivar chemlali growing in Tunisia. J Agric Food Chem, 53, 236–241.
- Chang CC, Yang MH, Wen HM, Chern JC. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal, 10, 178–182.
- Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. (2001). Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Biochem Pharmacol, 62, 1185–1191.
- Cuvrelier ME, Richard H, Berset C. (1992). Comparison of the antioxidant activity of some acid phenols, structure-activity relationship. Biosci Biotech Bioch, 56, 324–325.
- Derelanko MJ, Hollinger MA. (Eds.) (1995). CRC Handbook of Toxicology, 2nd edn. CRC Press Inc.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. (1999). Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci, 65, 337–353.
- Dimartino MJ, Griswold DE, Berkowitz BA, Poste G, Lewis AJ. (1987). Pharmacologic characterization of the anti-inflammatory properties of a new dual inhibor of lipoxygenase and cyclooxygenase. Agents Action, 20, 113–123.
- Fantone JC, Ward PA. (1982). Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol, 107, 395–418.
- Guardia T, Rotelli AE, Juarez AO, Pelzer LE. (2001). Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco, 56, 683–687.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. (1997). Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 275, 218–220.
- Kim HP, Son KH, Chang HW, Kang SS. (2004). Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci, 96, 229–245.
- Landolfi R, Mower RL, Steiner M. (1984). Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol, 33, 1525–1530.
- Larson RA. (1988). The antioxidants of higher plants. Phytochemistry, 27, 969–978.
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. (2005). Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem, 91, 571–577.
- Middleton E, Kandswami C. (1994). The Flavanoids: Advances in Research Since 1986, ed by JB Harborne 1st edn. Chapman & Hall, London. pp. 619–652.
- Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, Brissot P, Cillard P, Cillard J. (1993). Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol, 45, 13–19.
- Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, Kumazawa Y, Morikawa S. (2003). Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci, 74, 709–721.
- Moure A, Cruz JM, Franco D, Dominquez JM, Sineiro J, Dominquez H, Nunez MJ, Parajo JC. (2001). Natural antioxidants from residual sources. Food Chem, 72, 145–171.
- Pelzer LE, Guardia T, Osvaldo Juarez A, Guerreiro E. (1998). Acute and chronic anti-inflammatory effects of plant flavonoids. Farmaco, 53, 421–424.
- Rao TS, Basu N, Siddiqui HH. (1982). Anti-inflammatory activity of curcumin analogues. Indian J Med Res, 75, 574–578.
- Rice-Evans CA, Miller NJ, Paganga G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20, 933–956.
- Sánchez de Medina F, Vera B, Gálvez J, Zarzuelo A. (2002). Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci, 70, 3097–3108.
- Sato M, Miyazaki T, Kambe F, Maeda K, Seo H. (1997). Quercetin, a bioflavonoid, inhibits the induction of interleukin 8 and monocyte chemoattractant protein-1 expression by tumor necrosis factor-a in cultured human synovial cells. J Rheumatol, 24, 1680–1684.
- Schapoval EE, Vargas MR, Chaves CG, Bridi R, Zuanazzi JA, Henriques AT. (1998). Anti-inflammatory and antinociceptive activities of extracts and isolated compounds from Stachytarpheta cayennensis. J Ethnopharmacol, 60, 53–59.
- Segura L, Vila R, Gupta MP, Espósito-Avella M, Adzet T, Cañigueral S. (1998). Anti-inflammatory activity of Anthurium cerrocampanense Croat in rats and mice. J Ethnopharmacol, 61, 243–248.
- Shen SC, Lee WR, Lin HY, Huang HC, Ko CH, Yang LL, Chen YC. (2002). In vitro and in vivo inhibitory activities of rutin, wogonin, or quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production. Eur J Pharmacol, 446, 187–194.
- Singh RB, Downing D. (1995). Antioxidants and coronary artery disease. J Nutr Environ Med, 5, 219–224.
- Spácil Z, Nováková L, Solich P. (2008). Analysis of phenolic compounds by high performance liquid chromatography and ultra performance liquid chromatography. Talanta, 76, 189–199.
- Turkoğlu A, Kıvrak I, Mercan N, Duru ME, Gezer K, Turkoğlu H. (2006). Antioxidant and antimicrobial activities of Morchella conica Pers. Afr J Biotechnol, 5, 1146–1150.
- Unver N, Irem Kaya G, Tansel Ozturk H. (2005). Antimicrobial activity of Sternbergia sicula and Sternbergia lutea. Fitoterapia, 76, 226–229.
- van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. (1996). Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med, 20, 331–342.
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenan-induced edema in hind paw of rat as an assay for anti-inflammatory drugs. Prog Soc Biol Med, 11, 544–547.
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110.